Abstract
Historically, approaches designed to offer women diagnosed with cancer the prospects of having a genetically matched child after completion of their cytotoxic treatments focused on the existing oocyte population as the sole resource available for clinical management of infertility. In this regard, elective oocyte and embryo cryopreservation, as well as autologous ovarian cortical tissue grafting posttreatment, have gained widespread support as options for young girls and reproductive-age women who are faced with cancer to consider. In addition, the use of ovarian protective therapies, including gonadotropin-releasing hormone agonists and sphingosine-1-phosphate analogs, has been put forth as an alternative way to preserve fertility by shielding existing oocytes in the ovaries in vivo from the side-effect damage caused by radiotherapy and many chemotherapeutic regimens. This viewpoint changed with the publication of now numerous reports that adult ovaries of many mammalian species, including humans, contain a rare population of oocyte-producing germ cells—referred to as female germline or oogonial stem cells (OSCs). This new line of study has fueled research into the prospects of generating new oocytes, rather than working with existing oocytes, as a novel approach to sustain or restore fertility in female cancer survivors. Here, we overview the history of work from laboratories around the world focused on improving our understanding of the biology of OSCs and how these cells may be used to reconstitute “artificial” ovarian tissue in vitro or to regenerate damaged ovarian tissue in vivo as future fertility-preservation options.
Keywords: ovary, oocyte, stem cell, oogenesis, folliculogenesis, fertility, chemotherapy
Introduction: Current Fertility-Preservation Strategies
In women, the incidence of cancer increases dramatically from about 1 in 10,000 shortly after birth to about 1 in 300 as women reach the end of reproductive life during their mid-forties.1 It is well-documented that treatment of girls and of women before the age 45 for cancer with radiation, chemotherapeutic drugs or a combination of the two therapies can result in significant, and often irreversible, side-effect damage to the reproductive system.2–6 Oocytes are highly sensitive to cytotoxic therapies, with premature depletion of the ovarian follicle reserve frequently reported as an unintended consequence of anticancer treatments, especially those that involve pelvic radiotherapy or alkylating agents.7–11 At present, the cryopreservation of mature eggs and preimplantation embryos is the clinical standard of care for female cancer patients, although the patient’s age (adolescents), type of cancer (estrogen-responsive), or urgency of treatment initiation (aggressive cancers) may preclude the use of these approaches for all patients. Alternative strategies that involve the collection of immature (germinal vesicle-stage) oocytes for subsequent in-vitro maturation (IVM) and in-vitro fertilization (IVF) followed by embryo transfer,12–20 as well as the cryopreservation of ovarian cortical tissue strips for autologous transplantation after the anticancer treatments are completed,21–27 have gained increased attention as potential fertility-preservation options and are in clinical study around the world.
In addition to these approaches, which rely almost entirely on the cryopreservation of either oocytes (or embryos) or ovarian tissue strips containing oocytes before the initiation of treatments for cancer, parallel work has focused on minimizing ovarian damage in female cancer patients in vivo through pharmacologic protection of existing oocytes from cytotoxic insults during treatment.28–30 For example, building on early preclinical studies with rhesus monkeys showing that gonadotropin-releasing hormone (GnRH) agonists could reduce the loss of ovarian follicles caused by cyclophosphamide exposure,31 promising results have been obtained in some clinical studies of GnRH agonist treatment for ovarian protection in female cancer patients.32–40 However, other studies have questioned if use of GnRH analogs as a co-treatment in women receiving chemotherapy provides a clear benefit to maintaining specifically fertile potential in women.41–44 Although the consensus appears to be that GnRH agonist treatment can minimize ovarian damage in female cancer patients such that a resumption of menses and normal endocrine profiles are observed, the field remains divided over whether these outcomes actually lead to improvements in pregnancy success rates in women after cessation of treatment.
In parallel to studies of GnRH agonists, other work has explored the prospects of using anti-apoptotic small molecules as ovarian-protective agents in the face of radiotherapy and chemotherapy. This line of investigation arose from the observation that exposure of oocytes to chemotherapeutic drugs activates apoptosis or programmed cell death.45 Extensive genetic studies in mice, designed to define the key pathways utilized by oocytes to die,8 uncovered an indispensable role for ceramide generated by acid sphingomyelinase in triggering chemotherapy-induced oocyte apoptosis. In turn, a natural inhibitor of ceramide, termed sphingosine-1-phosphate (S1P), was shown in mice to prevent oocyte loss and infertility resulting from anticancer treatments.46,47 These findings of a protective effect of S1P on fertile potential in vivo after radiotherapy exposure were eventually extended to nonhuman primates, with the birth of healthy offspring free of any evidence of propagated cytogenetic damage.48
Although clinical studies of S1P or S1P analogs have not yet, to our knowledge, been pursued, several reports have provided evidence supporting a similar cytoprotective function of S1P in human ovaries. For example, two separate studies have shown that S1P reduces primordial follicle loss in human ovarian tissue xenografted in mice and exposed to cyclophosphamide as an in-vivo model of chemotherapy-induced ovarian damage.49,50 In addition and of direct relevance to clinical studies focused on restoration of fertility in women through orthotopic or heterotopic transplantation of cryopreserved-and-thawed ovarian cortical tissue strips,21–27 S1P has also been reported to minimize the loss of primordial follicles that occurs during vitrification and thawing of mouse ovarian tissue grafts51 as well as during the slow-freezing and thawing of human ovarian cortical tissue.52
These encouraging outcomes have prompted efforts to identify additional factors capable of minimizing ovarian damage caused by anticancer treatments, with one of the most recent being a preclinical study in rats reporting that injections of curcumin and capsaicin can offset cyclophosphamide-induced premature ovarian failure.53 In other studies, co-administration of imatinib (Gleevec), a 2-phenyl amino pyrimidine derivative that inhibits activity of the tyrosine kinase domains of c-Abl, c-Kit and platelet-derived growth factor receptor, has been reported to attenuate follicle depletion in mice caused by cisplatin treatment,54 the latter of which activates apoptosis in mouse oocytes through the TAp63 signaling pathway.55 However, a subsequent study with two different strains of mice failed to show protective effects of imatinib on either oocyte apoptosis (follicle depletion) or infertility resulting from cisplatin treatment,56 leaving open the question of whether small molecules such as imatinib, which specifically target receptor-associated tyrosine kinases, would be beneficial for fertility preservation in women exposed to cytotoxic agents. In fact, therapeutic strategies that interfere with c-Kit function may actually be counter-productive in efforts to maintain or restore female fertility, given the well-established importance of c-Kit to germ-cell development and survival.57,58
