Abstract
Background. Few Americans obtain all 41 guideline-recommended preventive services for nonpregnant adults. We assessed patient interest in prioritizing their preventive care needs. Methods. We conducted a mixed-methods study, with 4 focus groups (N = 28) at a single institution and a nationwide survey (N = 2,103). Participants were middle-aged and older adults with preventive care needs. We obtained reactions to written materials describing the magnitude of benefit from major preventive services, including both absolute and relative benefits. Recommendations were individualized for patient risk factors (“individualized preventive care recommendations”). Focus groups assessed patient interest, how patients would want to discuss individualized recommendations with their providers, and potential for individualized recommendations to influence patient decision making. Survey content was based on focus groups and analyzed with logistic regression. Results. Patients expressed strong interest in individualized recommendations. Among survey respondents, an adjusted 88.2% (95% confidence interval [CI] = 86.7% to 89.7%) found individualized recommendations very easy to understand, 77.2% (95% CI = 75.3% to 79.1%) considered them very useful, and 64.9% (95% CI = 62.8% to 67.0%) highly trustworthy (each ≥6/7 on Likert scale). Three quarters of participants wanted to receive their own individualized recommendations in upcoming primary care visits (adjusted proportion = 77.5%, 95% CI = 75.6% to 79.4%). Both focus group and survey participants supported shared decision making and reported that individualized recommendations would improve motivation to obtain preventive care. Half of survey respondents reported that they would be much more likely to visit their doctor if they knew individualized recommendations would be discussed, compared with 4.2% who would not be more likely to visit their doctor. Survey respondents already prioritized preventive services, stating they were most likely to choose quick/easy preventive services and least likely to choose expensive preventive services (adjusted proportions, 63.8% and 8.5%, respectively). Results were consistent in sensitivity analyses. Conclusions. Individualized preventive care recommendations are likely to be well received in primary care and might motivate patients to improve adherence to evidence-based care.
Keywords: Preventive Medicine, Preventive Health Services, Decision Making, Shared
Patients and providers face a myriad of preventive care decisions. The United States Preventive Services Task Force and Advisory Committee on Immunization Practices recommend 41 clinical services for nonpregnant adults.1,2 Yet recent work suggests that just 8% of adults in the United States obtain all recommended major preventive services.3
To improve preventive care delivery, a growing body of work has sought to help patients understand their preventive care options. Decision aids can improve decision quality and decision-making processes4,5 and are required for Medicare reimbursement of lung cancer screening.6 More recently, analytic models have been developed to individualize risks and benefits of cancer screenings7–14 and control of cardiovascular disease15–17 or diabetes.18–20 Nearly all of these studies focus on single decisions, such as when to obtain mammography.7–9
More realistically, patients face a range of decisions in clinical encounters. Some services, such as obesity reduction, offer large expected benefits but are difficult to achieve, while others, such as immunizations and cancer screenings, have smaller expected benefits but are easier to obtain. In choosing among these options, some patients lack time to follow all recommendations, especially those with more commitments to work, family, friends, and hobbies.21–25 Other patients may be unable to afford health care services or believe they are asked to do “too much”; diabetics are less likely to achieve glycemic control when asked to treat other chronic conditions such as asthma or arthritis.26–28 As a result, patients may unwittingly forego high-value services. In previous work, we found the rate of preventive care utilization was lower when the number of guideline recommendations was higher.29
Few studies seek to help patients prioritize among their preventive care options. Some work has considered the possibility of individualizing the risks and benefits of all major preventive services based on patient risk factors,30–33 and translating the results into an individualized increase in life expectancy attributable to preventive care.33,34 Others have ranked preventive services based on a combination of cost and potential to add healthy life-years to the US population.35–37 However, research remains limited and primarily theoretical. Little is known about patient interest in such models, optimal communication about the benefits of multiple interventions, or the ability of this information to influence patient decisions.
In this mixed-methods study, we explored patient interest in learning more about their magnitude of benefit from major preventive care services, individualized for their risk factors (“individualized preventive care recommendations”). The results were not intended to assess a ready-to-use tool but rather an early step toward communicating the tradeoffs between multiple decisions within a single clinical encounter.
Methods
Our study addressed three topics: interest in individualized recommendations, how health care providers should discuss individualized recommendations, and potential for individualized recommendations to affect decision making.
Study Design and Sample
This mixed-methods study used an exploratory sequential design. First, we conducted local focus groups at a single health system to obtain patient views on individualized preventive care recommendations and then fielded a nationwide survey to substantiate focus group results in a larger sample with geographic variation. Focus groups were based at the Cleveland Clinic Health System, which comprises a large academic medical center, 13 regional hospitals, 21 family health centers, and >75 outpatient locations. About 80% of primary care patients are from northeast Ohio. Eligible participants were aged 40 to 75 years, had a primary care provider, English literacy, and ≥2 of the following conditions (indicating high value of preventive care, based on chart review): current smoker; body mass index (BMI) ≥25 kg/m2; diagnosed hypertension, hyperlipidemia, or diabetes; or overdue for screening of lipid disorders (last panel >5 years ago), breast cancer, or colorectal cancer. Exclusion criteria were comorbidities severely limiting life expectancy, such as cancer (excluding non-melanoma skin; other specific types of cancer were not considered because of a large eligible cancer-free population), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and end-stage renal disease (ESRD). Heterogeneous purposive sampling was used to identify participants by chart review or clinical staff patient rosters, seeking variation by sex, race, and socioeconomic position. Participants were recruited by mail or in-person contact, with several options for focus group dates/times. Study personnel followed-up by telephone to confirm patient interest and availability.
Focus groups were conducted in April 2016, three at Cleveland Clinic’s main campus and one at a community health center for underserved patients in East Cleveland, Ohio. Each lasted 60 to 90 minutes and was audio recorded. Participants were compensated $50.
