Abstract
Introduction:
This study examines how many patients with distal radius fracture (DRF) eligible for bone health evaluation could potentially be screened using bone mineral density (BMD) estimation by L1 vertebra computed tomography (CT) attenuation obtained for other purposes.
Materials and Methods:
For all adult patients with DRF who presented over a 5-year period, we recorded the age, sex, dual-energy X-ray absorptiometry (DXA) results up to 3 years prior to injury or 1 year post-injury, and L1 CT attenuation on any CT including L1 that had been performed within 6 months of their fracture.1 We compared the availability of L1 CT attenuation measurement to the rate of DXA scan use. We calculated the percentage of patients with osteoporosis and compared attenuation results to DXA results in those patients where both tests were available.
Results:
Of 1853 patients with DRF, an L1 CT had been obtained in 195 patients. Of the 685 patients who met criteria for osteoporosis screening, 253 (37%) patients had undergone only DXA screening, 68 (10%) patients had an L1 CT only, and 18 (2%) patients had both tests. Of the 86 patients who met criteria for osteoporosis screening and had an adequate CT, 67 (78%) demonstrated L1 attenuation <135 HU, and 79 (92%) had CT attenuation <160 HU.
Discussion:
Our study found that 10% of patients with a distal radius fracture who met the criteria for osteoporosis screening had a CT scan that could be used to estimate bone density and that the majority of those patients met criteria for osteoporosis based on CT attenuation.
Conclusions:
Utilization of opportunistic BMD screening with L1 CT attenuation offers the potential to increase osteoporosis screening from 40% to 50% of eligible patients and make the diagnosis of osteoporosis in an additional 8% of patients with DRF at no additional cost.
Keywords: distal radius fracture, osteoporosis, bone mineral density, CT attenuation, screening
Introduction
Distal radius fractures (DRFs) are the most common fracture in adults.1 In older patients, many of these fractures occur after minor trauma and represent one of the first clinical signs of osteoporosis. Currently, most physicians diagnose osteoporosis based on low bone mineral density (BMD) on dual-energy X-ray absorptiometry (DXA) testing. Decreased BMD as measured by DXA correlates with increased future fracture risk.2 Unfortunately, relatively few patients receive testing or treatment for osteoporosis even after a DRF.3 According to the State of Healthcare Quality 2014 report, less than 30% of women aged 65 to 85 received evaluation or treatment for osteoporosis following a fracture.4
Although considered the “gold standard” test for osteoporosis, DXA does not provide the only assessment of bone health.5 Other advanced imaging techniques such as high-resolution peripheral quantitative computed tomography (HR-pQCT) and high-resolution magnetic resonance imaging correlate with fragility fractures in postmenopausal women independent of BMD as measured by DXA.6 This suggests that HR-pQCT detects differences in osseous architecture not measured by DXA scans alone.7 While HR-pQCT remains an excellent research tool to examine bone microarchitecture, this method requires special training, software, and equipment which limits its broader application.
Recently, Pickhardt et al used a measurement of attenuation of the L1 vertebral body on abdominal or chest computed tomography (CT) to estimate BMD. This straightforward measurement correlated with DXA results in majority of patients.8 This simple tool could potentially be used to diagnose osteoporosis in patients with DRF who undergo CT examination for other reasons without additional cost or radiation exposure. A subsequent study by Pompe et al demonstrated good interobserver reliability for this simple technique.9 Previous studies have compared CT to DXA in patients but have not calculated how many potential patients could be screened using this tool.
We examined what percentage of patients with DRF at our institution could be screened for osteoporosis opportunistically using L1 vertebral attenuation on CT scans obtained for other reasons. In addition, we examined how many patients with DRF eligible for osteoporosis screening underwent DXA testing at our institution.
Materials and Methods
Institutional review board approval was obtained for this study. We received no funds in support of this work, and there are no relevant financial activities outside the submitted work. Using billing records, we retrospectively identified adult patients (age ≥18 years) who sought care for distal radius fractures at our level 1 trauma center from September 1, 2008, to September 1, 2013. These records include both outpatients and inpatients who presented to either a clinic or emergency department that is part of our multi-site organization, including a level 1 trauma center and associated outpatient clinics. We obtained the patient’s age, sex, date of injury, and any DXA test results from up to 3 years prior to the injury or 1 year after the injury. We identified any patients who had an abdominal or chest CT scan that included a view of the L1 vertebra taken in the 6 months before or after the fracture. Patients with fractures through L1 were excluded. We measured CT attenuation of L1 as described by Pickhardt et al.8 This simple technique requires the evaluator to select a “region of interest” using the selector tool available on digital CT readers, excluding the vertebral cortex to avoid artificial inflation of CT attenuation. For each patient, we recorded the maximum, minimum, and mean CT attenuation in Hounsfield units (HUs) over the region of interest and utilized the mean value for further analysis. We used the Pearson correlation coefficient to determine the relationship between age and BMD for patients of all ages.