Discovery of Mammalian Oogonial Stem Cells
While the use, in some manner, of existing oocytes or the embryos generated from these oocytes through IVF as the traditional approach to female fertility preservation has yielded positive clinical results, progress toward the development of future technologies aimed at sustaining or restoring fertility in female cancer survivors would be severely constrained if existing oocytes were the only resource to work with. In 2004, this constraint was lifted by a report that identified the existence of mitotically active germ cells in postnatal mouse ovaries capable of supporting de-novo oogenesis and folliculogenesis during adult life.59–62 This report was met, not surprisingly, with a mix of cautious excitement along with outright disbelief.63–66 In short, it defied one of the foundational tenets in the field of reproductive science—female mammals are endowed with all of the oocytes they will ever have at birth, and this pool is not subject to renewal during postnatal life.67,68
Amid debate, continued studies on this topic from multiple laboratories around the world eventually produced a now large body of evidence substantiating the occurrence of postnatal oogenesis in mammals,69–76 as well as the characteristic features and functional properties of the germ cells responsible for continued oocyte formation.77 These cells, termed female germline or oogonial stem cells (OSCs), have been identified in, and isolated for study from, adult ovarian tissue of mice,75,76,78–102 rats,103 cows,104 pigs,105,106 nonhuman primates,107 and women.82,85,102,108–111 In evaluating OSCs from a functional perspective, the identity of these cells as bona-fide oocyte-producing germ cells has been independently verified by several groups using intragonadal transplantation-based approaches in rodent models,76,78,80,82,90,94,103 which are universally considered the litmus test for functional identity testing of the male-equivalent spermatogonial stem cells (SSCs) in the testis.112–114 These studies have collectively shown OSCs, expressing a fluorescent reporter for cell fate tracking, transplanted into ovaries of adult wild-type recipients generate oocytes that develop into mature eggs which fertilize to produce viable embryos and offspring.76,78,80,82,90,94,103 Extending these observations, two groups have also used genetic mouse models that enable inducible tracking of cell fate in vivo to discern the physiological significance, if any, of OSCs and postnatal oogenesis in adult females. These experiments confirmed the occurrence of germ cell meiotic entry and de-novo oogenesis in the ovaries during reproductive life,75,76 and further showed that oocytes formed during adulthood are used to produce offspring in natural mating trials.76 This considerable body of evidence, from multiple laboratories around the world, documenting the existence and functional properties of OSCs in adult mammalian ovaries across species should erase any residual doubts that the long-standing paradigm of a fixed pool of oocytes being endowed in ovaries of female mammals at birth has repeatedly been proven incorrect.77
Characteristics of Human OSCs
The isolation of OSCs from ovaries of women by at least four independent groups to date has enabled detailed comparative studies of these cells with OSCs in animal models, and by all accounts, the human cells display the fundamental characteristics of oocyte-generating germline stem cells.82,85,102,108–111 Although complete functional identity testing has not yet, at least to our knowledge, been performed with human OSCs, the cells have been tested for oocyte-forming capacity in a number of ways. For example, human OSCs maintained as pure germ cell cultures exhibit the same capacity as rodent OSCs to spontaneously generate in-vitro-derived (IVD) oocytes after passage and replating.82,85 Importantly, these human IVD oocytes express expected patterns of oocyte-specific genes82 and are able to complete meiotic progression to reach formal haploid status, as defined by flow cytometric analysis of DNA content per cell,82 as well as by fluorescence in-situ hybridization of chromosome numbers per cell.111 Building on these observations from cultured OSCs, introduction of enhanced green fluorescent protein (EGFP)-expressing human OSCs into adult human ovarian cortical tissue ex vivo has been used to trace the generation of new EGFP-positive oocytes that form primordial follicles capable of early development and growth.82,108 Moreover, similar outcomes can be achieved by simple reaggregation of EGFP-expressing human OSCs with cellular dispersates prepared from adult82 or fetal110 human ovarian tissue, collectively supporting that OSCs are fully capable of differentiating into oocytes that interact with appropriate ovarian somatic cell partners to form new follicles.
Although still early, these exciting findings offer a strong foundation on which to push forward with additional models and testing, including incorporation of existing technologies for in-vitro follicle growth115–119 along with those for IVM of germinal vesicle-stage oocytes for IVF and embryo transfer.12–20 The ultimate goal will be to establish an in-vitro platform that reliably enables the generation of developmentally competent eggs derived from human OSCs recombined in some manner with autologous ovarian somatic cells to facilitate oogenesis and folliculogenesis.77,120 Given the established ability of rodent OSCs to successfully differentiate into eggs that yield viable offspring,76,78,80,82,90,94,103 we feel that, with the appropriate environmental support, human OSCs will indeed be capable of achieving the same outcomes as their mouse and rat counterparts.
Reconstitution of Human Folliculogenesis in vitro
As discussed earlier, ovarian tissue cryopreservation and transplantation is now offered by some clinics as an option for fertility preservation in female cancer survivors.21–27 However, the live-birth success rate of the approach, even 15 years after initial reports of its use, remains fairly low.25 This limitation, coupled with the fact that the procedure presents significant risks associated with the multiple surgeries required to obtain and subsequently return the ovarian tissue used for cryopreservation and transplantation, highlights the need for additional technologies to adequately meet the family-planning hopes of these patients once their treatments are completed.121 Cryopreservation of eggs and embryos are now well-established alternatives for women diagnosed with cancer to consider for a future chance at having a baby; however, these approaches are not a viable option for all patients, especially those who are adolescent or suffer from premature ovarian insufficiency. With just these few options available, efforts by several groups to design and test new in-vitro and in-vivo platforms rooted in the principles of stem cell-based regenerative medicine may offer additional fertility solutions for cancer survivors.