Survey respondents were aged 45 to 70 years (at or close to middle-age), had ability to read English, and ≥1 of the following self-reported conditions: tobacco use in past 12 months; BMI ≥25 kg/m2 based on self-reported height/weight; ever told by a doctor that they had high blood pressure, high cholesterol, or diabetes (other than during pregnancy); never had breast cancer screening; never had colorectal cancer screening; no participation in exercise, sports, or physically active hobbies during the past week; ≥3 alcoholic drinks/day (males) or ≥2/day (females), or no regular fruit or vegetable consumption. (To reach a broad population, only one condition was needed, as compared with ≥2 for focus groups.) We excluded respondents with these comorbidities: ever been told by a doctor that they had COPD, emphysema, severe asthma that could not be controlled by medication, CHF, ESRD, or recent (within the past 5 years) cancer (other than non-melanoma skin). Individuals who failed to complete the survey or finished in <5 minutes were excluded. Respondents were recruited by Survey Sampling International (SSI; now Dynata), a commercial vendor with 3.5 million members who voluntarily agreed to receive email invitations for surveys.38
We asked SSI to match proportions of race/ethnicity, sex, and 5-year age group to US Census data, oversampling Asians, Blacks, and Hispanics in proportions consistent with the National Health and Nutrition Examination Survey.39 Respondents were eligible for cash prizes from the survey vendor.
Content
We developed a moderator guide to facilitate focus group discussion (Appendix 1; all appendixes are available online). Survey content was designed to be similar to focus group discussions and was finalized after the last focus group (Appendix 2).
Focus groups were moderated by an experienced qualitative researcher (MBM) and co-moderated by the principal investigator (GBT), who took notes and clarified details of the preventive care recommendations as needed. Focus groups were audio-recorded, transcribed verbatim, and verified for accuracy. To assess patient interest in individualized preventive care recommendations, we began focus groups and the survey by defining preventive care services and providing examples. Then, we presented a hypothetical patient, Mrs. Smith (or for male survey respondents, Mr. Smith), a middle-aged woman (man) with multiple chronic conditions (obesity, hypertension, hyperlipidemia, and tobacco use [focus groups] or diabetes [surveys]). Figure 1 shows examples of survey text. The patient’s doctor discussed her preventive care needs and, while emphasizing that all services were important, also understood that it may not be realistic to achieve them all at once. The doctor prepared some information to compare Mrs. Smith’s available choices, emphasizing that it had been created just for her and no other patient would receive the same information.
Figure 1.
Examples of survey text to help respondents imagine personalized information.
*Pronouns were individualized for each respondent’s self-reported sex.
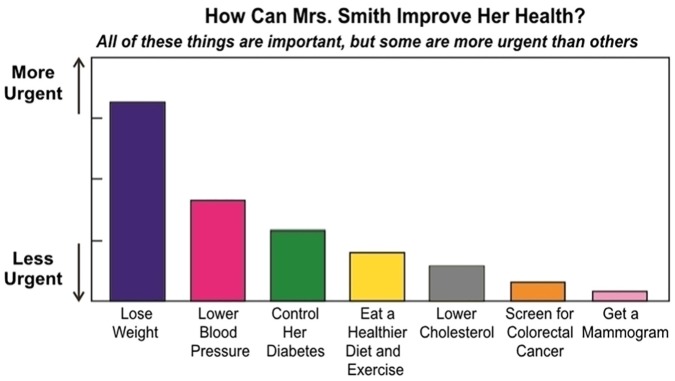
Focus groups elicited participants’ reactions to four examples of individualized preventive care recommendations: a bar chart showing the increase in life expectancy attributable to obtaining each preventive service (e.g., “Add 2 years”), a similar chart with a range from “More Urgent” to “Less Urgent” instead of numbers, a chart showing the improvement in true age (defined as the age most commonly associated with a patient’s life expectancy) (e.g., “2 years younger”), or a chart with horizontal (instead of vertical) bars. See Figure 2 (and Appendix 3). The magnitude of gain was based on a mathematical model that individualizes life expectancy gains for each preventive service rated grade A or B by the US Preventive Services Task Force (USPSTF),33,34 accounting for patient risk factors and their severity (e.g., smoking intensity). The model was largely derived from decision analyses informing USPSTF recommendations. For example, those analyses estimate that in the general population, colorectal cancer screening adds 270 life-years per 1,000 individuals,40 or a per-person gain of 3.2 months (270/1,000 life-years). In the mathematical model, benefits would be greater for patients with family history (based on number of relatives and age at diagnosis) but lower for patients with uncontrolled diabetes (because of lower baseline life expectancy). Because this way of thinking is not intuitive, for this study, we focused on overall patient impressions regarding relative benefit rather than specific magnitudes. For surveys, participants were randomized to one of six graphical formats (based on focus group feedback) (Figure 2 and Appendix 3). We assessed general comprehension by asking which preventive service was most likely to improve Mrs. (Mr.) Smith’s health (out of seven choices for females, six choices for males) and which was least likely to improve health.
Figure 2.
Example visual aid for individualized preventive care recommendations.
An example visual aid shown to focus group and survey participants. See Appendix 3 for alternatives. Pronouns were individualized for each respondent’s self-reported sex. “Get a Mammogram” was only shown to females. The height of the bars was proportionate to the estimated change in life expectancy associated with lifetime adherence to each preventive service, based on prior literature.
The survey also assessed whether patients currently prioritize among their preventive care options, a topic not addressed in focus groups, with two sets of questions. The first set asked participants to rate how likely they would be to do everything their doctor recommended in the next 4 weeks (7-point scale) if the doctor recommended one, two, three, five, or eight preventive services. We chose 4 weeks to illustrate a scenario where it may be difficult to follow all health care recommendations, rather than a critical time horizon. The second set of questions asked participants to imagine that their doctor recommended “too many” preventive care services, defined as more than they felt able to do. We then asked participants to rate 11 statements about actions they would take in response, such as doing nothing, considering the cost of preventive services, required effort (e.g., lab test v. lifestyle change), need to learn more about preventive services, and support from family/friends.