The US Preventive Task Force recommends that DXA evaluation be performed in all patients with a fracture risk equivalent to a 65-year-old Caucasian female with no other risk factors. 10 Using the FRAX tool, and including only previous fracture (the index distal radius fracture) as a risk factor, women ≥55 years and men ≥65 years with a known DRF have a fracture risk equivalent to a 65-year-old Caucasian female with no other risk factors. We determined the number of patients who met these criteria and how many of those patients underwent DXA testing and/or CT evaluation within the previously described time windows. Pickhardt et al proposed several different cutoff values for osteoporosis screening using CT attenuation.8 We performed separate analyses with both the 160 HU criteria (for balanced sensitivity and specificity in differentiating between osteoporosis and nonosteoporosis) and the more stringent 135-HU cutoff (very specific, but less sensitive). We compared the results of CT attenuation with the results of DXA testing for all patients who had both test results.
Results
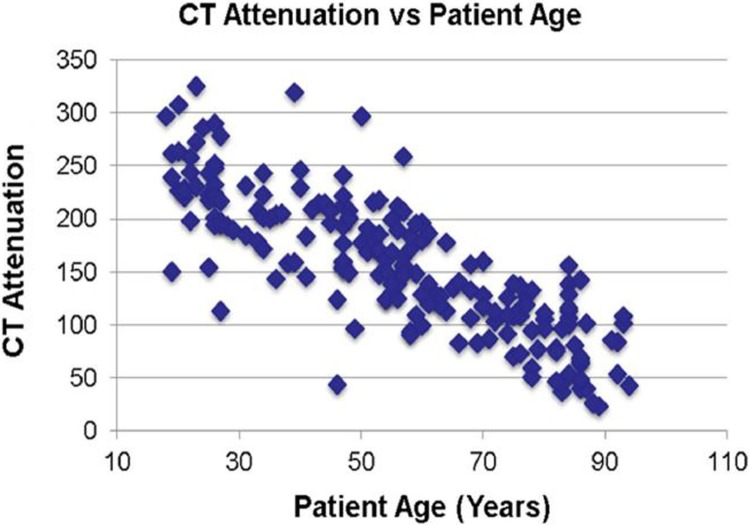
Over the 5-year period from 2008 to 2013, a total of 1853 patients presented with DRFs. A total of 195 (10.5%) patients also underwent a CT scan including L1 within 6 months of their DRF. Computed tomography attenuation closely correlated with age (Figure 1).
Figure 1.
Correlation of computed tomography (CT) attenuation and patient age (Pearson correlation coefficient = .81).
Of the patients with DRF, 685 met our usual criteria for DXA scan (females ≥55 years, and males ≥65 years; Table 1). Forty percent of patients underwent DXA scan: 124 patients before fracture, 130 patients after fracture, and 16 patients before and after fracture. Only 6 (8%) male patients underwent DXA testing, compared to 264 (43%) female patients.
Table 1.
Available Test Results in Patients Indicated for Bone Health Screening.
| Male, n = 75 | Female, n = 610 | |
|---|---|---|
| Adequate CT | 20 (27%) | 66 (10%) |
| DXA | 6 (8%) | 264 (43%) |
Abbreviations: CT, computed tomography; DXA, dual-energy X-ray absorptiometry.
