Abstract
Background:
Symptomatic osteochondral defects are difficult to manage, especially in patients with deep (>8-10 mm) empty defects. The restoration of articular congruence is crucial to avoid the progression to osteoarthritis (OA).
Purpose:
To describe the autologous chondrocyte implantation (ACI) “segmental-sandwich” technique for restoration of the osteochondral unit and to evaluate midterm outcomes in patients treated with this procedure. Correlations between magnetic resonance imaging (MRI) and radiographic findings with outcomes were assessed.
Study Design:
Case series; Level of evidence, 4.
Methods:
Outcomes were evaluated for a consecutive cohort of 15 patients with symptomatic deep (>8 mm) osteochondral lesions who underwent autologous bone grafting plus the ACI segmental-sandwich technique performed by a single surgeon between 2003 and 2011. Patients with a minimum 2-year follow-up were included. All patients completed validated clinical outcome scales and a patient satisfaction survey. The Kellgren-Lawrence (K-L) grade was assessed for the progression to OA. The repair site was evaluated with the MOCART (magnetic resonance observation of cartilage repair tissue) score. Filling and tissue characteristics of the bone defect were analyzed with MRI.
Results:
All patients (mean age at surgery, 31.0 ± 9.1 years) were available for follow-up (mean follow-up, 7.8 ± 3.0 years; range, 2-15 years). The mean chondral lesion size was 6.0 ± 3.5 cm2 (range, 1.5-13.5 cm2), with a mean bone defect area of 1.7 cm2 (27%-40% of overall surface area treated by ACI) and depth of 1.0 cm. All patients had successful clinical outcomes, and all functional scores improved significantly (P < .05). Patients reported a very high satisfaction rate (93%). The K-L grade demonstrated no significant progression to OA over a mean follow-up of 4.7 years. For 12 patients with MRI results available, the mean MOCART score at a mean of 3.3 years was 64.2 ± 19.9, with complete or near-complete (≥75% of defect volume) chondral defect filling (83%) and complete integration to adjacent cartilage (83%). Bone defects were completely filled in 83% of patients.
Conclusion:
The ACI segmental-sandwich technique provides significant functional improvements at midterm follow-up and excellent survival rates. This unique treatment allows for the resurfacing of cartilage defects and the repair of underlying segmental bone lesions.
Keywords: autologous chondrocyte implantation, osteochondral lesion, osteochondral unit, autologous bone graft, articular, cartilage
Osteochondral lesions in the knee joints do not heal spontaneously, and if left untreated, they can lead to osteoarthritis (OA). Several studies have shown that fragment removal of an osteochondritis dissecans (OCD) lesion can induce the development of OA because of the presence of an incongruous joint surface.27,41 Surgeons are occasionally confronted with chondral lesions that are accompanied by bone defects from OCD, bone cysts, or failed and collapsed osteochondral allografts. Although a variety of surgical procedures have been developed to manage such lesions, the optimal surgical technique is still under debate.10,14,23 Techniques such as fragment removal,1,2,35,48 debridement,24,35 drilling,25 abrasion chondroplasty,20 and microfracture43 have been shown to result in the formation of fibrocartilage,36 a tissue with mechanical properties that are inferior to those of hyaline cartilage. However, these techniques may be acceptable in the short term for small (<1.5 cm2) and shallow lesions (<5-8 mm deep) without sclerotic subchondral bone or subchondral cystic changes.
Research has demonstrated promising midterm results for autologous chondrocyte implantation (ACI) in the treatment of shallow osteochondral lesions.40 However, once defects become deeper (>8-15 mm), the osteochondral unit needs to be restored as a single functional unit. Moreover, several studies have reported an increased failure rate after ACI for defects that have associated subchondral cystic and sclerotic bone changes, which are often seen after bone marrow stimulation procedures.33,39 For the treatment of deep osteochondral defects, several options are available, including osteochondral autograft transplantation (OAT),18 fresh-matched osteochondral allograft transplantation (OCA),13,16,17 autologous bone grafting (ABG),21,46 and the ACI “sandwich” technique.4,5,31,34,47
For the ACI sandwich technique, first described by Jones and Peterson,22 ABG and ACI are performed simultaneously. Cultured chondrocytes are separated from the ABG site and marrow space by “sandwiching” the cells between 2 periosteal or collagen membranes on the surface for single-stage autologous reconstruction of the osteochondral unit. The ACI sandwich technique includes 2 types: the ACI “full-sandwich” technique,34 in which ACI cartilage repair covers the full area of bone grafted, and the ACI “segmental-sandwich” technique, in which the bone graft covers just a segment of the overall defect area treated by ACI. In short, the ACI “segmental-sandwich” technique consists of ACI with ABG to repair an osseous defect as part of a larger chondral surface repair procedure (Figure 1). A 2018 study demonstrated superior results with the ACI full-sandwich technique versus ABG alone.34 However, the ACI segmental-sandwich technique has not been fully described, and clinical outcomes with this technique are still unclear. Thus, the purpose of this study was to describe the ACI segmental-sandwich technique and to evaluate clinical outcomes in patients treated with this procedure.