To consider the optimal way to communicate the benefits of multiple interventions, focus groups asked about the perceived utility of individualized recommendations to facilitate discussions between patients and providers. For the survey, we included a modified version of a validated shared decision-making questionnaire, the SDM-Q-9 (Appendix 2).41 Validated questions stating “my doctor” were replaced with “Mrs. (Mr.) Smith’s doctor” and verbs in the past tense were replaced with the conditional tense (e.g., “should discuss”) (Appendix 2). Respondents then were asked to imagine that their doctor had prepared similar information created just for them, based on their own health care needs. We asked about their interest and experience with individualized recommendations, and how they would envision a conversation with their doctor.
To evaluate the potential ability of individualized preventive care recommendations to influence patient decision making, both focus groups and surveys asked about perceived impact on behavior change and barriers and facilitators for implementation.
Finally, as covariates, we asked about demographics (age, sex, race/ethnicity, education, marital status [survey only], state [survey only]). Covariates were intended to adjust for differences in interest or comprehension across population subgroups. The survey also evaluated numeracy through the Subjective Numeracy Scale (SNS)42 and asked six questions about two pictographs, defining the percent of correct answers as “graphical literacy.”43
Data Analysis
To analyze focus group data, we used interpretive description,44 entailing an iterative and progressive process of data immersion, coding, and theme identification with the transcripts. Content domains were identified using the moderator guide and data immersion, and a coding tree was created to organize data (NVivo Version 8, Burlington, MA). Coding nodes were clustered by themes that emerged during data coding and comparison. Data coding was performed by an experienced qualitative researcher (MBM). The principal investigator (GBT) and MBM reviewed, verified, and summarized themes into reportable findings.
To analyze survey results, we computed the proportion of responses that were strongly favorable (≥6 on 7-point Likert-type scale), strongly unfavorable (≤2/7) or (for comprehension questions) correct answers. To do so, we conducted logistic regression and estimated predictive margins at the mean values of all covariates.
This study was approved by Cleveland Clinic’s Institutional Review Board.
Results
Twenty-eight subjects participated in focus groups, primarily females (n = 18, 64%) and Blacks or African Americans (n = 18, 64%). Almost half had completed some college (n = 12, 43%) (Table 1).
Table 1.
Description of Focus Group Participants (N = 28) and Survey Respondents (N = 2,103)a
| Characteristic | Focus Groups, n (%) | Survey, n (%) | US Population, (%)55–59b |
|---|---|---|---|
| Variables collected from both focus groups and survey | |||
| Age group (years) | |||
| <45 | 4 (14) | — | — |
| 45–49 | 3 (11) | 436 (21) | (20) |
| 50–54 | 5 (18) | 457 (22) | (20) |
| 55–59 | 1 (4) | 432 (21) | (21) |
| 60–64 | 7 (25) | 396 (19) | (20) |
| 65–70 | 7 (25) | 382 (18) | (20) |
| Missing | 1 (4) | — | — |
| Sex | |||
| Female | 18 (64) | 1,105 (52) | (52) |
| Male | 10 (36) | 998 (48) | (48) |
| Race/ethnicity | |||
| Non-Hispanic White | 9 (32) | 1,022 (49) | (67) |
| Non-Hispanic Black | 18 (64) | 442 (21) | (12) |
| Hispanic White | —c | 404 (19) | (12) |
| Hispanic Black | —c | 15 (1) | (1) |
| Asian | — | 190 (9) | (6) |
| Other | 1 (4) | 30 (1) | (3) |
| Education | |||
| Less than high school | 4 (14) | 54 (3) | (12) |
| High school diploma or GED | 4 (14) | 401 (19) | (32) |
| Trade school | — | 93 (4) | (4) |
| Some college or associate’s degree | 12 (43) | 761 (36) | (22) |
| Bachelor’s degree | 4 (14) | 525 (25) | (18) |
| Graduate or professional degree | 4 (14) | 269 (13) | (12) |
| Variables only collected from survey | |||
| Annual household income | |||
| Less than $20,000 | 330 (16) | (10) | |
| $20,000-$34,999 | 364 (17) | (9) | |
| $35,000-$49,999 | 326 (16) | (10) | |
| $50,000-$74,999 | 369 (18) | (16) | |
| $75,000-$99,999 | 267 (13) | (14) | |
| $100,000-$149,999 | 233 (11) | (19) | |
| $150,000 or more | 132 (6) | (23) | |
| Prefer not to answer | 82 (4) | — | |
| Marital status | |||
| Married or living with a civil/domestic partner | 1,201 (57) | (65) | |
| Widowed | 120 (6) | (5) | |
| Divorced | 346 (16) | (16) | |
| Separated | 55 (3) | (2) | |
| Never married or never in a civil/domestic partnership | 381 (18) | (12) | |
| Geographic division | |||
| New England | 94 (4) | (5) | |
| Middle Atlantic | 312 (15) | (14) | |
| East North Central | 306 (15) | (15) | |
| West North Central | 118 (6) | (7) | |
| South Atlantic | 490 (23) | (20) | |
| East South Central | 97 (5) | (6) | |
| West South Central | 212 (10) | (11) | |
| Mountain | 138 (7) | (7) | |
| Pacific | 336 (16) | (16) | |
| Subjective Numeracy Scale (mean, SD)d | 70.7 (16.0) | — | |
| Graphical Literacy (mean, SD)e | 69.8 (32.5) | — | |
Data for the US population are shown for comparison. Because of rounding, percentages in some categories may not sum to 100%.
Estimates as of December 2018 for age group, 2016 for marital status, and 2017 for all other variables. Following available data from the US Census Bureau, education is shown for ages ≥55 years, annual household income and geographic division are each shown for ages 45 to 64 years, and marital status is shown for ages 45 to 69 years. For marital status, US Census Bureau data did not include civil/domestic partnership in Married category.
Hispanics comprised 1% of the health system’s eligible primary care population.
Subjective Numeracy Scale converted to a 100-point range.
Maximum possible score, 100.
Of 2,915 individuals who started the survey, 438 did not meet eligibility criteria. Among the remaining 2,477 respondents, 366 were excluded due to an incomplete survey and 8 due to taking <5 minutes (84.9% survey completion rate; N = 2,103). Table 1 provides summary statistics. Individuals from all 50 states and Washington, D.C. completed the survey; median response time was 22 minutes.