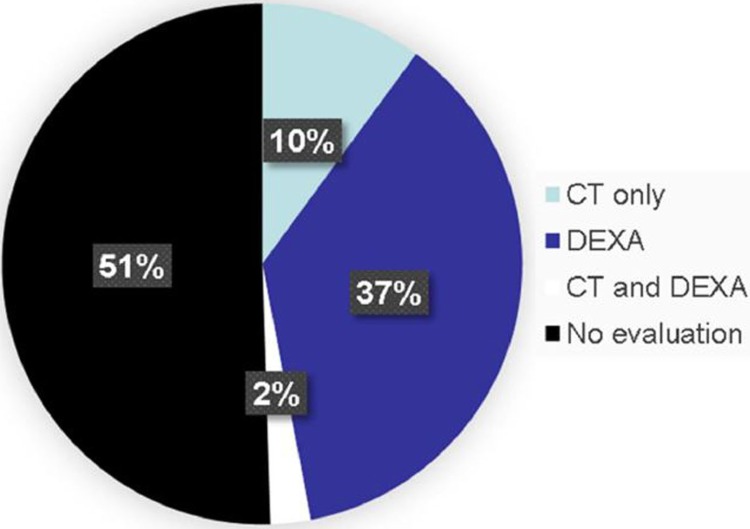
Of those who would meet criteria for osteoporosis screening, we identified 68 (10%) patients with a L1 CT scan within 6 months of their injury and no DXA testing, and an additional 18 patients who had both CT and DXA results. Twenty (27%) male patients and 66 (10%) female patients had L1 CT scans (Figure 2). We found a mean CT attenuation of 106.6 HU in this cohort (range 23-169 HU) for these 86 patients.
Figure 2.
Tests performed in patients indicated for bone health screening.
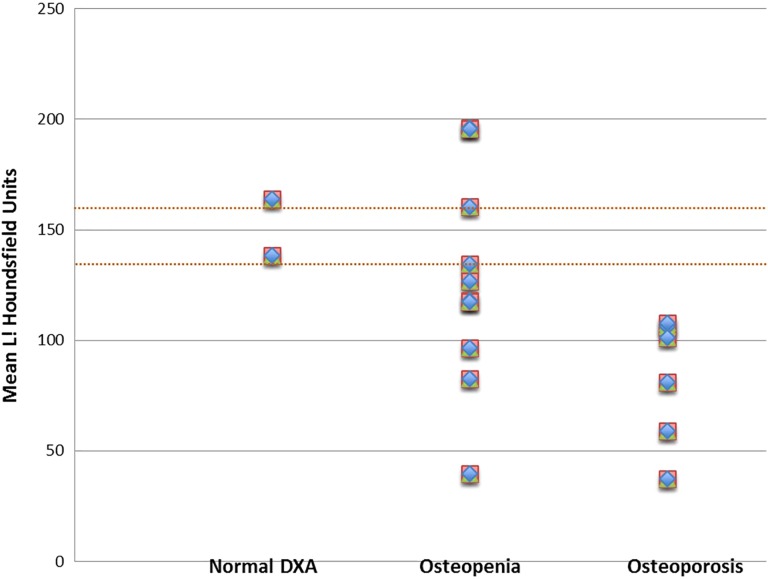
Using the 160 HU threshold, 79 (92%) of 86 patients met the criteria for osteoporosis. Sixty-seven (78%) of 86 patients fell below the more specific 135 HU threshhold. Of the 18 patients with both tests, CT attenuation did not always correlate with DXA results (Figure 3) as 6 of 10 patients with osteopenia by DXA met the criteria for osteoporosis by L1 CT scans by falling below the 135 HU threshold for CT attenuation.
Figure 3.
L1 computed tomography (CT) attenuation for patients with dual-energy X-ray absorptiometry (DXA) test results.
Of the remaining 109 patients with L1 CT scans (females <55 years and males <65 years), the mean CT attenuation was 167 HU (range 43-325 HU). Thirteen patients displayed CT attenuation <135 HU, and 16 additional patients had CT attenuation between 135 and 160 HU. Only 1 patient in this group underwent DXA testing, with a resulting diagnosis of osteoporosis; that patient also met criteria for osteoporosis by CT attenuation, with a value of 96 HU.
Discussion
Distal radius fractures are often the presenting clinical sign of osteoporosis and provide an opportunity to initiate bone health evaluation. At this time, most physicians utilize DXA testing to determine whether a patient has osteoporosis. Dual-energy X-ray absorptiometry testing is widely available and requires minimal radiation. Despite recommendations for BMD testing in older patients with DRFs, relatively few patients undergo DXA scans.4 Rozental et al found that 21% of patients underwent DXA testing within 39 months of their distal radius fracture.3 In that study, DXA scan rates improved substantially if the treating orthopedic surgeon ordered the DXA scan rather than referring to the primary care physician. According to the National Committee for Quality Assurance data, we fail to initiate osteoporosis evaluation or treatment in 70% of women older than 65 with a fracture.4 Given the significant burden of osteoporotic fractures on the health-care system, and the significant morbidity for patients, identifying osteoporosis and offering treatment options to prevent future fractures remain a priority for orthopedic surgeons.
