Abstract
This multicenter, randomized, double-blind, placebo-controlled study assessed ambrisentan or placebo in patients with inoperable chronic thromboembolic pulmonary hypertension. Futility of enrollment led to early termination. Trends of improvement in favor of ambrisentan versus placebo in the primary and some secondary endpoints were observed; adverse event profiles were similar between groups.
Keywords: Six-minute walk distance, 6MWD, AMBER 1, Group 4 pulmonary hypertension
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a life-threatening condition characterized by major pulmonary vessel fibrotic obstruction as a consequence of venous thromboembolism, resulting in increased pulmonary vascular resistance (PVR), pulmonary hypertension (PH), and right ventricular (RV) dysfunction.1,2 The current gold standard treatment is surgical pulmonary endarterectomy (PEA).3
Ambrisentan is an endothelin receptor antagonist approved to treat pulmonary arterial hypertension (PAH).4 Given the similar histopathological changes in small pulmonary vessels of patients with CTEPH and those with PAH, together with evidence that endothelin-1 levels are elevated in patients with CTEPH,5 it was hypothesized that ambrisentan may provide clinical benefit in patients with inoperable CTEPH.
Here we present results of the AMBER 1 study and its open-label extension, assessing the efficacy and safety of ambrisentan in patients with inoperable CTEPH. The objective of this publication is to share the results in a transparent manner.
Methods
AMBER 1 was a 16-week, multicenter, randomized, double-blind, placebo-controlled phase IIIa study (NCT01884675) conducted between 12 September 2013 and 30 March 2015, with a subsequent open-label extension (NCT01894022) conducted between 23 January 2014 and 18 November 2015. A diagnosis of inoperability was due to surgical inaccessibility of the vascular obstruction (i.e. distal disease) and an unfavorable risk–benefit ratio, based on routine clinical and surgical assessments including a ventilation/perfusion lung scan, computed tomography angiogram and pulmonary angiogram, and confirmed at an expert center or by a central adjudication committee. Expert centers were required to have at least one cardiology or respiratory consultant plus a consultant PEA surgeon. Further details of eligibility criteria and protocol approvals are presented in the Supplementary Material. Written informed consent was obtained from all patients before participation.
Eligible patients were randomized 1:1 to the indicated ambrisentan starting dose of 5 mg once daily (QD)4 or placebo for 16 weeks. Patients who completed the double-blind study could enroll in the open-label extension, to receive ambrisentan 5 mg or 10 mg QD for ≤18 months. The registered dose for ambrisentan is 5 mg, which can be increased to 10 mg if necessary.4
The primary endpoint (double-blind study) was change from baseline in 6-min walk distance (6MWD)6 at week 16. Main secondary endpoints included change from baseline at Week 16 in N-terminal pro-B-type natriuretic peptide (NT-proBNP) and PVR. Endpoints (except for PVR, which was assessed only at week 16) were assessed every four weeks (double-blind study) and every three months (open-label extension). Safety assessments (both studies) included monitoring of treatment-emergent adverse events (TEAEs), serious AEs (SAEs), clinical laboratory parameters, physical examinations, and vital signs.
Sample size calculations and population definitions are presented in the Supplementary Material. As the study was terminated early due to futility of enrollment, data are presented descriptively and no formal statistical comparisons or imputations for missing efficacy data were performed. Efficacy data are presented only from the main AMBER 1 study, as the equivalent data from the open-label extension were considered inconclusive due to low patient numbers.
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Results
Patient population
In total, 33 patients were randomized in the double-blind study, of whom 28 (85%; 15 ambrisentan and 13 placebo) completed the 16-week treatment period; five were withdrawn as a result of study termination. Of the 33 patients, 19 (58%; 10 ambrisentan, 9 placebo) entered the open-label extension. Patient demographic and baseline characteristics were similar between treatment groups (including 6MWD, NT-proBNP, and PVR) (Suppl. Table 1). All patients received at least one of the following concomitant therapies during the study period – CTEPH-specific medications: anticoagulants (ambrisentan = 94%; placebo - 100%) and non-CTEPH specific medications: diuretics (ambrisentan = 71%; placebo = 94%), calcium channel blockers (ambrisentan = 18%; placebo = 25%), and oxygen (ambrisentan = 0%; placebo = 6%).