Patient Interest in Individualized Recommendations
Table 2 presents key findings.
Table 2.
Illustrative Quotes From Focus Groups and Key Findings From Surveya
| Focus Group Theme and Illustrative Quotes | Survey Result | Survey Adjusted Proportion (95% CI) |
|---|---|---|
| Patient interest in individualized recommendations | ||
| “I mean you look at [individualized recommendations], you’re like, ‘Geez, 64? I’m really not doing very well for my age. There are some things I can do to definitely put myself in a better health.’” (FG 3, main campus) “I think the graph is good because it’s a constant reminder of what your needs are; what you need to do to make your health better.” (FG 4, community health center) “I would know what to work on and actually, I would work on more than one of these simultaneously. But I’d know which one was the most important or that I really had to focus on immediately.” (FG 1, main campus) “I like the way it’s presented. The height of the bars tells you which one gives you the most impact, if you quit doing it, or do it.” (FG 1, main campus) |
• Very easy to understand | 88.2% (86.7% to 89.7%) |
| • Very useful to compare preventive care services based on their ability to improve your health | 77.2% (75.3% to 79.1%) | |
| • Very trustworthy way to present information | 64.9% (62.8% to 67.0%) | |
| • Correctly identified which preventive care service was most likely to improve health or longevity* | 81.3% (79.6% to 83.1%) | |
| • Correctly identified which preventive care service was least likely to improve health or longevityb | 77.3% (75.4% to 79.2%) | |
| Optimal communication about multiple interventions | ||
| “But we still need the conversation. You still need to feel that your doctor cares more than the piece of paper or the data that supports it. I think the data does not overweigh your doctor’s opinion and how he cares about your health.” (FG 3, main campus) “What the doctor says to you in connection with the graph. I really think that that is more likely to have a more significant impact. It really is going to depend on how the doctor frames it when the doctor gives you the graph.” (FG 3, main campus) “I think I would feel more comfortable [reviewing the graph] with my personal primary physician- the relationship is there already.” (FG 2, main campus) “I think it’s a great idea, because if all you’re trying to do is start a conversation, but [your doctor] might want to take it to the next step and say, ‘we can accomplish a couple of these quite easily, at least at the outset.’” (FG 3, main campus) “I think it depends on how it’s explained; what the doctor says to you in connection with the graph. I can see it as more likely to have a more significant impact depending on how the doctor frames it.” (FG 1, main campus) |
• Very interested in talking about individualized preventive care recommendations with your doctor | 77.5% (75.6% to 79.4%) |
| • Talking about individualized preventive care recommendations with your doctor would be very helpful | 75.5% (73.6% to 77.4%) | |
| Mrs. Smith’s doctor should . . .c | ||
| • . . . make clear that a decision needs to be made about her preventive care | 76.6% (74.8% to 78.5%) | |
| • . . . ask exactly how she wants to be involved in making a decision about her preventive care | 74.1% (72.1% to 76.0%) | |
| • . . . tell her there are different options for her preventive care | 77.7% (75.9% to 79.5%) | |
| • . . . precisely explain the advantages and disadvantages of the preventive care options | 82.3% (80.5% to 84.0%) | |
| • . . . help her understand all the information | 86.2% (84.6% to 87.8%) | |
| • . . . ask which preventive care options she prefers | 67.4% (65.3% to 69.4%) | |
| Mrs. Smith and her doctor should . . .c | ||
| • . . . thoroughly weigh the different preventive care options | 77.3% (75.5% to 79.2%) | |
| • . . . select preventive care options together | 75.8% (73.9% to 77.7%) | |
| • . . . reach an agreement on how to proceed | 81.0% (79.2% to 82.7%) | |
| Potential to impact patient decision-making | ||
| “It would actually motivate me. It would push me.” (FG 4, community health center) “[I]f you go to the doctor every three months, and they give you one of these graphs, well, it’s gonna tell you where you were three months ago [murmurs of agreement] and if you follow the chart as it specifically is laid out, it’s gonna tell you, well look, I was here, three months ago, but look where I am now.” (FG 4, community health center) “I’d put it on my refrigerator. I’d look at it every day, every time I go in the refrigerator and I’d know exactly what not to do and what I should do.” (FG 1, main campus) “I would [like it ahead of time] because if there’s something that’s of interest, then I would write it down and talk to the doctor about it. You’ve had time to reflect on it before your appointment.” (FG 2, main campus) “I’d like it during the appointment because if I don’t understand something or if I got some questions right then and there, because I freak out.” (FG 1, main campus) |
• How helpful do you think talking about this information with your doctor would be in motivating you to improve your health? | 75.5% (73.6% to 77.4%) |
| • Would you be more likely to visit your doctor if you knew you were going to see the chart and talk about it? | 52.2% (50.0% to 54.4%) | |
| • Would like to see individualized preventive care recommendations before your doctor visit | 57.7% (55.6% to 59.9%) | |
| • Enough time to during typical check-up to discuss individualized preventive care recommendations | 56.6% (54.1% to 59.1%) | |
| • Would definitely consider discussing individualized preventive care recommendations with a nurse | 44.5%(42.3% to 46.7%) | |
CI, confidence interval; FG, focus group.
This table summarizes key findings from the study. Unless otherwise noted, survey results show the proportion of participants choosing ≥6 on a 7-point Likert-type scale, adjusted for all variables in Table 1. See Appendix 1 for the focus group moderator guide and Appendix 2 for the survey.
Adjusted proportion of participants choosing the correct answer (out of six choices for males or seven choices for females).
Modified SDM-Q-9 questionnaire. Pronouns were individualized for each respondent’s self-reported sex.
Focus Groups
Most focus group participants liked the simple visual presentation and found individualized recommendations easy to understand. As one focus group participant stated, “I think it’s pretty self-explanatory. It’s pretty simple to understand” (Group 2, main campus). Overall, participants viewed individualized recommendations as a source of information about their preventive care needs. “I think the graph is good because it’s a constant reminder of what your needs are; what you need to do to make your health better” (Group 4, East Cleveland). Some participants viewed individualized recommendations as useful to help prioritize preventive care services. “I would know what to work on and actually, I would work on more than one of these simultaneously. But I’d know which one was the most important or that I really had to focus on immediately” (Group 1, main campus).