Figure 1.
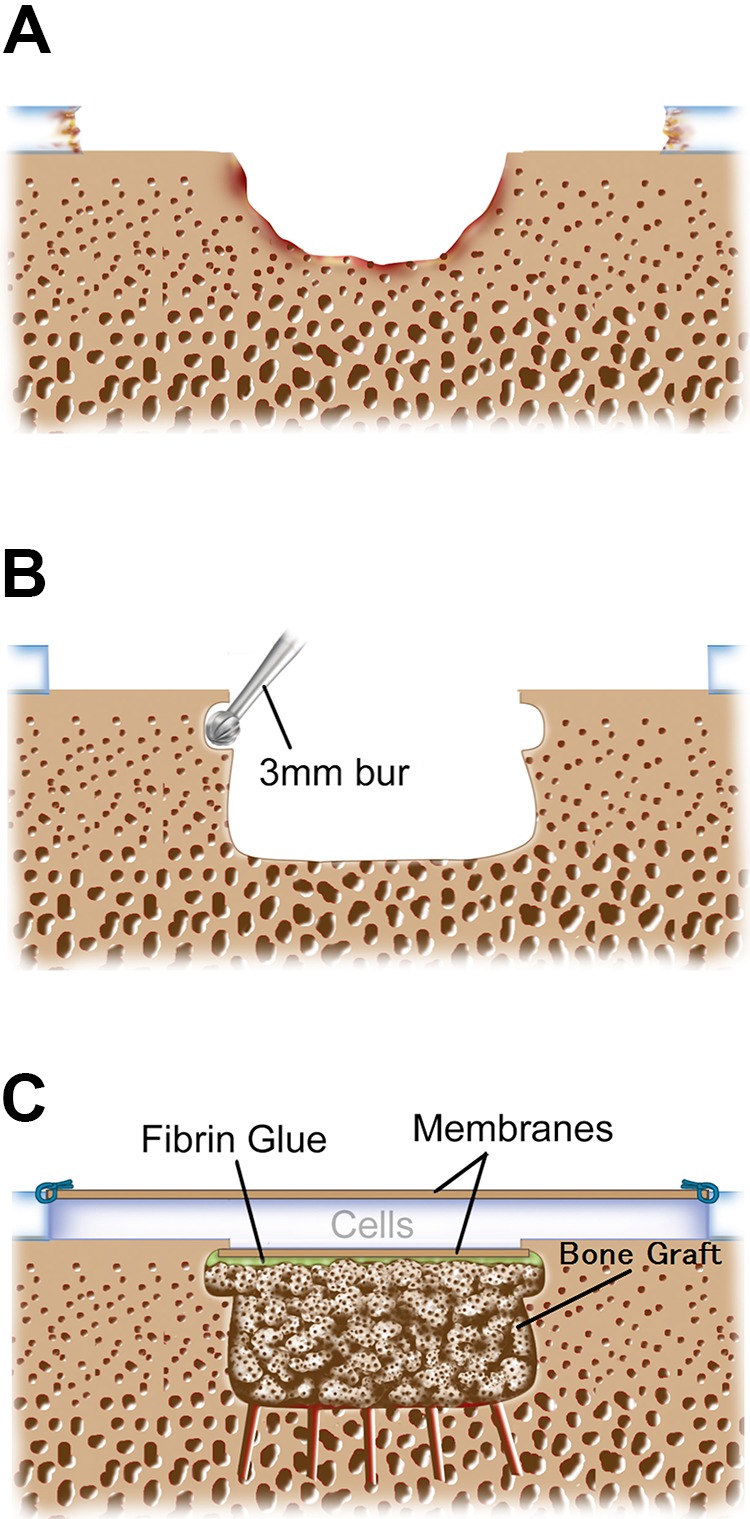
Autologous chondrocyte implantation segmental-sandwich technique. (A) Osteochondral defect: the bone defect is smaller than the overlying chondral defect. (B) Preparation of the bone defect: a high-speed bur, usually 8 mm in diameter, removed all subchondral sclerotic bone back to healthy-appearing spongy bone. Then, a 3 mm–diameter bur undermined the subchondral bone to secure the membrane when it was glued to the graft with overlying gentle pressure. (C) Fibrin glue was applied over the bone graft, and the membrane was secured. The second membrane was then sutured to the surface with the tourniquet down and with a dry defect bed. The cultured chondrocytes were then injected into the sealed cavity. The bone grafted area was smaller than the overlying chondral defect.