To that end, significant strides have been made over the past several years in the generation of functional eggs from stem cells entirely outside of the body, using mice as an exploratory model system. This line of investigation dates back to the early work of Hübner et al,122 who first reported the in-vitro derivation of oocyte-like cells and follicle-like structures from mouse embryonic stem cells (ESCs) in culture. Almost a decade later, Hayashi et al123 successfully specified primordial germ cell-like cells (PGC-LCs) from mouse ESCs and induced pluripotent stem cells (iPSCs). When the PGC-LCs were aggregated with fetal gonadal somatic cells and then grafted to ovaries of adult female recipients, the cells differentiated into immature oocytes that yielded viable offspring following IVM, IVF, and embryo transfer.123 These experiments were further refined to eventually remove the need for in-vivo tissue grafting, ultimately providing a platform for complete reconstitution of female gametogenesis from ESCs and iPSCs entirely ex vivo.124 Parallel studies of human ESCs and iPSCs have provided evidence that, like their murine counterparts, these cells are capable of generating PGC-LCs as well125,126 and that ovarian follicle-like structures can be formed from human ESCs in culture.127 Collectively, these studies have prompted widespread, but in our view quite premature, speculation that technologies involving ESCs and iPSCs will one day in the not-too-distant future offer women in need of fertility assistance the opportunity to produce essentially unlimited eggs for assisted reproduction.128–130
However, it is critical to emphasize several key points when evaluating the potential of ESCs or iPSCs to solve human female infertility in the future. The first of these is the apparent obligate need for PGC-LCs to interact with fetal gonadal somatic cells for differentiation into oocytes that can mature into competent eggs,123,124 a concept reinforced by previous studies with mice showing that PGC-LCs introduced in adult ovarian tissue generate only immature oocytes that arrest and degenerate at very early stages of follicle development.128 It is therefore unclear, if only for practical reasons, how human fetal gonadal tissue would be obtained and made available for use in any type of clinical platform involving gametogenesis from pluripotent stem cells. Even if this major obstacle is overcome, the issue is further complicated by the fact that non-autologous fetal ovarian tissue would be needed to produce eggs from iPSC-derived germline cells of another individual. A second stumbling block to human translation of the mouse work with iPSCs pertains to regulatory issues surrounding genetic manipulation of the parental somatic cells to obtain iPSCs in the first place, whether or not one employs nonintegrating genetic approaches for nuclear reprogramming. Finally, it remains to be determined, even in mice, if nuclear reprogramming of differentiated somatic cells into iPSCs for subsequent generation of PGC-LCs generates germline cells that effectively carry out the process of maternal mitochondrial inheritance. In other words, studies must be performed to demonstrate that offspring produced from iPSC-derived eggs are not burdened from the outset with compromised mitochondrial genomes that are pre-existent in the parental somatic cells reprogrammed into iPSCs for germ cell specification.131
In comparison, OSCs represent an alternative source of oocyte-generating stem cells present in the ovaries of women which are free of these limitations and complications, but exhibit the same capacity as ESCs or iPSCs in mouse studies to generate fully functional eggs that fertilize and produce viable offspring.76,78,80,83,90,94,103 Unlike iPSCs, however, the use of OSCs does not require any genetic manipulation or reprogramming since OSCs are already germline stem cells naturally wired to produce oocytes in adult ovaries.76 Moreover, OSCs are capable of generating mature oocytes when introduced into adult ovarian tissue,76,78,80,83,90,94,103 removing the need for fetal ovarian somatic cells that ESC- or iPSC-derived PGC-LCs depend on to achieve full functionality as egg precursor cells.123,124 Despite these advantages, however, OSCs are not capable of differentiation into fully functional eggs alone, and any technology platform that uses these cells, like all other stem cells, will require parallel incorporation of appropriate somatic cell partners for success.120
Without question, the most important of these partners are primitive or undifferentiated granulosa cells (sometimes referred to as pregranulosa cells), which are capable of interacting with newly generated oocytes to form primordial follicle-like structures. While previous studies have reported the existence and functional characterization of ovarian somatic stem cells that can give rise to thecal-interstitial cells,132,133 we are not aware of any publication to date that has definitively identified a putative granulosa stem cell population in postnatal ovarian tissue. The possibility that primitive granulosa cells differentiate from multi-potent stem cells present in the ovarian surface epithelium was proposed many years ago, based largely on indirect in-vitro studies of undefined (heterogeneous) ovarian cell cultures.134,135 However, rigorous follow-up experiments that have functionally tested this hypothesized lineage relationship, or that have confirmed multi-potent stem cells in adult mammalian ovaries are a physiological source of pregranulosa cells for de-novo folliculogenesis in vivo, are lacking. In fact, it is still unclear if primitive granulosa cells can even be isolated from adult ovarian tissue and successfully expanded in vitro in an undifferentiated state for use in biomimetic stem cell-based platforms aimed at achieving folliculogenesis and, ultimately, ex-vivo egg generation.120 By employing fluorescence-activated cell sorting (FACS), we have very recently isolated a distinct population of ovarian somatic cells that express stem cell-associated genes (e.g. POU domain class 5 transcription factor 1 or Pou5f1, and Nanog), can be stably expanded in-vitro over many passages, and exhibit several key features of primitive granulosa cells. The latter includes expression of the Forkhead box L2 (Foxl2) and Wingless-type MMTV integration site family member 4 (Wnt4) genes, as well as inducible expression of follicle-stimulating hormone (FSH) receptor and the molecular machinery required for steroidogenesis (T. Akahori, D.C. Woods and J.L. Tilly, unpublished data). While we await the outcome of functional characterization studies that are underway in our laboratories to unequivocally establish the identity of these cells, we will turn our attention back to published studies on potential sources of primitive granulosa cells.
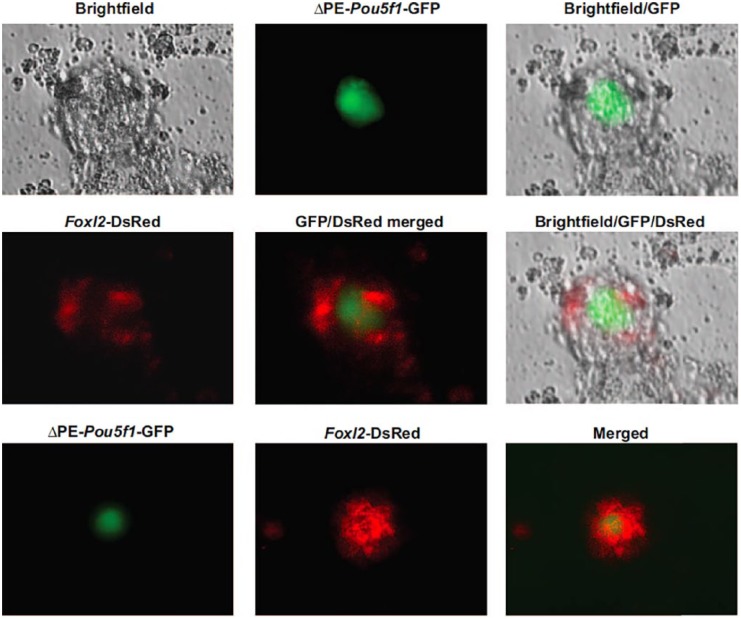
Building on earlier observations of Hübner et al122 that follicle-like structures, capable of steroidogenesis and extrusion of oocyte-like cells, can be observed in cultures in mouse ESCs, other groups reported similar outcomes using mouse ESCs with some disagreement on “normalcy” of the oocyte-like cells contained within the follicle-like structures.136–138 Moreover, all of these reports focused almost entirely on the germline side of the story, with comparatively little attention placed on the putative granulosa cells also present in these structures. In 2013, Woods et al139 reported the first in-depth study of primitive granulosa cell specification from a dual-reporter mouse ESC line engineered to express EGFP driven by a modified Pou5f1 gene promoter and red fluorescent protein (DsRed) under control of the Foxl2 gene promoter to simultaneously track germ cell and granulosa cell formation, respectively. After confirming the generation of follicle-like structures containing EGFP-positive germ (oocyte-like) cells surrounded by DsRed-positive somatic (pre-granulosa) cells in ESC cultures (Figure 1), the DsRed-positive cells were isolated by FACS at various time points postspecification and analyzed by several approaches.
Figure 1.