Although subjects expressed interest in all visual presentation formats, 10 focus group participants (38%) were skeptical about life expectancy. This was partly due to not knowing the sources of information, impressions that increases in life expectancy from preventive care seemed low, and the unpredictability of death. “I like statistics, but I think these are kind of hocus pocus things. Where’d they get this? How do they know this?” (Group 1). Participants responded favorably to adding information on where data originated, which they perceived as adding credibility.
Survey
On a scale of 1 (not at all easy) to 7 (extremely easy), 88.2% of survey respondents (adjusted proportion = 88.2%, 95% confidence interval [CI] = 86.7% to 89.7%) reported an ease of use ≥6. Impressions were consistent across visual presentation formats (P = 0.64). Three quarters of survey respondents (adjusted proportion = 77.2%, 95% CI = 75.3% to 79.1%) reported that it was very useful to compare preventive care services based on their ability to improve health or longevity (≥6/7 on Likert-type scale), with nearly half (46%) reporting that it was extremely useful (7/7). General comprehension of individualized recommendations was high, with 81.3% (95% CI = 79.6% to 83.1%) of respondents correctly stating which preventive service was most likely to improve health and 77.3% (95% CI = 75.4% to 79.2%) correctly stating which was least likely to improve health.
Two thirds of survey respondents considered individualized recommendations highly trustworthy (adjusted proportion ≥6/7 = 64.9%, 95% CI = 62.8% to 67.0%) whereas <1% considered them not trustworthy (adjusted proportion ≤2/7 = 0.1%, 95% CI = 0.5% to 1.5%). When asked, “[W]hich type of chart would be more trustworthy, a chart that compares preventive care services based on their ability to help you live longer or based on their ability to improve your quality of life?” an adjusted 35.6% chose quality of life (≥6/7) versus 20.4% for length of life (≤2/7) (P < 0.001).
When shown all presentation formats and asked their first choice, survey respondents generally preferred the more urgent/less urgent format that summarized individualized recommendations without numbers (Figure 2 and Appendix 3 Figure 1C) (adjusted proportion = 38.2%, v. 32.7% for life expectancy [Appendix 3 Figure 1A and D] and 29.1% for true age [Appendix 3 Figure 1B and E]) followed by true age (adjusted proportion for second choice = 40.1%, v. 24.3% for more urgent/less urgent and 35.6% for life expectancy).
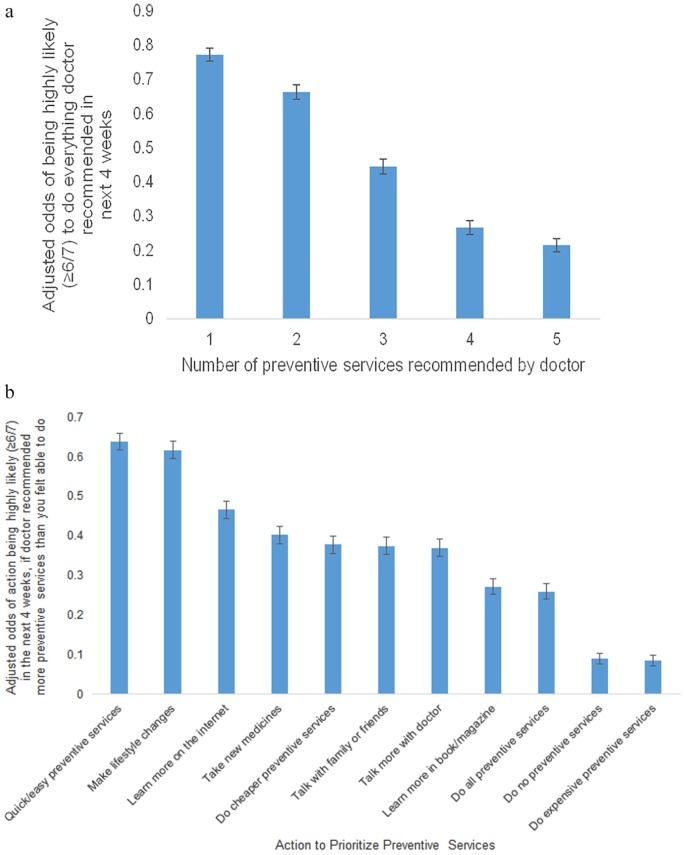
Survey results suggested that patients already prioritize preventive services (Figure 3). The adjusted proportion of respondents stating that they were highly likely to do everything the doctor recommended in the next 4 weeks (≥6/7) declined as the number of recommended preventive services increased, ranging from 77.2% (95% CI = 75.3% to 79.1%) for 1 preventive service to 44.5% (95% CI = 42.3% to 46.7%) for 3 preventive services and 21.5% (95% CI = 19.6% to 23.4%) for 8 preventive services (Figure 3A). Respondents stated that they were most likely to choose quick/easy preventive services and least likely to do expensive preventive services (adjusted proportions, 63.8%, 95% CI = 61.7% to 65.9%, and 8.5%, 95% CI = 7.2% to 9.9%, respectively) (Figure 3B). Doing cheaper preventive services was similarly-ranked to taking new medicines, talking with family/friends, and making another appointment to learn more (adjusted proportions, 37.0% to 40.2%). Respondents with high deductible health insurance plans were especially sensitive to price, with 61% higher odds of being highly likely to choose cheaper preventive services (adjusted odds ratio = 1.61, 95% CI = 1.27 to 2.04, P < 0.001) (data not shown).
Figure 3.
Patient prioritization of preventive care services. (A) Prioritization based on number of recommended preventive services. (B) Actions taken to prioritize preventive care services.