Methods
Patient Selection
This study was approved by an institutional review board, and informed consent was obtained from all patients. Data were prospectively collected. Between July 2003 and June 2011, a total of 15 consecutive patients were treated with the ACI segmental-sandwich technique; of these patients, those who had at least 2 years of follow-up were included in the study. Before surgery, patients underwent a physical examination, radiography, computed tomography, magnetic resonance imaging (MRI), and arthroscopic surgery. Indications for surgery included osteochondral defects larger than 1.5 cm2 in size and deeper than approximately 8 to 10 mm, with symptoms matching the defect location. Surgery was indicated only in patients whose lesions were resistant to nonoperative therapies, including physical therapy and injections. Contraindications to surgery included the presence of inflammatory joint disease, unresolved or recent septic arthritis, and metabolic or crystal disorders.
Articular comorbidities such as malalignment and patellar maltracking were corrected at the time of surgery. Tibiofemoral malalignment >2° to 3° was corrected via opening wedge high tibial osteotomy, with correction of the mechanical axis to neutral. Patellofemoral maltracking was addressed with anteromedialization tibial tubercle osteotomy to centralize patellar tracking12,32; proximal soft tissue balancing (lateral release, vastus medialis obliquus advancement) was performed as necessary to centralize the extensor mechanism.
Surgical Technique
A single surgeon (T.M.) performed all of the procedures. A periosteal patch was used in patients who underwent the procedure before May 2007 (n = 5), whereas a type I/III bilayer collagen membrane derived from porcine peritoneum and skin (Bio-Gide; Geistlich Pharma) was used in patients who underwent the procedure after May 2007 (n = 10). When a periosteal patch was used in conjunction with ABG, the first periosteal patch was glued with Tisseel fibrin glue (Baxter BioSurgery), and a few tacking sutures (No. 6-0 resorbable sutures) were used circumferentially over the bone graft with the cambium layer facing out. The periosteum was then covered with a neural patty, the leg was brought into full extension, and the tourniquet was let down. The knee was then gently flexed up, the neural patty was gently removed, and visual inspection was performed to ensure that the base of the defect was dry with no marrow-derived blood present. A second periosteal patch was then microsutured on the articular surface at intervals of 3 to 5 mm circumferentially, with the cambium layer facing the defect. The margins were then sealed watertight with Tisseel fibrin glue, and autologous cultured chondrocytes were injected between the 2 membranes, where they were sandwiched between the cambium layers of the periosteum or collagen membranes (Figures 1 and 2).
Figure 2.
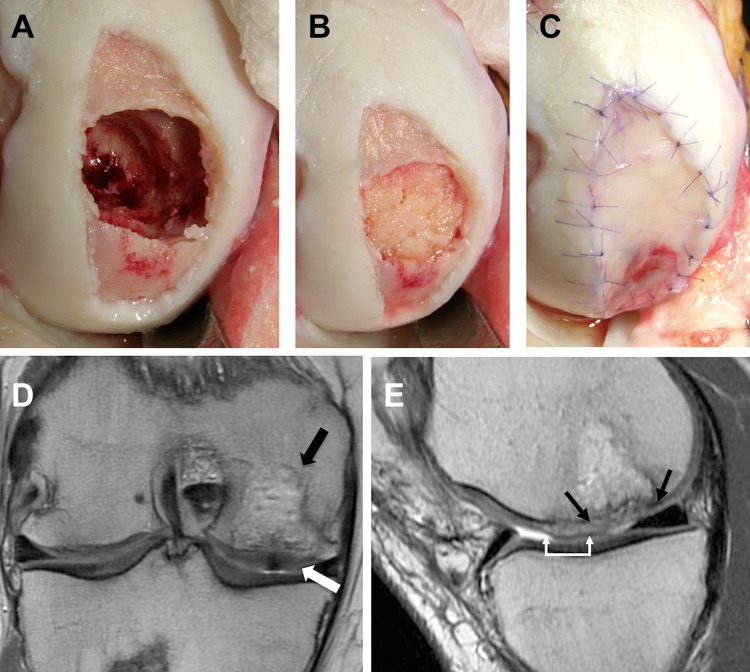
Intraoperative photographs and postoperative magnetic resonance imaging (MRI). A 22-year-old male football player who underwent prior treatment of a medial femoral condyle (MFC) defect with a fresh osteochondral allograft that failed by resorption and collapse of the allograft. (A) Debrided defect of the MFC with osseous deficiency from removal of the allograft and extension of chondral degeneration around it, producing a segmental bone defect of the surface chondral area. (B) Osseous defect bone grafted with autologous cancellous bone to the level of the adjacent subchondral bone. (C) The osseous bone grafted area was then covered with fibrin glue and a membrane, the area was covered with a neural patty, and the tourniquet was let down. The overall area was then covered with a second membrane that was sutured and filled with cells. (D) Coronal view (T1-weighted) showing complete osseous defect filling (black arrow) and complete chondral defect filling with a congruent articular surface (white arrow) at 6 months postoperatively. (E) Sagittal view showing complete chondral defect filling (over the bone grafted [black arrows] and non–bone grafted [white arrows] areas). This case was included in the present study; however, the MRI results were excluded, as postoperative MRI was performed at 6 months postoperatively and did not meet the inclusion criteria of MRI evaluations (>1 year after index surgery).