Specification of primitive ovarian granulosa cells from ESCs in vitro, which are capable of interacting with early germ cells to initiate folliculogenesis. Differentiation of mouse ESCs engineered to express green fluorescent protein (GFP) driven by a ΔPE-Pou5f1 gene promoter (germ cell marker) and DsRed driven by a Foxl2 gene promoter (primitive granulosa cell marker) leads to the in-vitro formation of ovarian follicle-like structures containing GFP-positive oocytes surrounded by DsRed-expressing granulosa cells. Reproduced from Woods et al.139
Of several interesting findings presented in this study, DsRed-expressing cells collected relatively early after specification from ESCs exhibited a gene expression profile consistent with an in-vivo pregranulosa cell phenotype, as defined by expression of Foxl2, Wnt4, Follistatin (Fst), and Kit ligand (Kitl), among other genes.139 As the culture time was extended, the gene expression profile in the DsRed-positive cells changed to one more in line with granulosa cells at an early stage of differentiation, as demonstrated by the activation of FSH receptor (Fshr), anti-Müllerian hormone (Amh), cytochrome P450 family 19 subfamily a polypeptide 1 (Cyp19a1), and steroidogenic acute regulatory protein (Star) gene expression. Perhaps the most convincing evidence of a granulosa cell identity, however, was derived from in-vivo transplantation studies of DsRed-positive cells isolated from these ESC cultures, in which the fate of the cells in ovaries was traced to incorporation in the granulosa cell layer of immature follicles.139 The findings reported in this study, which showed that Foxl2-expressing somatic cells formed from differentiating ESCs express granulosa cell markers, actively associate with germ cells in vitro, synthesize steroids, respond to FSH, and participate in folliculogenesis in vivo,139 have been repeated and extended by others.140–142
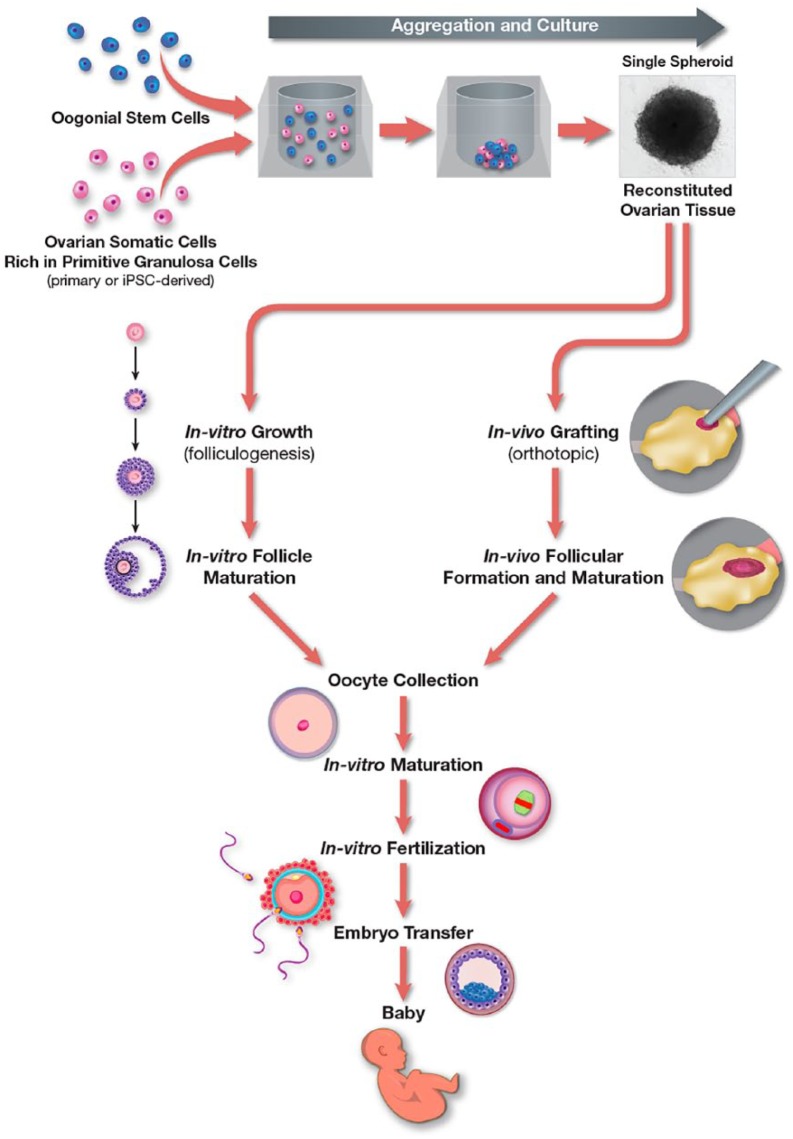
Collectively, these observations offer a strong impetus to consider the use of patient-derived iPSCs to generate autologous primitive or pregranulosa cells for aggregation with OSCs to successfully reconstitute the process of oogenesis and folliculogenesis in vitro143 (Figure 2). It is also worth mentioning that several studies have reported the generation of steroidogenic cells from iPSCs derived from reprogrammed granulosa cells.144–146 In one of these studies, it was further concluded that generation of iPSCs from the cell type that one seeks to ultimately specify in vitro may prove advantageous due to epigenetic memory of the parental cells being carried through the reprogramming and lineage specification process.145 In any case, the prospect of designing an in-vitro platform for the generation of mature human eggs through stem cell-based bioengineering, while still early in development, is clearly inching closer to reality.
Figure 2.
Working model for ex-vivo reconstitution of autologous human ovarian tissue. Aggregation of OSCs with primitive granulosa cells, specified from iPSCs or isolated from ovarian tissue during OSC purification, enables de-novo oogenesis and folliculogenesis in the reconstituted tissue in vitro. The tissue containing new follicles is then used for orthotopic grafting to the ovaries for in-vivo growth to produce maturing follicles for oocyte aspiration or for in-vitro follicle culture to generate oocytes. Oocytes obtained from either approach are subjected to in-vitro maturation and in-vitro fertilization to generate blastocysts for embryo transfer and establishment of successful pregnancies.