The survey assessed whether and how respondents already prioritize among their preventive care options. Panel A stated, “It can be hard when your doctor asks you to make a lot of changes to improve your health. When answering the questions below, please think about how many changes you could make in your life in the next 4 weeks, while also maintaining your relationships with your family and friends, your work, and your hobbies. In your opinion: In the next 4 weeks, how likely would you be to do everything your doctor recommended if your doctor recommended [1, 2, 3, 5, or 8] preventive care services?” Panel B stated, “Imagine that you visit your doctor today, and he or she recommends too many preventive care services (more than you feel able to do). In your opinion, which of the following would you be likely to do in the next 4 weeks? Both panels utilized a 7-point Likert-type scale from “not at all likely” to “very likely”. Error bars denote 95% confidence intervals.
Optimal Communication About Multiple Interventions
Focus Groups
Participants emphasized that the graph should supplement, not replace, preventive care discussions between patients and physicians. They viewed it as a tool to initiate or focus discussion:
But we still need the conversation. You still need to feel that your doctor cares more than the piece of paper or the data that supports it. I think the data does not overweigh your doctor’s opinion and how he cares about your health. (Group 3, main campus)
Participants consistently emphasized the need to develop an action plan for behavioral change:
[I]f you come up with a personal plan, with goals, objectives, and then you, in your next visit, look back on your goals . . . how did you do, and what do you need to do for the next set of goals? (Group 3)
Others acknowledged a need to discuss available resources and life circumstances that may be barriers to action.
Survey
Similarly, three quarters (adjusted proportion = 77.5%, 95% CI = 75.6% to 79.4%) of survey respondents expressed strong interest in discussing the graph with their physician (≥6/7) and nearly half (48.8%) were extremely interested (7/7). Participants expressed interest in shared decision making to discuss the information; the mean score on the modified SDM-Q-9 questionnaire was 87.4/100 (SD 13.4).
Potential to Impact Patient Decision Making
Focus Groups
Some focus group participants saw the potential for this conversation to improve motivation. For example, one participant said the graph “will plant a seed” (Group 1), suggesting that viewing the graph will take root and inspire behavior change.
Survey
Survey participants agreed; 75.5% (95% CI = 73.6% to 77.4%) believed that individualized recommendations would be very helpful in motivating them to improve their health (≥6/7) and 45.1% perceived them as extremely helpful (7/7). Half reported that they would be much more likely to visit their doctor if they knew individualized recommendations would be discussed (adjusted proportion ≥6/7 = 52.2%, 95% CI = 50.0% to 54.4%), as compared with only 4.2% of survey respondents who reported that they would not be more likely to visit their doctor (≤2/7, 95% CI = 3.2% to 5.2%).
More respondents thought there would be enough time to discuss individualized recommendations during annual check-ups (adjusted proportion = 56.6%) than during a routine visit, defined in the survey as “something other than a check-up, such as a cough or fever” (adjusted proportion = 38.3%). When asked if they would consider discussing individualized recommendations with a nurse who might have more time than their doctor, an adjusted 44.5% strongly agreed (≥6/7) while only 8.3% strongly disagreed (≤2/7).
Subgroup Analysis
Responses were similar by age, sex, race/ethnicity, income, geography, and preference for quality versus length of life (Appendix 4). Participants who obtained regular health maintenance exams (a common venue for preventive care) were more interested than other participants.
Discussion
In this mixed-methods study, we conducted local focus groups followed by a national survey to confirm the results. Subjects expressed strong interest in development of individualized preventive care recommendations for use in primary care. Among survey respondents, 88.2% found our prototype of individualized recommendations very easy to understand, 77.2% considered them very useful, and 74.2% wanted to see their own individualized recommendations (≥6 on 7-point Likert-type scale). Comprehension was high and visual aids were perceived as a highly useful tool in conjunction with a primary care visit.
For nearly 30 years, at least 45% of US deaths have been attributable to modifiable risk factors.45–48 Because only 8% of US adults attain all preventive care recommendations,3 patients need help understanding their options and the tradeoffs between them. They cannot solely rely on physicians, who spend just 27% of their day in direct clinical care,49 a fraction of the time needed to fully evaluate and implement all preventive care guidelines.50 Moreover, patients who want preventive care routinely misunderstand benefits and risks,51–53 so they cannot appropriately decide which services to obtain, and may be less likely to follow guidelines when they are eligible for more services.29 Thus, according to former surgeon general David Satcher, “The ranking of clinical preventive services is an invaluable translational guide to deliver recommended quality services, improve the health of individuals, eliminate health disparities, and use resources responsibly.”54 We evaluated patient views on the potential for this approach, expressed as a graphical ranking of preventive care recommendations individualized for each patient based on their risk factors, to improve delivery.
There is growing consensus of a need for personalized health care, ranging from genetic (precision) medicine to health risk assessments to predictive modeling. Many of these frameworks are informational; for example, a patient who learns she/he is at high risk for Alzheimer’s disease must accept rather than mitigate this risk. Similarly, while evidence-based guidelines abound, most offer only rudimentary tailoring for risk factors, primarily age and sex. As the field of personalized medicine grows, investigators should consider context. For patients facing one to two decisions, it is likely reasonable to discuss information in detail. Decision aids typically show two pictographs per decision (one for benefits and another for risks), so a patient contemplating two decisions would receive four graphs. But for patients facing a large number of decisions, such as those with multiple risk factors or multiple chronic conditions, medicine should offer science to help patients quickly understand tradeoffs and—when the patient is unwilling or unable to follow all recommendations—prioritize. Survey results suggested that patients already prioritize their preventive care; an evidence-based approach may help patients to do so better.