Postoperative Course
Postoperatively, patients were instructed to use a continuous passive motion machine for 6 to 8 hours daily for 6 weeks. Patients were encouraged to start riding a stationary bicycle with no resistance as early as 3 weeks after surgery and to begin to increase resistance 6 weeks after surgery if there was no joint crepitus or pain. Patients were flat-foot touchdown weightbearing for 6 weeks, with a gradual progression to full weightbearing at 7 to 12 weeks. Patients were permitted to return to most activities of daily living after 3 months and to noncontact inline sporting activities without cutting movements (eg, outdoor biking, treadmill walking, elliptical training, swimming, rollerblading, and hiking) after 4 to 6 months. After 12 to 14 months, inline jogging was permitted if there was no swelling or pain. Pivoting activities were permitted at 14 to 18 months after surgery. The postoperative recovery protocol was individually adjusted based on the defect location, use of concurrent procedures, degree of graft maturation, and patient’s previous activity level.
Failure Definition
Treatment failure was defined as recurrent symptoms of pain and catching, with MRI or arthroscopic surgery demonstrating partial or complete displaced delamination with a full-thickness defect necessitating repeat biological osteochondral debridement or repair or conversion to prosthetic arthroplasty.
Clinical Outcome Evaluation
Outcome measures included the modified Cincinnati Knee Rating System (Figure 3),9,30 the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC),6 a visual analog scale (VAS) for pain, and the 36-Item Short Form Health Survey (SF-36).8 Patients also self-reported knee function and satisfaction with their condition. Scores were collected preoperatively and at the latest follow-up during consultations or via a mailed questionnaire.
Figure 3.
Modified Cincinnati Knee Rating System: overall condition.
Radiographic Evaluation
Anteroposterior and lateral standing radiographs of the knee were assessed at baseline and at the latest radiographic follow-up in accordance with the Kellgren-Lawrence (K-L) grade26 to determine whether there was any OA progression from baseline.
MRI Evaluation
All MRI was performed on a 1.5- or 3-T system (GE Healthcare or Siemens Medical Solutions) with an extremity coil and using a 14- to 16-cm field of view. The repair sites were evaluated using the MOCART (magnetic resonance observation of cartilage repair tissue) score29,30 for cartilage restoration; separate grading was used to determine the success of bone defect repair. Cartilage defect filling was defined as the overall volume of repair tissue, in quartiles, relative to the volume of the original cartilage defect (ie, new tissue above the expected level of the subchondral bone plate and below the expected articular surface [not including any hypertrophic tissue]). Bone defect filling was defined as the overall volume of repair tissue, in quartiles, relative to the volume of the original bone defect filled by the segmental-sandwich bone graft (ie, new tissue below the expected level of the subchondral bone plate [not including any intralesional osteophytes]). Bone and cartilage defect filling 75% to 100% were considered near-complete filling, whereas complete filling was defined as defects that were entirely filled with repair tissue. Hypertrophy and intralesional osteophytes were graded separately. Bone defect repair tissue was characterized by the percentage of filling, in quartiles, that had MRI characteristics of bone rather than soft tissue, with a score of 100% indicating that new bone completely filled the bony defect to the expected level of the subchondral plate. The presence and signal intensity (SI) of any edema-like marrow signal within the bone repair site were scored as follows: no edema, mild edema (SI ≤ red marrow), moderate edema (SI = muscle), or severe edema (SI > muscle and/or dark on T1-weighted imaging).
A musculoskeletal radiologist (C.S.W.) with 22 years of experience in assessing images of cartilage repair evaluated all MRI scans; the radiologist was blinded to patient demographics and clinical outcomes.
Statistical Analysis
The Wilcoxon signed-rank test was used to compare differences in functional scores (obtained from the modified Cincinnati Knee Rating System, VAS for pain, WOMAC, and SF-36) between the 2 time points (preoperatively and at last follow-up). The Spearman rank-order correlation test was used to analyze correlations between MRI findings and clinical outcomes at final follow-up. The level of significance was set a priori at P < .05. All statistical analyses were performed with Stata (v 13; StataCorp).
