Abstract
Patients with isolated right ventricular (RV) failure have poor outcomes and minimal treatment options. We report a case where a durable RV assist device (RVAD) was implanted for end-stage RV failure from combined pre- and postcapillary pulmonary hypertension (PH) due in part to chronic thromboembolic PH using a temporary percutaneous RVAD as a bridging strategy. While the patient ultimately died from non-cardiovascular causes, there was significant improvement in markers of cardiogenic shock and hemodynamic RV function parameters without adverse effects from increased pulmonary artery pressures. More research is needed to identify an appropriate long-term mechanical support strategy for this patient population.
Keywords: RVAD, PH, CTEPH, mechanical circulatory support, Protek Duo, RV failure
Introduction
A right ventricular assist device (RVAD) using the HVAD (HeartWare, Inc., Framingham, MA, USA) has been used in conjunction with a left ventricular assist device (LVAD)1 for patients with combined LV and RV failure. However, RVAD implantation for isolated RV failure from pulmonary hypertension (PH) is rare.2,3 We report a case of an isolated durable RVAD implantation for refractory RV failure due in part to chronic thromboembolic pulmonary hypertension (CTEPH).
A 57-year-old man without significant medical history began to develop progressively worsening New York Heart Association (NYHA) class III symptoms of dyspnea on exertion. A work-up revealed CTEPH with baseline hemodynamics demonstrating a central venous pressure (CVP) of 5 mmHg, pulmonary artery pressure (PAP) of 97/30 (64) mmHg, LV end diastolic pressure of 16 mmHg, cardiac index of 2.1 L/min/m2, and pulmonary vascular resistance of 8.6 Woods units. An echocardiogram demonstrated a severely dilated RV with a basal diameter of 6.7 cm that was severely dysfunctional qualitatively. He underwent a successful pulmonary thromboendarterectomy but RV function failed to normalize on echocardiogram and he developed right heart failure associated with a large pericardial effusion five months postoperatively. He improved after pericardiocentesis and underwent a pericardial window to prevent recurrence. One year later, he developed refractory RV failure. A pulmonary angiogram demonstrated only a residual left upper lobe stenosis and moderate distal right middle lobe stenosis without other significant filling defects. Cardiac magnetic resonance imaging (MRI) revealed a thickened pericardium that enhanced with gadolinium and respirophasic ventricular interdependence consistent with constrictive pericarditis which failed to improve with medical therapy. The MRI also confirmed RV dysfunction with an ejection fraction of 26%. He underwent a pericardial stripping, biatrial MAZE for paroxysmal atrial fibrillation, and a mitral valve repair due to bileaflet mitral valve prolapse with severe mitral regurgitation. His preoperative hemodynamics were notable for a CVP of 22 mmHg and a PAP of 50/20 mmHg suggestive of significant RV dysfunction with persistent PH present before surgery.
His postoperative course was complicated by cardiogenic shock requiring multiple vasopressors and oliguric renal failure requiring continuous veno-venous hemofiltration (CVVH). Echocardiograms revealed normal LV function and severe RV dysfunction. Because he failed to improve, and with no other treatment options, a percutaneous RVAD was implanted. A 29Fr Protek Duo catheter (CardiacAssist, Inc., Pittsburgh, PA, USA) was inserted in the right internal jugular vein and connected to a TandemHeart (CardiacAssist, Inc., Pittsburgh, PA, USA) pump. Flow was increased until optimized at 3.7 L/min. The CVP declined from 22 mmHg to 11 mmHg, the RV stroke work index increased from 5.6 g/m2/beat to 8.0 g/m2/beat, and the PA pulsatility index (PA pulse pressure/CVP) increased from 0.7 to 1.3. While vasopressor requirements decreased but were unable to be completely weaned after 12 days, the decision was made to implant a durable RVAD as destination therapy for end-stage RV failure.