Human Ovarian Regeneration in vivo
In parallel to the efforts outlined above for in-vitro reconstitution of human oogenesis and folliculogenesis from stem cells, a growing body of evidence supports that a reintroduction of OSCs back into ovarian tissue following chemotherapy may enable at least some recovery of normal ovarian function and fertility. The first observations made in this regard came from mouse studies a decade ago, in which transplantation of EGFP-expressing OSCs into ovaries of adult wild-type female mice previously conditioned with chemotherapy was used to demonstrate that the cells can successfully differentiate into GFP-positive oocytes which complete maturation and produce viable offspring in natural mating trials.78 These observations from OSC transplantation have been independently confirmed by others using chemotherapy-treated mice as a model90 and are in keeping with the well-established ability of SSC transplantation to restore fertility in male mice conditioned with busulfan.114
A different way to look at the question of ovarian regeneration in vivo could entail therapeutic “reactivation” of resident OSCs, which perhaps undergo some type of protective dormancy in the face of cytotoxic insults, to resume normal function once the anticancer treatments are completed. However, such an approach to female fertility preservation or restoration would depend on several factors to realize success. One of these would be the identification and validation of deliverable “oogenic” factors capable of driving OSC differentiation into oocytes. Unfortunately, the list of these factors is quite small at present and includes histone deacetylase inhibitors,73 bone morphogenetic protein 4 (BMP4),84 Hippo signaling pathway components,95 and as-yet unidentified factors present in the peripheral circulation of males.72 Nevertheless, the ability of OSCs to generate IVD oocytes in culture76,82,84,85,102,111 provides a firm foundation on which to perform high-throughput screening of candidate oogenic factors that can then be rigorously tested for their ability to expand the ovarian reserve in vivo.120
A second point worth considering here is the type of anticancer treatment employed, since those regimens known to present a high risk for premature ovarian failure—such as radiotherapy or alkylating agents, may destroy resident OSCs. However, other chemotherapeutic agents, such as doxorubicin (Adriamycin), may not kill off OSCs and thus might allow for a spontaneous recovery of oocyte numbers and ovarian function, depending on dose, mode of delivery and duration of the treatment.69 Likewise, a recent study of age-matched ovarian tissue collected from women without and with previous treatment with an adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD)-based chemotherapeutic regimen uncovered a significant increase in nongrowing follicle density in ABVD-exposed ovaries.147 Although the mechanistic basis of this surprising observation remains to be determined, it was postulated that OSCs were possibly recruited into action following the initial damage to the ovaries, leading to enhanced de-novo oogenesis and folliculogenesis after the ABVD treatments were ceased.147
Any approach designed to achieve ovarian regeneration in vivo should also take into account methods that entail delivery of various somatic cell preparations capable of broadly repairing ovaries damaged by anticancer treatments, possibly enabling resident stem cells to resume normal function. For example, early studies by Johnson et al69 demonstrated that systemic bone marrow transplantation (BMT) into adult female mice conditioned beforehand with a combination of cyclophosphamide and busulfan could partially restore oocyte numbers and ovarian function compromised by the chemotherapy regimen. Evaluation of the ovaries of the wild type mice receiving BMT using EGFP-expressing transgenic female mice as donors further indicated the presence of EGFP-positive oocytes contained in immature follicles.69 However, like the developmental arrest observed in oocytes generated from ESC-derived PGC-LCs transplanted into adult ovaries,128 bone marrow stem cell-derived oocytes exhibit a similar developmental arrest and do not contribute to the pool of ovulated eggs used for reproduction.148,149
Nonetheless, in rodent models, BMT has a clear beneficial effect on fertility preservation in chemotherapy-treated adult females,149 and these restorative outcomes are also observed in untreated female mice that exhibit natural aging-associated impairments in fertility and reproductive outcomes in natural mating trials.150 Although it remains unresolved if BMT exerts similar protective effects in women subjected to chemotherapy or radiotherapy,151 recent studies using human bone marrow-derived stem cells infused into immunodeficient female mice treated with chemotherapy to induce ovarian damage indicate that human bone marrow cells can, like their mouse counterparts, significantly improve follicle development and fertility.152 These types of studies support that stem cell-based ovarian regeneration in women is, in all likelihood, possible, but that the approach will need to encompass a restoration of oogenesis and follicular development69,149,152 along with repair of the ovarian stroma and microvascular beds which are known to be negatively impacted by chemotherapy.153
Concluding Remarks and Future Directions
The field of fertility preservation has made tremendous strides over the years in bringing new hope to survivors of cancer that they can have genetically matched children once their treatments are completed. Progress in human oocyte, embryo, and ovarian tissue cryopreservation, coupled with continued improvements in IVM of human oocytes and in efforts to therapeutically protect ovarian function in vivo during the course of cytotoxic treatments aimed at killing cancer cells, offers several options for women to consider. However, many limitations exist, both in technology and in application, which support the need for development of additional approaches to achieve fertility preservation or restoration. Of the various scientific directions currently being pursued, the relatively recent introduction of regenerative medicine-based technologies into efforts designed to improve natural and assisted reproduction is one of the most exciting, and potentially the most high-impact, areas of investigation.77,120,143,154,155
In this regard, it is important to emphasize that the successful reconstitution of female gametogenesis in mice from mitotically active germ cells to functional eggs entirely in vitro123,124—an accomplishment that only a decade ago was incomprehensible to most scientists in the field of reproductive biology, conceptually epitomizes the power of stem cell biology for potentially addressing unmet needs in reproductive medicine. Likewise, the existence of oocyte-producing stem cells in the ovaries of mammals,59–62 which are fully capable of supporting de-novo oogenesis in adult life,76,78,80,82,90,94,103 was not even considered a possibility 15 years ago because of five decades of previous dogmatic thinking arguing in support of fixed endowment of oocytes at birth.67,68 With human OSCs now identified and under rigorous investigation by several groups,82,85,102,108–111 scientists have tools to work with today that have the inherent capacity to forever change the landscape of human reproduction and infertility. These types of research investigations, coupled with those in other exciting arenas of reproductive medicine and health, combine to offer great hope for the future of female fertility preservation.77,120,143,154–157
Acknowledgments
The authors thank Michael Cooper (Cooper Graphics) for expert graphic arts assistance with the preparation of Figure 2. Work conducted by the authors discussed herein was supported by grants from the U.S. National Institutes of Health (grant nos.: R01-AG012279 and R21-HD072280) and the Glenn Foundation for Medical Research.
Footnotes
Author Contributions: All authors contributed to the writing and preparation of this article, and approved its final submission.
Funding:Work conducted by the authors discussed herein was supported by grants from the National Institutes of Health (grant nos.: R01-AG012279 and R01-HD072280) and the Glenn Foundation for Medical Research.
Declaration of conflicting interest:T.A. declares no competing interests. D.C.W. declares interest in intellectual property described in U.S. Patent 8,642,329, U.S. Patent 8,647,869 and U.S. Patent 9,150,830. J.LT declares interest in intellectual property described in U.S. Patent 7,195,775, U.S. Patent 7,850,984, U.S. Patent 7,955,846, U.S. Patent 8,642,329, U.S. Patent 8,647,869, U.S. Patent 8,652,840, U.S. Patent 9,150,830, U.S. Patent 9,267,111 and U.S. Patent 9,845,482.