Results
Patient Demographics and Lesion Characteristics
All 15 patients had at least 2 years of follow-up after surgery (mean, 7.8 ± 3.0 years; range, 2-15 years). The mean age at the time of surgery was 31.0 ± 9.1 years (range, 16-43 years), and the mean body mass index (BMI) was 26.2 ± 4.8 kg/m2 (range, 18.6-36.9 kg/m2). The mean chondral lesion size was 6.0 ± 3.5 cm2 (range, 1.5-13.5 cm2), with a mean bone defect area of 1.7 cm2 (27%-40% of overall surface area treated by ACI) and depth of 1.0 cm. In addition to the osteochondral lesions, 8 patients had a total of 10 chondral defects, with a mean defect size of 4.5 ± 2.2 cm2 (range, 1.5-7.5 cm2); these secondary defects were treated with ACI during the index surgery. Twelve patients underwent concomitant osteotomy during the index surgery (Table 1). Before the index surgery, 12 of the 15 patients had undergone a mean of 2.7 surgical procedures (range, 1-5). The bone graft was harvested from the proximal tibia in 8 patients, from the tibial wedge for high tibial osteotomy in 4 patients, from the distal femur in 2 patients, and from the iliac crest in 1 patient. Data on the previous duration of symptoms were available from 12 of the 15 patients, and the mean duration was 5.2 ± 2.4 years (range, 1.5-9.4 years).
TABLE 1.
Patient Demographics and Lesion Characteristics (N = 15)a
| Patient | Age, y (Sex) | Cause | Segmental Sandwich Location/Type of Membrane | Chondral Lesion Area, cm2 | Bone Defect Area, cm2 | Bone Defect Depth, cm | Bone Defect Size of Chondral Lesion, % | Additional Chondral Lesions, Location/Size, cm2 | Concomitant Surgery |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 (M) | OCD | MFC/periosteum | 12.0 | 1.8 | 0.5 | 15 | None | HTO |
| 2 | 39 (M) | OCD | MFC/periosteum | 13.5 | 3.8 | 0.8 | 28 | None | HTO |
| 3 | 22 (F) | OCD | MFC/periosteum | 3.8 | 0.5 | 0.5 | 13 | None | HTO + TTO |
| 4 | 22 (F) | Failed OAT | MFC/periosteum | 3.8 | 1.0 | 1.0 | 27 | LFC/2.5, trochlea/5.5 | TTO |
| 5 | 18 (M) | OCD | MFC/periosteum | 7.0 | 1.0 | 1.0 | 14 | None | None |
| 6 | 38 (M) | OCD | Trochlea/collagen | 7.8 | 1.0 | 1.0 | 13 | None | TTO |
| 7 | 33 (M) | Cyst | Trochlea/collagen | 2.2 | 1.0 | 1.0 | 45 | Patella/3.0 | None |
| 8 | 24 (M) | Failed MFX, OAT, and OCA | MFC/collagen | 9.0 | 4.0 | 2.5 | 44 | LFC/4.8, trochlea/7.5 | TTO |
| 9 | 34 (M) | Failed OAT | Patella/collagen | 6.2 | 3.5 | 0.9 | 57 | LFC/5.3 | TTO |
| 10 | 42 (M) | Failed OAT | Trochlea/collagen | 2.5 | 1.0 | 1.0 | 40 | MFC/7.0 | HTO + TTO |
| 11 | 32 (M) | Cyst | MFC/collagen | 6.4 | 0.6 | 1.0 | 10 | None | HTO |
| 12 | 43 (M) | Cyst | Patella/collagen | 4.0 | 0.6 | 0.8 | 16 | LFC/1.8 | TTO |
| 13 | 16 (M) | OCD | Trochlea/collagen | 1.5 | 1.0 | 1.0 | 67 | Patella/1.5 | TTO |
| 14 | 35 (F) | Cyst | Trochlea/collagen | 3.6 | 1.5 | 0.8 | 42 | Patella/6.3 | TTO |
| 15 | 22 (M) | Cyst | MFC/collagen | 6.8 | 2.6 | 0.8 | 39 | None | None |
aF, female; HTO, high tibial osteotomy; LFC, lateral femoral condyle; M, male; MFC, medial femoral condyle; MFX, microfracture; OAT, osteochondral autograft transplantation; OCA, osteochondral allograft transplantation; OCD, osteochondritis dissecans; TTO, tibial tubercle osteotomy.