Efficacy
At week 16, the mean change ± standard deviation (SD) from baseline in 6MWD was 28.3 ± 41.7 m with ambrisentan and 6.8 ± 67.5 m in the placebo group (Fig. 1a; Supplementary Table 2). On the secondary efficacy measures, the following changes from baseline at week 16 were observed (ambrisentan versus placebo): geometric mean NT-proBNP concentration (% baseline) = –29.4% vs. + 14.1%; and mean PVR ± SD = –212.5 ± 392.8 dyn.s/cm5 vs. –108.5 ± 51.3 dyn.s/cm5 (Fig. 1b; Supplementary Table 2). Changes were generally observed at four weeks and maintained for the remainder of the double-blind study.
Fig. 1.
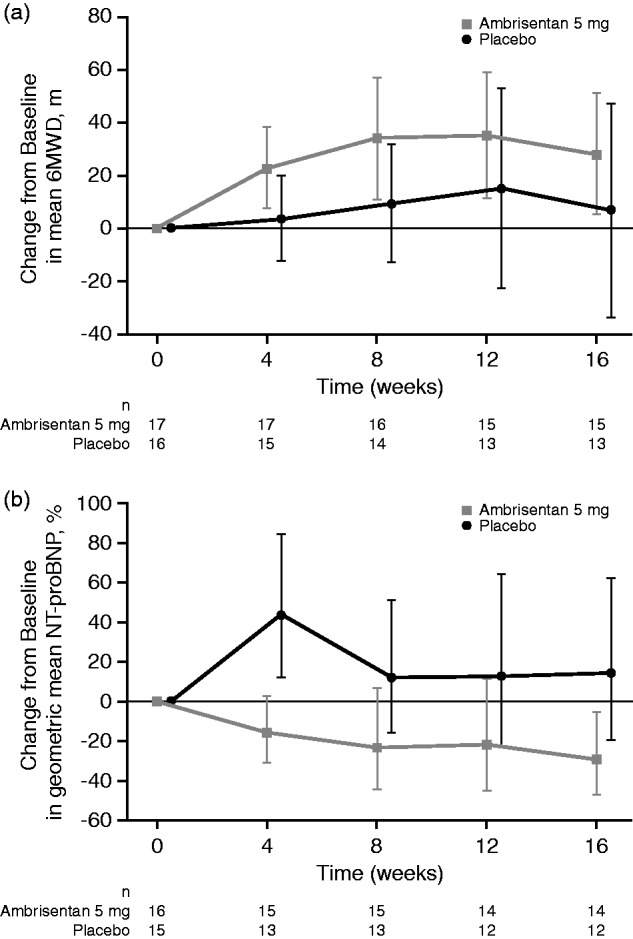
Change from baseline over 16 weeks in (a) 6MWD and (b) NT-proBNP in the double-blind study (observed case data, ITT population). Error bars represent 95% CI values. For NT-proBNP, values shown are the percent change in the geometric mean ratio of week 16 to baseline. 6MWD, 6-min walk distance; CI, confidence interval; ITT, intent-to-treat; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Safety
All TEAEs reported during AMBER I and its extension are presented in Suppl. Tables 3 and 4. TEAEs occurring in >10% of ambrisentan-treated patients (double-blind study) were peripheral edema (ambrisentan = 3/17; placebo = 2/16) and headache (ambrisentan = 2/17; placebo = 3/16). Drug-related TEAEs were not more often observed with ambrisentan (4/17) than with placebo (5/16) in the double-blind study; no new safety signals were identified during the open-label extension. One patient in the double-blind study (placebo group) and three patients in the open-label extension (all previously received double-blind placebo) experienced SAEs. No safety events led to withdrawal during the double-blind study. Of the 19 patients in the open-label extension, one died of cardiac failure (not drug-related) and one withdrew due to cardiac failure (drug-related). Additional safety information is detailed in the Supplementary Materials.