References
- 1. Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006;11:590–601. [DOI] [PubMed] [Google Scholar]
- 2. Waxman J. Chemotherapy and the adult gonad: a review. J R Soc Med. 1983;76:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reichman BS, Green KB. Breast cancer in young women: effect of chemotherapy on ovarian function, fertility and birth defects. J Natl Cancer Inst Monogr. 1994;16:125–129. [PubMed] [Google Scholar]
- 4. Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91:1723–1728. [DOI] [PubMed] [Google Scholar]
- 5. Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–896. [DOI] [PubMed] [Google Scholar]
- 6. Levine JM, Kelvin JF, Quinn GP, Gracia CR. Infertility in reproductive-age female cancer survivors. Cancer. 2015;121:1532–1539. [DOI] [PubMed] [Google Scholar]
- 7. Tilly JL, Johnson AL. Chemotherapy and apoptosis in the ovary. Treatment comes with a price. In: Hickman JA, Dive C, eds. Apoptosis and Cancer Chemotherapy. Totowa, NJ: Humana Press; 1999:257–273. [Google Scholar]
- 8. Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–848. [DOI] [PubMed] [Google Scholar]
- 9. Johnston RJ, Wallace WH. Normal ovarian function and assessment of ovarian reserve in the survivor of childhood cancer. Pediatr Blood Cancer. 2009;53:296–302. [DOI] [PubMed] [Google Scholar]
- 10. Gracia CR, Sammel MD, Freeman E, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353–362. [DOI] [PubMed] [Google Scholar]
- 13. Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109–113. [DOI] [PubMed] [Google Scholar]
- 14. Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103–120. [DOI] [PubMed] [Google Scholar]
- 15. Kim BK, Lee SC, Kim KJ, Han CH, Kim JH. In-vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil Steril. 2000;74:1153–1158. [DOI] [PubMed] [Google Scholar]
- 16. Son WY, Chung JT, Demirtas E, et al. Comparison of in-vitro maturation cycles with and without in-vivo matured oocytes retrieved. Reprod Biomed Online. 2008;17:59–67. [DOI] [PubMed] [Google Scholar]
- 17. Oktay K, Buyuk E, Rodriguez-Wallberg KA, Sahin G. In vitro maturation improves oocyte or embryo cryopreservation outcome in breast cancer patients undergoing ovarian stimulation for fertility preservation. Reprod Biomed Online 2010;20:634–638. [DOI] [PubMed] [Google Scholar]
- 18. Fadini R, Dal Canto M, Mignini Renzini M, et al. Embryo transfer following in vitro maturation and cryopreservation of oocytes recovered from antral follicles during conservative surgery for ovarian cancer. J Assist Reprod Genet. 2012;29:779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang EM, Song HS, Lee DR, Lee WS, Yoon TK. In vitro maturation of human oocytes: its role in infertility treatment and new possibilities. Clin Exp Reprod Med. 2014;41:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prasath EB, Chan ML, Wong WH, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–278. [DOI] [PubMed] [Google Scholar]
- 21. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. [DOI] [PubMed] [Google Scholar]
- 22. Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. [DOI] [PubMed] [Google Scholar]
- 23. Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. [DOI] [PubMed] [Google Scholar]
- 24. Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. [DOI] [PubMed] [Google Scholar]
- 25. Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24:1111–1120. [DOI] [PubMed] [Google Scholar]
- 28. Tilly JL. Pharmacological protection of female fertility. In: Tulandi T, Gosden RG, eds. Fertility Preservation. London: Taylor & Francis; 2004:65–75. [Google Scholar]
- 29. Selesniemi KL, Tilly JL. Therapeutic targeting of apoptosis in female reproductive biology. In: Reed JC, Green DR, eds. Apoptosis: Physiology and Pathology. New York: Cambridge University Press; 2011:273–282. [Google Scholar]
- 30. Roness H, Kashi O, Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil Steril. 2016;105:20–29. [DOI] [PubMed] [Google Scholar]
- 31. Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52:365–372. [DOI] [PubMed] [Google Scholar]
- 32. Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel with chemotherapy. Hum Reprod. 1996;11:1620–1626. [DOI] [PubMed] [Google Scholar]
- 33. Del Mastro L, Catzeddu T, Boni L, et al. Prevention of chemotherapy-induced menopause by temporary ovarian suppression with goserelin in young, early breast cancer patients. Ann Oncol. 2006;17:74–78. [DOI] [PubMed] [Google Scholar]
- 34. Recchia F, Saggio G, Amiconi G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514–523. [DOI] [PubMed] [Google Scholar]
- 35. Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–1054. [DOI] [PubMed] [Google Scholar]
- 36. Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008;14:543–552. [DOI] [PubMed] [Google Scholar]
- 37. Urruticoechea A, Arnedos M, Walsh G, Dowsett M, Smith IE. Ovarian protection with goserelin during adjuvant chemotherapy for pre-menopausal women with early breast cancer (EBC). Breast Cancer Res Treat. 2008;110:411–416. [DOI] [PubMed] [Google Scholar]
- 38. Clowse ME, Behera MA, Anders CK, et al. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. J Womens Health (Larchmt). 2009;18:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Del Mastro L, Boni L, Michelotti A, et al. Effect of gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced menopause on premenopausal women with breast cancer: a randomized trial. J Am Med Assoc. 2011;306:269–276. [DOI] [PubMed] [Google Scholar]
- 40. Lambertini M, Ceppi M, Poggio F, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015;26:2408–2419. [DOI] [PubMed] [Google Scholar]
- 41. Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Pharmacological interventions for fertility preservation during chemotherapy: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010;122:803–811. [DOI] [PubMed] [Google Scholar]
- 42. Kim SS, Lee JR, Jee BC, et al. Use of hormonal protection for chemotherapy-induced gonadotoxicity. Clin Obstet Gynecol. 2010;53:740–752. [DOI] [PubMed] [Google Scholar]
- 43. Bedaiwy MA, Abou-Setta AM, Desai N, et al. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: a systematic review and meta-analysis. Fertil Steril. 2011;95:906–914. [DOI] [PubMed] [Google Scholar]
- 44. Yang B, Shi W, Yang J, et al. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a meta-analysis of randomized controlled trials. Breast. 2013;22:150–157. [DOI] [PubMed] [Google Scholar]
- 45. Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228–1232. [DOI] [PubMed] [Google Scholar]
- 46. Morita Y, Perez GI, Paris F, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–1114. [DOI] [PubMed] [Google Scholar]
- 47. Paris F, Perez GI, Fuks Z, et al. Sphingosine-1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8:901–902. [DOI] [PubMed] [Google Scholar]
- 48. Zelinski MB, Murphy MK, Lawson MS, et al. In-vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female non-human primates. Fertil Steril. 2011;95:1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meng Y, Xu Z, Wu F, et al. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in mice. Fertil Steril. 2014;102:871–877. [DOI] [PubMed] [Google Scholar]
- 50. Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod. 2014;29:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsai TC, Tzeng CR, Wang CW, Hsu MI, Tan SJ, Chen CH. Antiapoptotic agent sphingosine-1-phosphate protects vitrified murine ovarian grafts. Reprod Sci. 2014;21:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guzel Y, Bildik G, Dilege E, Oktem O. Sphingosine-1-phosphate reduces atresia of primordial follicles occurring during slow-freezing and thawing of human ovarian cortical strips. Mol Reprod Dev. 2018;85:858–864. [DOI] [PubMed] [Google Scholar]
- 53. Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res. 2018;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-Tap63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. [DOI] [PubMed] [Google Scholar]
- 55. Kim SY, Cordeiro MH, Serna VA, et al. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20:987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kerr JB, Hutt KJ, Cook M, et al. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat Med. 2012;18:1170–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carlsson IB, Laitinen MP, Scott JE, et al. Kit ligand and c-Kit are expressed during early human ovarian follicular development and their interaction is required for the survival of follicles in long-term culture. Reproduction. 2006;131:641–649. [DOI] [PubMed] [Google Scholar]