Survival Analysis, Clinical Outcomes, and Satisfaction Survey
There were no cases of treatment failure, and the survival rate over the study period was 100%. All functional scores significantly and clinically meaningfully improved after surgery (Table 2). All patients were satisfied with the procedure, and all indicated that they would undergo the same surgery again. Thirteen patients (87%) rated the outcomes for their knees as good or excellent (Table 3).
TABLE 2.
Preoperative and Final Follow-up Clinical Outcomesa
| Preoperative | Final Follow-up | P | |
|---|---|---|---|
| Modified Cincinnati Knee Rating System | 2.9 ± 1.1 | 6.4 ± 1.8 | <.001 |
| VAS for pain | 7.3 ± 1.9 | 2.8 ± 1.5 | <.001 |
| WOMAC | |||
| Total | 50.5 ± 17.8 | 16.4 ± 9.3 | <.001 |
| Pain | 11.2 ± 4.3 | 3.7 ± 2.8 | <.001 |
| Stiffness | 4.3 ± 1.7 | 2.1 ± 1.8 | <.001 |
| Function | 34.9 ± 13.0 | 10.7 ± 6.7 | <.001 |
| Short Form–36 | |||
| PCS | 36.3 ± 8.9 | 47.8 ± 8.6 | <.001 |
| MCS | 47.3 ± 7.9 | 53.8 ± 6.6 | .0116 |
aData are reported as mean ± SD. MCS, mental component summary; PCS, physical component summary; Short Form–36, 36-Item Short Form Health Survey; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
TABLE 3.
Satisfaction With the Procedure at Final Follow-up (N = 15)
| Question | n (%) |
|---|---|
| Compared with before surgery, how would you rate the operated joint now? | |
| Better | 14 (93) |
| About the same | 0 (0) |
| Worse | 1 (7) |
| What is your overall satisfaction level with the joint surgical procedure? | |
| Satisfied | 14 (93) |
| Neutral | 1 (7) |
| Dissatisfied | 0 (0) |
| If you could go back in time and make the decision again, would you choose to undergo your joint surgery? | |
| Yes | 15 (100) |
| Uncertain | 0 (0) |
| No | 0 (0) |
| How would you rate the results of your joint surgery? | |
| Good/excellent | 13 (87) |
| Fair | 2 (13) |
| Poor | 0 (0) |
Subsequent Surgical Procedures
Eight patients (53%) required a mean of 1.4 subsequent surgical procedures (range, 1-2) at a mean of 1.4 years (range, 1 month to 4.8 years) after surgery. Most of these procedures (92%) were performed arthroscopically and were successfully managed. Subsequent surgical procedures were for adhesions in 7 cases, graft hypertrophy in 2 cases, debridement of a membrane flap in 1 case, and hemarthrosis in 1 case (Table 4). There was no statistical difference in the rate of subsequent surgical procedures required between patients with periosteal versus collagen membranes (P = .608).
TABLE 4.
Subsequent Surgical Proceduresa
| Periosteal Membrane Patch Group (n = 2/5; 40%) | Collagen Membrane Patch Group (n = 6/10; 60%) | Total (n = 8/15; 53%) | |
|---|---|---|---|
| Adhesions | 1 | 6 | 7 |
| Graft hypertrophy | 2 | 0 | 2 |
| Membrane flap debridement | 0 | 1 | 1 |
| Hemarthrosis | 0 | 1 | 1 |
| Total | 3 | 8 | 11 |
aData are reported as No.
Radiographic Outcomes
Ten patients (67%) were available for a radiographic evaluation at a mean of 4.7 years after surgery (with a minimum of 2 years after surgery; range, 2.0-7.6 years). OA based on the K-L grade did not increase except in 1 knee; in this case, the K-L grade increased from 1 to 2 at 7.6 years after surgery.
MRI Outcomes
Twelve patients (80%) were available for an MRI evaluation at a mean of 3.3 years after surgery (with a minimum of 1 year after surgery; range, 1.1-7.8 years) (Table 5). The mean MOCART score was 64.2 ± 19.9; seven patients (58%) had a score higher than 64 points. Ten sites (83%) showed complete or near-complete filling of the cartilage defect, with complete defect filling for 8 repair sites (67%); none of the repair sites exhibited subchondral exposure, and none of the sites demonstrated repair tissue hypertrophy. Two of the 15 patients in this study had graft hypertrophy postoperatively. However, MRI findings from 1 of these 2 patients were available. It should be noted that MRI of this patient was performed after debridement of hypertrophy, which resulted in no hypertrophy in the MRI evaluation. Within the limits of the MRI technique, we could not identify a gap or fissure between the repair tissue and surrounding intact cartilage in most of the patients (n = 10; 83%) (Table 6). Irregular constitution of the surface on MRI was significantly correlated with a poor WOMAC function score (rho = –0.599; P = .040), whereas better structure of the repair tissue was significantly correlated with a better SF-36 mental component summary score (rho = –0.579; P = .048). An edge cartilage flap was observed in 1 patient, but the remaining tissue was in situ. In the MRI evaluation of bone defects, all of the repair sites exhibited complete or near-complete filling, with complete bone filling for 10 repair sites (83%). The composition of the filled bone defect was inhomogeneous, with varying degrees of marrow edema. There was no significant correlation between each bone defect variable on MRI and functional outcomes at final follow-up. However, there was a significant correlation between SI of the edema-like marrow signal within the bone repair site and the total MOCART score (rho = 0.7143; P = .0091).