Dual-energy X-ray absorptiometry scan may not identify all patients with increased fracture risk, but the greatest limitation to DXA remains failure to obtain the test.11 Despite a significant effort at our institution to initiate bone health evaluation, only 40% of patients with DRF underwent DXA scans during the 5-year study period, and male patients were much less likely than female patients to undergo testing.
This study demonstrates that opportunistic screening of BMD by L1 CT attenuation may provide another mechanism to evaluate bone health. Pickhardt et al introduced measurement of CT attenuation as a simple, inexpensive estimate of BMD in patients who already have a CT including L1.8 They examined CT bone attenuation in 1867 patients who had undergone both CT and DXA testing and found a strong correlation between CT attenuation and BMD as calculated by DXA. In fact, CT bone attenuation may be more sensitive and specific than DXA scan, as Pickhardt et al identified 62 patients with vertebral compression fractures who did not meet DXA criteria for osteoporosis (false negatives).8
Although all studies to date show a correlation between BMD and CT attenuation, this method has not been widely adopted. Lack of agreement about a specific CT attenuation cutoff for osteoporosis limits the usefulness of this technique. Pickhardt defined 3 potential cutoffs: 190 HU for greatest sensitivity but poor specificity, 160 HU for balanced sensitivity and specificity, and 135 HU for lowest sensitivity and greatest specificity.8 When comparing those few patients who had both CT and DXA results, we found that all patients with osteoporosis by DXA were well under the 135 HU cutoff as well as 6 of 10 patients diagnosed with osteopenia by DXA scan.
At this time, CT attenuation is not included in the FRAX tool.12 Further studies correlating CT attenuation directly with fracture risk are necessary. In addition, measuring CT attenuation is not a routine part of radiology evaluation, and reporting this value would require system changes for radiologists.11 However, orthopedic surgeons with an available CT scan can perform this reading without additional equipment or software.8
One limitation of this study is that our patients presented to the orthopedic department of a single health system in an urban area and may not be representative of the general population. We may have underestimated the actual DXA scan or CT rates, as we cannot account for patients who had a DXA scan or CT not recorded in our health system electronic medical record.
Conclusions
In conclusion, we found that an additional 10% of patients with DRF could have BMD estimated by CT attenuation without additional cost or radiation. Further research is needed to clarify the relationship between fracture risk and BMD by CT attenuation and establish appropriate diagnostic criteria for osteoporosis.
Footnotes
Authors’ Note: Mark A. Arnold is now affiliated with Department of Otolaryngology, Upstate Medical University, Syracuse, NY, USA. Osa Emohare is now affiliated with Department of Anesthesiology, Duke University Medical Center, Durham, NC, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Christina M. Ward, MD  https://orcid.org/0000-0003-4138-2175
https://orcid.org/0000-0003-4138-2175
References
- 1. Karl JW, Olson PR, Rosenwasser MP. The epidemiology of upper extremity fractures in the United States, 2009. J Orthop Trauma. 2015;29(8):e242–e244. [DOI] [PubMed] [Google Scholar]
- 2. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the rotterdam study. Bone. 2004;34(1):195–202. [DOI] [PubMed] [Google Scholar]
- 3. Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953–961. [DOI] [PubMed] [Google Scholar]
- 4. The National Committee for Quality Assurance. The state of healthcare quality: reform, the quality agenda and resource use. 2014. http://ncqa.org/tabid/836/Default.aspx. Accessed April 24, 2015.
- 5. Stone KL, Seeley DG, Lui LY, et al. Osteoporotic fractures research group. BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res. 2003;18(11):1947–1954. [DOI] [PubMed] [Google Scholar]
- 6. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–6515. [DOI] [PubMed] [Google Scholar]
- 7. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23(3):392–399. [DOI] [PubMed] [Google Scholar]
- 8. Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pompe E, de Jong PA, de Jong WU, et al. Interobserver and interexamination variability of manual vertebral bone attenuation measurements on computed tomography. Eur Radiol 2016;26:3046–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Preventive Services Task Force. Screening for osteoporosis: recommendation statement. Am Fam Physician 2011;83(10):197–200. [PubMed] [Google Scholar]
- 11. Majumdar SR, Leslie WD. Conventional computed tomography imaging and bone mineral density: opportunistic screening or “incidentaloporosis”? Ann Intern Med. 2013;158(8):630–631. [DOI] [PubMed] [Google Scholar]
- 12. Kanis JA, McCloskey EV, Johansson H, Oden A, Ström O, Borgström F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21(suppl 2):S407–S413. [DOI] [PubMed] [Google Scholar]