How can health care decisions be combined into an easy-to-understand, individualized format? One solution is to draw on existing decision analytic models of population- and public-health management. Decision analytic models combine multiple diverse outcomes with different probabilities of occurrence into a single metric, quality-adjusted life-years. For individual patients who face multiple preventive care recommendations, prior proof-of-concept work allows them to compare outcomes using life expectancy.33,34 The current study suggests that patients find this information easy-to-understand and valuable. Further study is needed to determine whether such information can be developed in ways that are accurate and helpful to motivate and change patient behavior, and its impact on physician time. More complex models, accounting for numerous disease states, can be used to compare policy implications at the population level. A good example is the Evidera (formerly Archimedes) framework, which facilitates cardiometabolic risk reduction and diabetes management.30
Our exploratory study had several limitations. First, subjects did not receive their own individualized preventive care recommendations. Instead, they were shown examples based on a hypothetical patient. Assuming that people are more interested in seeing their own data than hypothetical data, our results may underestimate true interest. Second, our illustration portrayed expectations (means) rather than the range of uncertain outcomes more realistically encountered in preventive care. This represents a simplification, but is still be more informative than current practice, which except for single-topic decision aids, rarely provides numeric estimates of benefit to patients. Third, one third of participants strongly preferred information on quality of life whereas the example visual aid portrayed changes in life expectancy. This observation did not affect their strong support for individualized recommendations. Fourth, examples did not describe the extent of weight loss, blood pressure, and so on, that would be required of the hypothetical patient. This was another simplification to elicit overall impressions and avoid overwhelming participants with numbers. The scenario also explained that Mrs. Smith and her doctor would discuss the recommendations. Fifth, we showed a single graphic of net benefits rather than separate information on benefits and risks. This might affect patient preferences and warrants further research. Sixth, focus groups were conducted at one institution, but comprised a diverse group of patients. The results were tested in a nationwide sample. Seventh, surveys were conducted among individuals who had previously chosen to receive email invitations. If people willing to take surveys are more interested in thinking about new ideas than the general population, then survey results, including confidence intervals, may be overestimates.
Conclusion
In focus groups and a nationwide survey, we found strong interest in development of individualized preventive care recommendations for use in primary care. Future work should consider optimal design and barriers and facilitators to implementation in primary care settings, as well as measure the impact of such information on patient decision-making.
Supplemental Material
Supplemental material, Appendix_1_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice
Supplemental Material
Supplemental material, Appendix_2_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice
Supplemental Material
Supplemental material, Appendix_3_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice
Supplemental Material
Supplemental material, Appendix_4_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. This study was approved by the Institutional Review Board of Cleveland Clinic.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding support for this study was provided by Grants R21AG052849 (from the National Institute on Aging) and KL2TR000440 (from the National Center for Advancing Translational Sciences and Clinical and Translational Science Collaborative of Cleveland). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at https://journals.sagepub.com/home/mpp.
ORCID iD: Glen B. Taksler  https://orcid.org/0000-0002-7566-7104
https://orcid.org/0000-0002-7566-7104
Contributor Information
Glen B. Taksler, Medicine Institute, Division of Clinical Epidemiology, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, Ohio; Center for Health Care Policy and Research, Case Western Reserve University at MetroHealth Medical Center, Cleveland, Ohio.
Mary Beth Mercer, Office of Patient Experience, Cleveland Clinic, Cleveland, Ohio.
Angela Fagerlin, Department of Population Health Sciences, University of Utah, Salt Lake City, Utah; Salt Lake City VA Informatics Decision-Enhancement and Analytic Sciences (IDEAS 2.0) Center for Innovation, Salt Lake City, Utah.
Michael B. Rothberg, Medicine Institute, Cleveland Clinic, Cleveland, Ohio
References
- 1. US Preventive Services Task Force. USPSTF A and B recommendations [cited July 10, 2018]. Available from: http://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/
- 2. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67(5):158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borsky A, Zhan C, Miller T, Ngo-Metzger Q, Bierman AS, Meyers D. Few Americans receive all high-priority, appropriate clinical preventive services. Health Aff (Millwood). 2018;37(6):925–8. [DOI] [PubMed] [Google Scholar]
- 4. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stacey D, Légaré F, Lewis KB. Patient decision aids to engage adults in treatment or screening decisions. JAMA. 2017;318(7):657–8. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) [cited July 12, 2018]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 7. Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trentham-Dietz A, Kerlikowske K, Stout NK, et al. Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med. 2016;165(10):700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Broek JJ, van Ravesteyn NT, Heijnsdijk EA, de Koning HJ. Simulating the impact of risk-based screening and treatment on breast cancer outcomes with MISCAN-Fadia. Med Decis Making. 2018;38(1 Suppl.):54S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70(1):96–108,e1–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Subramanian S, Bobashev G, Morris RJ, Hoover S. Personalized medicine for prevention: can risk stratified screening decrease colorectal cancer mortality at an acceptable cost? Cancer Causes Control. 2017;28(4):299–308. [DOI] [PubMed] [Google Scholar]
- 12. Taksler GB, Perzynski AT, Kattan MW. Modeling individual patient preferences for colorectal cancer screening based on their tolerance for complications risk. Med Decis Making. 2017;37(3):204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149(6):1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gulati R, Inoue LY, Gore JL, Katcher J, Etzioni R. Individualized estimates of overdiagnosis in screen-detected prostate cancer. J Natl Cancer Inst. 2014;106(2):djt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med. 2011;154(9):627–34. [DOI] [PubMed] [Google Scholar]