TABLE 5.
Characteristics of Repaired Cartilage (MOCART Score) and Bonea
| Patient | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 6 | 7 | 9 | 10 | 11 | 12 | 13 | 14 | |
| MRI after surgery, y | 1.5 | 4.9 | 1.3 | 1.4 | 7.8 | 6.7 | 3.6 | 4.7 | 3.3 | 1.1 | 1.9 | 2.1 |
| MOCART score | ||||||||||||
| Degree of defect repair and filling of defect | 20 | 20 | 20 | 20 | 10 | 20 | 20 | 10 | 20 | 10 | 20 | 10 |
| Integration to border zone | 15 | 15 | 15 | 15 | 5 | 10 | 15 | 15 | 15 | 15 | 15 | 15 |
| Surface of repair tissue | 5 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 5 | 10 | 0 |
| Structure of repair tissue | 5 | 5 | 5 | 5 | 0 | 5 | 5 | 0 | 0 | 0 | 0 | 0 |
| Signal intensity of repair tissue | 10 | 30 | 10 | 10 | 0 | 10 | 30 | 10 | 10 | 10 | 10 | 0 |
| Subchondral lamina | 0 | 5 | 5 | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 0 | 0 |
| Subchondral bone | 0 | 5 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Adhesions | 5 | 5 | 5 | 5 | 0 | 5 | 5 | 5 | 5 | 5 | 55 | 5 |
| Effusion | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 | 5 | 5 | 0 |
| Total score | 60 | 95 | 70 | 65 | 25 | 75 | 90 | 60 | 70 | 55 | 70 | 35 |
| Degree of filling of bone defect, % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 75-100 | 100 | 100 | 75-100 |
| Bone defect fill composition | ||||||||||||
| Bone defect fill that is bone (not soft tissue), % | 0-25 | 100 | 75-100 | 50-75 | 100 | 100 | 100 | 100 | 75-100 | 100 | 50-75 | 25-50 |
| Edema-like marrow signal intensity in bone fill | Severe | No edema | Severe | Mild | Severe | No edema | No edema | No edema | Mild | No edema | No edema | Moderate |
aMOCART, magnetic resonance observation of cartilage repair tissue; MRI, magnetic resonance imaging.
TABLE 6.
MOCART and MRI Evaluation of Repaired Cartilagea
| Points | n (%) | |
|---|---|---|
| Degree of defect repair and filling of defect | ||
| Complete | 20 | 8 (66.6) |
| Hypertrophy | 15 | 0 (0.0) |
| Incomplete | ||
| 75% to 100% of adjacent cartilageb | 10 | 2 (16.6) |
| 50% to <75% of adjacent cartilageb | 10 | 2 (16.6) |
| <50% of adjacent cartilage | 5 | 0 (0.0) |
| Subchondral bone exposed | 0 | 0 (0.0) |
| Integration to border zone | ||
| Complete | 15 | 10 (83.3) |
| Incomplete | ||
| Demarcating border visible (splitlike) | 10 | 1 (8.3) |
| Defect visible | ||
| <50% of length of repair tissue | 5 | 1 (8.3) |
| ≥50% of length of repair tissue | 0 | 0 (0.0) |
| Surface of repair tissue | ||
| Surface intact | 10 | 10 (83.3) |
| Surface damaged | ||
| <50% of repair tissue depth | 5 | 2 (16.7) |
| ≥50% of repair tissue depth or total degeneration | 0 | 0 (0.0) |
| Structure of repair tissue | ||
| Homogeneous | 5 | 6 (50.0) |
| Inhomogeneous or cleft formation | 0 | 6 (50.0) |
| Signal intensity of repair tissue | ||
| Isointense | 30 | 2 (16.7) |
| Moderately hyperintense | 10 | 8 (66.6) |
| Markedly hyperintense | 0 | 2 (16.7) |
| Subchondral lamina | ||
| Intact | 5 | 4 (33.3) |
| Not intact | 0 | 8 (66.6) |
| Subchondral bone | ||
| Intact | 5 | 8 (66.6) |
| Granulation tissue, cyst, sclerosis | 0 | 4 (33.3) |
| Adhesions | ||
| No | 5 | 11 (91.7) |
| Yes | 0 | 1 (8.3) |
| Effusion | ||
| No | 5 | 4 (33.3) |
| Yes | 0 | 8 (66.6) |
aMOCART, magnetic resonance observation of cartilage repair tissue; MRI, magnetic resonance imaging.
bModified from the original 2-dimensional MOCART score.