- 58. Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2016;12:61–69. [DOI] [PubMed] [Google Scholar]
- 59. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. [DOI] [PubMed] [Google Scholar]
- 60. Tilly JL, Johnson J. Compositions comprising female germline stem cells and methods of use thereof. U.S. patent 7,955,846. June 7, 2011. [Google Scholar]
- 61. Tilly JL, Johnson J. Method for obtaining female germline stem cells and uses thereof. U.S. patent 8,652,840. February 18, 2014. [Google Scholar]
- 62. Tilly JL, Johnson J. Isolated populations of female germline stem cells and cell preparations and compositions thereof. U.S. patent 9,962,411. May 8, 2018. [Google Scholar]
- 63. Skaznik-Wikiel M, Tilly JC, Lee HJ, et al. Serious doubts over “Eggs Forever?”. Differentiation. 2007;75:93–99. [DOI] [PubMed] [Google Scholar]
- 64. Tilly JL, Johnson J. Recent arguments against germ cell renewal in the adult human ovary. Is an absence of marker gene expression really acceptable evidence of an absence of oogenesis? Cell Cycle. 2007;6:879–883. [DOI] [PubMed] [Google Scholar]
- 65. Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism. Biol Reprod. 2009;80:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Woods DC, White YAR, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view towards the future. Reprod Sci. 2013;20:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zuckerman S. The number of oocytes in the mature ovary. Rec Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 68. Franchi LL, Mandl AM, Zuckerman S. The development of the ovary and the process of oogenesis. In: Zuckerman S, ed. The Ovary. New York: Academic Press; 1962:1–88. [Google Scholar]
- 69. Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells derived from bone marrow and peripheral blood. Cell. 2005;122:303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. [DOI] [PubMed] [Google Scholar]
- 71. Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany NY). 2009;1:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Niikura Y, Niikura T, Wang N, Satirapod C, Tilly JL. Systemic signals in aged males exert potent rejuvenating effects on the ovarian follicle reserve in mammalian females. Aging (Albany NY). 2010;2:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Woods DC, Telfer EE, Tilly JL. Oocyte family trees: old branches or new stems? PLoS Genet. 2012;8:e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guo K, Li CH, Wang XY, He DJ, Zheng P. Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod. 2016;22:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang N, Satirapod C, Ohguchi Y, Park ES, Woods DC, Tilly JL. Genetic studies in mice directly link oocytes produced during adulthood to ovarian function and natural fertility. Sci Rep. 2017;7:10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martin JJ, Woods DC, Tilly JL. Implications and current limitations of oogenesis from female germline or oogonial stem cells in adult mammalian ovaries. Cells. 2019;8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. [DOI] [PubMed] [Google Scholar]
- 79. Pacchiarotti J, Maki C, Ramos T, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–170. [DOI] [PubMed] [Google Scholar]
- 80. Zhang Y, Yang Z, Yang Y, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–141. [DOI] [PubMed] [Google Scholar]
- 81. Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using Fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20:2197–2204. [DOI] [PubMed] [Google Scholar]
- 82. White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Imudia AN, Wang N, Tanaka Y, White YA, Woods DC, Tilly JL. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil Steril. 2013;100:1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park ES, Woods DC, Tilly JL. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil Steril. 2013;100:1468–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8:966–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang H, Jiang M, Bi H, et al. Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol. 2014;6:164–171. [DOI] [PubMed] [Google Scholar]
- 87. Xie W, Wang H, Wu J. Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep. 2014;4:5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khosravi-Farsani S, Amidi F, Habibi Roudkenar M, Sobhani A. Isolation and enrichment of mouse female germ line stem cells. Cell J. 2015;16:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Park ES, Tilly JL. Use of DEAD-box polypeptide 4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in postnatal mouse ovaries. Mol Hum Reprod. 2015;21:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xiong J, Lu Z, Wu M, et al. Intraovarian transplantation of female germline stem cells rescues ovarian function in chemotherapy injured ovaries. PLoS ONE. 2015;10:e0139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lu Z, Wu M, Zhang J, et al. Improvement in isolation and identification of mouse oogonial stem cells. Stem Cells Int. 2016;2016:2749461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang C, Wu J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Hum Reprod. 2016;22:457–464. [DOI] [PubMed] [Google Scholar]
- 93. Zhang XL, Wu J, Wang J, et al. Integrative epigenomic analysis reveals unique epigenetic signatures involved in unipotency of mouse female germline stem cells. Genome Biol. 2016;17:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wu C, Xu B, Li X, et al. Tracing and characterizing the development of transplanted female germline stem cells in vivo. Mol Ther. 2017;25:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ye H, Li X, Zheng T, et al. The Hippo signaling pathway regulates ovarian function via the proliferation of ovarian germline stem cells. Cell Physiol Biochem. 2017;41:1051–1062. [DOI] [PubMed] [Google Scholar]
- 96. Gu Y, Wu J, Yang W, et al. STAT3 is required for proliferation and exerts a cell type-specific binding preference in mouse female germline stem cells. Mol Omics. 2018;14:95–102. [DOI] [PubMed] [Google Scholar]
- 97. Wang J, Gong X, Tian GG, et al. Long noncoding RNA growth arrest-specific 5 promotes proliferation and survival of female germline stem cells in vitro. Gene. 2018;653:14–21. [DOI] [PubMed] [Google Scholar]
- 98. Wu M, Xiong J, Ma L, et al. Enrichment of female germline stem cells from mouse ovaries using the differential adhesion method. Cell Physiol Biochem. 2018;46:2114–2126. [DOI] [PubMed] [Google Scholar]
- 99. Yang H, Yao X, Tang F, Wei Y, Hua J, Peng S. Characterization of female germline stem cells from adult mouse ovaries and the role of rapamycin on them. Cytotechnology. 2018;70:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang HY, Yang Y, Xia Q, et al. Cadherin 22 participates in the self-renewal of mouse female germline stem cells via interaction with JAK2 and ß-catenin. Cell Mol Life Sci. 2018;75:1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhu X, Tian GG, Yu B, Yang Y, Wu J. Effects of bisphenol A on ovarian follicular development and female germline stem cells. Arch Toxicol. 2018;92:1581–1591. [DOI] [PubMed] [Google Scholar]
- 102. MacDonald JA, Takai Y, Ishihara O, Seki H, Woods DC, Tilly JL. Extracellular matrix signaling activates differentiation of adult ovary-derived oogonial stem cells in a species-specific manner. Fertil Steril. 2019;111:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhou L, Wang L, Kang JX, et al. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2014;20:271–281. [DOI] [PubMed] [Google Scholar]
- 104. de Souza GB, Costa J, da Cunha EV, et al. Bovine ovarian stem cells differentiate into germ cells and oocyte-like structures after culture in vitro. Reprod Domest Anim. 2017;52:243–250. [DOI] [PubMed] [Google Scholar]
- 105. Tsai TS, Johnson J, White Y, John JC. The molecular characterization of porcine egg precursor cells. Oncotarget. 2017;8:63484–63505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hou L, Wang J, Li X, et al. Characteristics of female germline stem cells from porcine ovaries at sexual maturity. Cell Transplant. 2018;27:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Woods DC, Tilly JL. Reply to adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med. 2015;21:1118–1121. [DOI] [PubMed] [Google Scholar]