- 16. Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferket BS, van Kempen BJ, Heeringa J, et al. Personalized prediction of lifetime benefits with statin therapy for asymptomatic individuals: a modeling study. PLoS Med. 2012;9(12):e1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laiteerapong N, Cooper JM, Skandari MR, et al. Individualized glycemic control for US adults with type 2 diabetes: a cost-effectiveness analysis. Ann Intern Med. 2018;168(3):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laiteerapong N, Fairchild PC, Chou CH, Chin MH, Huang ES. Revisiting disparities in quality of care among US adults with diabetes in the era of individualized care, NHANES 2007–2010. Med Care. 2015;53(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174(8):1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeRigne L, Stoddard-Dare P, Collins C, Quinn L. Paid sick leave and preventive health care service use among US working adults. Prev Med. 2017;99:58–62. [DOI] [PubMed] [Google Scholar]
- 22. Jaen CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859–63. [PubMed] [Google Scholar]
- 23. Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38(2):166–71. [PubMed] [Google Scholar]
- 24. Jaen CR, Stange KC, Tumiel LM, Nutting P. Missed opportunities for prevention: smoking cessation counseling and the competing demands of practice. J Fam Pract. 1997;45(4):348–54. [PubMed] [Google Scholar]
- 25. Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: what influences mammography recommendations? J Am Board Fam Pract. 2001;14(5):352–61. [PubMed] [Google Scholar]
- 26. Piette JD, Richardson C, Valenstein M. Addressing the needs of patients with multiple chronic illnesses: the case of diabetes and depression. Am J Manag Care. 2004;10(2 Pt. 2):152–62. [PubMed] [Google Scholar]
- 27. Pentakota SR, Rajan M, Fincke BG, et al. Does diabetes care differ by type of chronic comorbidity? An evaluation of the Piette and Kerr framework. Diabetes Care. 2012;35(6):1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aung E, Donald M, Coll J, Dower J, Williams GM, Doi SA. The impact of concordant and discordant comorbidities on patient-assessed quality of diabetes care. Health Expect. 2015;18(5):1621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taksler GB, Pfoh ER, Stange KC, Rothberg MB. Association between number of preventive care guidelines and preventive care utilization by patients. Am J Prev Med. 2018;55(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagykaldi Z, Aspy CB, Chou A, Mold JW. Impact of a wellness portal on the delivery of patient-centered preventive care. J Am Board Fam Med. 2012;25(2):158–67. [DOI] [PubMed] [Google Scholar]
- 31. Nagykaldi ZJ, Voncken-Brewster V, Aspy CB, Mold JW. Novel computerized health risk appraisal may improve longitudinal health and wellness in primary care: a pilot study. Appl Clin Inform. 2013;4(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krist AH, Woolf SH, Rothemich SF, et al. Interactive preventive health record to enhance delivery of recommended care: a randomized trial. Ann Fam Med. 2012;10(4):312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taksler GB, Keshner M, Fagerlin A, Hajizadeh N, Braithwaite RS. Personalized estimates of benefit from preventive care guidelines: a proof of concept. Ann Intern Med. 2013;159(3):161–8. [DOI] [PubMed] [Google Scholar]
- 34. Owens DK, Goldhaber-Fiebert JD. Prioritizing guideline-recommended interventions. Ann Intern Med. 2013;159(3):223–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maciosek MV, LaFrance AB, Dehmer SP, et al. Updated priorities among effective clinical preventive services. Ann Fam Med. 2017;15(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Isham G, Sanchez E, Jones WA, Teutsch S, Woolf S, Haddix A. Prevention priorities: guidance for value-driven health improvement. Ann Fam Med. 2017;15(1):6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Connor PJ, Sperl-Hillen JM, Margolis KL, Kottke TE. Strategies to prioritize clinical options in primary care. Ann Fam Med. 2017;15(1):10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dynata. Available from: http://www.surveysampling.com
- 39. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey [cited May 4, 2019]. Available from: http://www.cdc.gov/nchs/nhanes.htm [Google Scholar]
- 40. Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. 2010;80(1):94–9. [DOI] [PubMed] [Google Scholar]
- 42. Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27(5):672–80. [DOI] [PubMed] [Google Scholar]
- 43. Garcia-Retamero R, Cokely ET, Ghazal S, Joeris A. Measuring graph literacy without a test: a brief subjective assessment. Med Decis Making. 2016;36(7):854–67. [DOI] [PubMed] [Google Scholar]
- 44. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. 3rd ed. Thousand Oaks: Sage; 2014. [Google Scholar]
- 45. Rhodes HG; Committee on Population; Board on Health Care Services. Measuring the Risks and Causes of Premature Death: Summary of Workshops. Washington: National Academies Press; 2015. [PubMed] [Google Scholar]
- 46. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. [DOI] [PubMed] [Google Scholar]
- 47. McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270(18):2207–12. [PubMed] [Google Scholar]
- 48. US Burden of Disease Collaborators; Mokdad AH, Ballestros K, et al. The State of US Health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753–60. [DOI] [PubMed] [Google Scholar]
- 50. Yarnall KS, Pollak KI, Østbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274–86. [DOI] [PubMed] [Google Scholar]
- 52. Hudson B, Zarifeh A, Young L, Wells JE. Patients’ expectations of screening and preventive treatments. Ann Fam Med. 2012;10(6):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Metcalfe KA, Narod SA. Breast cancer risk perception among women who have undergone prophylactic bilateral mastectomy. J Natl Cancer Inst. 2002;94(20):1564–9. [DOI] [PubMed] [Google Scholar]
- 54. Satcher D. Preventive interventions: an immediate priority. Ann Fam Med. 2017;15(1):8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. US Census Bureau. ALLDATA: monthly population estimates by age, sex, race and Hispanic origin for the United States: April 1, 2010 to July 1, 2017 (with short-term projections to December 2018) [cited December 17, 2018]. Available from: https://www.census.gov/newsroom/press-kits/2018/estimates-characteristics.html [Google Scholar]
- 56. US Census Bureau. Educational attainment in the United States: 2017. Table 3. Detailed years of school completed by people 25 years and over by sex, age groups, race and Hispanic origin: 2017 [cited December 17, 2018]. Available from: https://www.census.gov/data/tables/2017/demo/education-attainment/cps-detailed-tables.html [Google Scholar]
- 57. US Census Bureau. HINC-03. People in households—households, by total money income in 2017, age, race and Hispanic origin of householder. Current Population Survey (CPS) Annual Social and Economic (ASEC) Supplement [cited December 17, 2018]. Available from: https://www.census.gov/data/tables/time-series/demo/income-poverty/cps-hinc/hinc-03.html [Google Scholar]
- 58. US Census Bureau. Age and sex composition in the United States: 2016. Table 2. Marital status of the population 15 years and over by sex and age: 2016 [cited December 17, 2018]. Available from: https://www.census.gov/data/tables/2016/demo/age-and-sex/2016-age-sex-composition.html
- 59. US Census Bureau. DP05. ACS Demographic and Housing Estimates. 2013–2017 American Community Survey 5-year estimates [cited December 17, 2018]. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_17_5YR_DP05 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice
Supplemental material, Appendix_2_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice
Supplemental material, Appendix_3_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice
Supplemental material, Appendix_4_online_supp for Assessing Patient Interest in Individualized Preventive Care Recommendations by Glen B. Taksler, Mary Beth Mercer, Angela Fagerlin and Michael B. Rothberg in MDM Policy & Practice