Discussion
Based on the recommendations of the Independent Data Monitoring Committee, AMBER I and its open-label extension were terminated early due to futility of enrollment. This was due to several factors, including an unexpectedly low screening rate (∼20% of expected) and high screening failure rate (approaching 60%, mostly due to concerns regarding inoperability raised by the central adjudication committee). The low screening rate may have been due to the market release of a new medication (riociguat)7,8 and a new percutaneous intervention (pulmonary balloon angioplasty) for CTEPH after the study had started recruiting.1 Furthermore, it is possible that had we undertaken a more ambitious clinical trial design, a greater number of doctors may have chosen to participate in the study.
Due to early termination and a small sample size, the double-blind study was underpowered to detect differences in efficacy outcomes. Nevertheless, the double-blind study data may indicate positive trends towards benefits on outcomes with ambrisentan versus placebo. In addition, the overall safety profile of ambrisentan appeared similar to previous studies and current labeling.4,9,10
In conclusion, the preliminary signals from AMBER I suggest an encouraging trend towards an efficacy of ambrisentan in the treatment of CTEPH; however, this should be considered inconclusive due to the limited sample size.
Supplemental Material
Supplemental Material for Ambrisentan for treatment of inoperable chronic thromboembolic pulmonary hypertension (CTEPH) by Pilar Escribano-Subias, Hakim Bendjenana, Paula S. Curtis, Irene Lang and Anton Vonk Noordegraaf in Pulmonary Circulation
Acknowledgments
The authors thank Dr Marius Hoeper for his contribution to this study. Katy Tucker PhD, CMPP, and Andrew Smith PhD of Fishawack Indicia, provided medical writing support, which was funded by GSK, but did not contribute to the conception/design of these studies, or the acquisition, analysis, or interpretation of data.
Conflict of interest
HB and PSC are employees of and hold stocks in GSK. PE-S has received grants from Actelion, Bayer, and GSK, and fees for acting as a speaker, consulting, and serving on advisory boards from Actelion, Bayer, and GSK. IL has relationships with the following: AOP Orphan Pharmaceuticals, Actelion, AstraZeneca, Bayer Pharma AG, Cordis, GSK, Medtronic, MSD, Pfizer, Sanofi, Servier, and Ferrer. AVN has received speaker’s fees from Actelion, Pfizer, MSD, and Bayer.
Funding
These studies were funded by GSK (NCT01884675, AMB115811; NCT01894022, AMB116457), who were involved in the study design, analysis, and interpretation of data. Employees of GSK are authors of the article and were therefore involved in the writing and final decision to submit for publication.
References
- 1.Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014; 130: 508–518. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper MM, Madani MM, Nakanishi N, et al. Chronic thromboembolic pulmonary hypertension. Lancet Respir Med 2014; 2: 573–582. [DOI] [PubMed] [Google Scholar]
- 3.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 2003; 76: 1457–1462. discussion 62–64. [DOI] [PubMed] [Google Scholar]
- 4.Gilead Sciences Inc. Letairis prescribing information. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/cardiovascular/letairis/letairis_pi.pdf (accessed April 2019).
- 5.Reesink HJ, Meijer RC, Lutter R, et al. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J 2006; 70: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 6.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985; 132: 919–923. [PMC free article] [PubMed] [Google Scholar]
- 7.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 8.Bayer HealthCare Pharmaceuticals Inc. Adempas® prescribing information. 2013. Available at: http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf (accessed April 2017).
- 9.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–3019. [DOI] [PubMed] [Google Scholar]
- 10.Oudiz RJ, Galie N, Olschewski H, et al. Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: 1971–1981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Ambrisentan for treatment of inoperable chronic thromboembolic pulmonary hypertension (CTEPH) by Pilar Escribano-Subias, Hakim Bendjenana, Paula S. Curtis, Irene Lang and Anton Vonk Noordegraaf in Pulmonary Circulation