- 108. Ding X, Liu G, Xu B, et al. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. 2016;6:28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bothun A, Gao Y, Takai Y, et al. Quantitative proteomic profiling of the human ovary from early to mid-gestation reveals protein expression dynamics of oogenesis and folliculogenesis. Stem Cells Dev. 2018;27:723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Clarkson YL, McLaughlin M, Waterfall M, et al. Initial characterisation of adult human ovarian cell populations isolated by DDX4 expression and aldehyde dehydrogenase activity. Sci Rep. 2018;8:6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Silvestris E, Cafforio P, D’Oronzo S, Felici C, Silvestris F, Loverro G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: single-cell isolation and molecular characterization. Hum Reprod. 2018;33:464–473. [DOI] [PubMed] [Google Scholar]
- 112. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kanatsu-Shinohara M, Morimoto H, Shinohara T. Fertility of male germline stem cells following spermatogonial transplantation in infertile mouse models. Biol Reprod. 2016;94:112. [DOI] [PubMed] [Google Scholar]
- 115. Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. [DOI] [PubMed] [Google Scholar]
- 116. McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH in a two-step serum-free culture system. Reproduction. 2010;139:971–978. [DOI] [PubMed] [Google Scholar]
- 117. Xu M, Fazleabas A, Shikanov A, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Telfer EE, McLaughlin M. Strategies to support human oocyte development in vitro. Int J Dev Biol. 2012;56:901–907. [DOI] [PubMed] [Google Scholar]
- 119. McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod. 2018;24:135–142. [DOI] [PubMed] [Google Scholar]
- 120. Woods DC, Tilly JL. Germline stem cells in adult mammalian ovaries. In: Sanders S, ed. Ten Critical Topics in Reproductive Medicine. Washington, DC: Science/AAAS; 2013:10–12. [Google Scholar]
- 121. Takai Y. Recent advances in oncofertility care worldwide and in Japan. Reprod Med Biol. 2018;17:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hübner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. [DOI] [PubMed] [Google Scholar]
- 123. Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–975. [DOI] [PubMed] [Google Scholar]
- 124. Hikabe O, Hamazaki N, Nagamatsu G, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539:299–303. [DOI] [PubMed] [Google Scholar]
- 125. Panula S, Medrano JV, Kee K, et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yamashiro C, Sasaki K, Yabuta Y, et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science. 2018;362:356–360. [DOI] [PubMed] [Google Scholar]
- 127. Jung D, Xiong J, Ye M, et al. In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat Commun 2017; 8:15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nicholas CR, Haston KM, Grewall AK, Longacre TA, Reijo Pera RA. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum Mol Genet. 2009;18:4376–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Smitz JE, Gilchrist RB. Are human oocytes from stem cells next? Nat Biotechnol. 2016;34:1247–1248. [DOI] [PubMed] [Google Scholar]
- 130. Cohen IG, Daley GQ, Adashi EY. Disruptive reproductive technologies. Sci Transl Med. 2017;9:eaag2959. [DOI] [PubMed] [Google Scholar]
- 131. Woods DC, Khrapko K, Tilly JL. Influence of maternal aging on mitochondrial heterogeneity, inheritance, and function in oocytes and preimplantation embryos. Genes (Basel). 2018;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Honda A, Hirose M, Hara K, et al. Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. Proc Natl Acad Sci USA. 2007;104:12389–12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dalman A, Totonchi M, Valojerdi MR. Establishment and characterization of human theca stem cells and their differentiation into theca progenitor cells. J Cell Biochem. 2018;119:9853–9865. [DOI] [PubMed] [Google Scholar]
- 134. Bukovsky A, Caudle MR, Svetlikova M, Upadhyaya NB. Origin of germ cells and formation of new primary follicles in adult human ovaries. Reprod Biol Endocrinol. 2004;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol. 2005;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Novak I, Lightfoot DA, Wang H, Eriksson A, Mahady E, Höög C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24:1931–1936. [DOI] [PubMed] [Google Scholar]
- 137. Salvador LM, Silva CP, Kostetskii I, Radice GL, Strauss III JF. The promoter of the oocyte-specific gene, Gdf9, is active in population of cultured mouse embryonic stem cells with an oocyte-like phenotype. Methods. 2008;45:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Psathaki OE, Hübner K, Sabour D, et al. Ultrastructural characterization of mouse embryonic stem cell-derived oocytes and granulosa cells. Stem Cells Dev. 2011;20:2205–2215. [DOI] [PubMed] [Google Scholar]
- 139. Woods DC, White YAR, Niikura Y, Kiatpongsan S, Lee H-J, Tilly JL. Embryonic stem cell-derived granulosa cells participate in follicle formation in vitro and in vivo. Reprod Sci. 2013;20:524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang J, Li H, Tan X, Liu F, Huang X, Fang X. Differentiation of rat iPS cells and ES cells into granulosa cell-like cells in vitro. Acta Biochim Biophys Sin (Shanghai). 2013;45:289–295. [DOI] [PubMed] [Google Scholar]
- 141. Liu T, Li Q, Wang S, Chen C, Zheng J. Transplantation of ovarian granulosa-like cells derived from human induced pluripotent stem cells for the treatment of murine premature ovarian failure. Mol Med Rep. 2016;13:5053–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lipskind S, Lindsey JS, Gerami-Naini B, et al. An embryonic and induced pluripotent stem cell model for ovarian granulosa cell development and steroidogenesis. Reprod Sci. 2017;25:712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Truman AM, Tilly JL, Woods DC. Ovarian regeneration: the potential for stem cell contribution in the postnatal ovary to sustained endocrine function. Mol Cell Endocrinol. 2017;445:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mao J, Zhang Q, Ye X, Liu K, Liu L. Efficient induction of pluripotent stem cells from granulosa cells by Oct4 and Sox2. Stem Cells Dev. 2014;23:779–789. [DOI] [PubMed] [Google Scholar]
- 145. Anchan R, Gerami-Naini B, Lindsey JS, et al. Efficient differentiation of steroidogenic and germ-like cells from epigenetically-related iPSCs derived from ovarian granulosa cells. PLoS ONE. 2015;10:e0119275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Mao J, Liu L. Generation if iPS cells from granulosa cells. Methods Mol Biol. 2016;1357:451–464. [DOI] [PubMed] [Google Scholar]
- 147. McLaughlin M, Kelsey TW, Wallace WHB, Anderson RA, Telfer EE. Non-growing follicle density is increased following adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy in the adult human ovary. Hum Reprod. 2017;32:165–174. [DOI] [PubMed] [Google Scholar]
- 148. Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2005;441:1109–1114. [DOI] [PubMed] [Google Scholar]
- 149. Lee HJ, Selesniemi K, Niikura Y, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–3204. [DOI] [PubMed] [Google Scholar]
- 150. Selesniemi K, Lee HJ, Niikura T, Tilly JL. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging (Albany NY). 2008;1:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jadoul P, Donnez J. How does bone marrow transplantation affect ovarian function and fertility? Curr Opin Obstet Gynecol. 2012;24:164–171. [DOI] [PubMed] [Google Scholar]
- 152. Herraiz S, Buigues A, Diaz-Garcia C, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109:908–918. [DOI] [PubMed] [Google Scholar]
- 153. Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Woods DC, Tilly JL. The next (re)generation of human ovarian biology and female fertility: is current science tomorrow’s practice? Fertil Steril. 2012;98:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Woods DC, Tilly JL. Autologous germline mitochondrial energy transfer (AUGMENT) in human assisted reproduction. Semin Reprod Med. 2015;33:410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Park A. The incredible, surprising, controversial new way to make a baby. Time. April 13, 2015:185. [PubMed] [Google Scholar]
- 157. Park A. The next frontier in fertility treatments. Time. January 14, 2019:34–37. [Google Scholar]