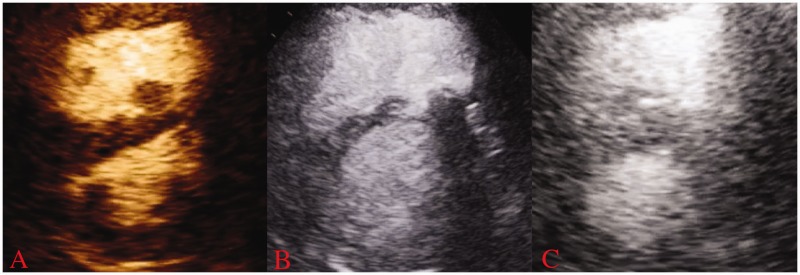
An HVAD was surgically implanted. The inflow cannula was placed in the right atrium and the outflow cannula in the proximal pulmonary artery. He was extubated on postoperative day (POD) 3 and pressor support continuously reduced. HVAD flow was maintained in the range of 5–6 L/min and pump speeds at 2400–2600 rpm without any evidence of pulmonary edema or hypoxia to suggest excessive preload of the left ventricle. Serial echocardiograms showed improvement in RV pressure and volume overload after RVAD implantation (Fig. 1). PAPs reduced slightly and the pulmonary wedge pressure remained unchanged. He was beginning to ambulate short distances, but on POD 7 he acutely worsened due to pneumonia requiring intubation and escalation of vasopressors. On POD 9, he developed severe upper gastrointestinal (GI) bleeding without an identified source, requiring temporary cessation of anticoagulation. On POD 13, he had an acute rise in LDH to 3900 U/L and power spikes along with worsening shock and signs of end organ perfusion due to RVAD pump thrombosis.
Fig. 1.
Transthoracic echocardiogram images obtained in the parasternal short-axis view in diastole from the patient. (a) Before any right ventricular mechanical support and demonstrates marked RV dilation and interventricular septal flattening. (b) After placement of the Protek Duo device showing partial improvement in septal shift and (c) After placement of durable RVAD and highlights significant improvement in RV dimensions and septal position.
He underwent urgent RVAD exchange where a thrombus was confirmed intraoperatively in the inflow cannula. His elevated transaminases resolved and serum lactic acid level decreased from 8.4 mMol/L to 1.9 mMol/L within 48 h. Echocardiography showed that his LV function remained normal and that the RV remained effectively unloaded. He continued to require vasopressor support, however, and his course was further complicated by several recurrent episodes of severe GI bleeding over the next 10 days, during which serial endoscopies could not determine a source of bleeding. On POD 24, following another severe GI bleed with superimposed sepsis, he and his family elected to withdraw care and he died shortly after from hemorrhagic and vasodilatory shock.
While the patient failed to survive the hospitalization, his death was attributed to his uncontrollable GI bleeding. Importantly, the implantation of a durable RVAD improved many markers of cardiogenic shock and unloaded the RV as indicated by reduction in RV size and CVP while improving cardiac output. Adverse events that might occur with a durable RVAD in patients with PH were not seen, including increased PAPs, increased LV preload, and pulmonary hemorrhage from increased pulmonary blood flow. Pulmonic regurgitation related to placement of the outflow cannula in the proximal pulmonary artery was also not seen.
This case highlights the use of a temporary RVAD as an effective bridge to a durable RVAD. More studies are needed to determine whether the efficacy of a temporary RVAD should be established before placement of a durable device. While there are promising indicators, further investigation is needed to demonstrate the safety and efficacy of this novel use of a RVAD in patients with PH and isolated RV failure who otherwise have no long-term therapeutic options.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Shehab S, Macdonald PS, Keogh AM, et al. Long-term biventricular HeartWare ventricular assist device support–Case series of right atrial and right ventricular implantation outcomes. J Heart Lung Transplant 2016; 35: 466–473. [DOI] [PubMed] [Google Scholar]
- 2.Bernhardt AM, De By TM, Reichenspurner H, et al. Isolated permanent right ventricular assist device implantation with the HeartWare continuous-flow ventricular assist device: first results from the European Registry for Patients with Mechanical Circulatory Support. Eur J Cardiothor Surg 2015; 48: 158–162. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzweig EB, Chicotka S, Bacchetta M. Right ventricular assist device use in ventricular failure due to pulmonary arterial hypertension: Lessons learned. J Heart Lung Transplant 2016; 35: 1272–1274. [DOI] [PubMed] [Google Scholar]