Discussion
In this retrospective analysis of prospectively collected data, we evaluated outcomes for patients who underwent the ACI segmental-sandwich technique. Our study showed that this technique provides excellent survival rates, with significant and clinically meaningful improvements in pain and function as well as very high patient satisfaction rates at midterm follow-up. Within the limits of the MRI technique, MRI showed that the majority of repair tissues were completely filled and integrated to adjacent cartilage with a smooth surface. Moreover, most repair sites displayed complete filling of the bone defect.
Several previous studies have reported good outcomes when ACI was combined with bone grafting to restore the osteochondral unit in patients with an osteochondral defect or OCD.5,7,34 All of these procedures addressed chondral lesions, with ACI cartilage repair covering the full area of bone grafted. However, the ACI segmental-sandwich technique can address bone lesions treated with ABG for only part of the chondral lesion. Other options for treating osseous defects as a part of chondral lesions do exist, but data regarding outcomes with these procedures are scarce. In one study, Sharpe et al42 reported good clinical outcomes over a mean follow-up period of 3 years in 13 patients who underwent a combination of ACI and OAT for the treatment of degenerative large cartilage lesions. Fibrillation of new cartilage between the cores was observed arthroscopically after 1 year, perhaps produced by mesenchymal stem cells derived from bone marrow during the OAT procedure. The ACI segmental-sandwich technique is unique in that it aims to separate autologous cultured chondrocytes from the bone graft and mesenchymal stem cells by covering the bone area with a membrane; however, histological studies are needed to confirm that the covering membrane is truly able to separate the bone marrow–derived stem cells.
Recently, OCA has been gaining in popularity for the reconstruction of both chondral and bone lesions. However, when bone lesions are limited under larger chondral lesions, unnecessarily sacrificing the intact portion of the subchondral bone is concerning. In such cases, the ACI segmental-sandwich technique might allow clinicians to decrease the amount of subchondral bone volume affected by the surgical procedure. Moreover, while OCA has demonstrated unfavorable outcomes for lesions in the patellofemoral joints,3,19 the ACI segmental-sandwich technique provided good clinical outcomes in 7 patients (47%) in our study who had patellofemoral defects.
According to previous studies, empty OCD lesions lead to degenerative changes in the joint at long-term follow-up.27,41,45 Sanders et al41 showed that the cumulative incidence of OA was 12.0% at 5 years after the removal of fragments, and risk factors for the progression of OA included a BMI greater than 25 kg/m2 and adult patients. In our cohort with a mean BMI of 26.2 kg/m2, most of the patients were adults, and our results showed that only 1 of 10 patients exhibited a progression of OA based on the K-L grade; we believe that using the ACI segmental-sandwich technique for these osteochondral lesions may have delayed or prevented the development of OA over a mean of 4.7 years by restoring congruence of the osteochondral unit. Because of the very small sample size and short follow-up of our study however, further evaluations with a larger sample size and a longer follow-up will be needed to confirm this observation.
For the assessment of cartilage repair tissue, the MOCART score was used, as this system is a reliable evaluation method that has demonstrated excellent intraobserver reproducibility.29 Although several studies have reported significant correlations between the same parameters of the MOCART score and functional outcomes,28,44 reported correlations between the MOCART score and clinical outcomes have been variable and remain controversial.11 For our ACI segmental-sandwich repair procedures, the surface and structure of repair tissue were significantly correlated with the WOMAC function score and SF-36 mental component summary score, respectively. We also evaluated filling of the bone defect and marrow edema intensity in bone filling with a novel evaluation system. Notably, the marrow edema intensity in bone filling was significantly correlated with the total MOCART score. Although this correlation did not allow us to establish causality between the severity of bone marrow edema and the quality of repair cartilage tissue assessed by the total MOCART score, this finding does suggest that the severity of bone marrow edema has an important association with the outcomes of cartilage repair. Indeed, several previous studies have demonstrated the negative association of bone marrow edema on cartilage repair.37,38 A prospective longitudinal study is needed to determine whether there is any bidirectional association between these parameters. Additionally, the clinical significance of bone marrow edema is still unclear. In our study, analysis of all bone defect variables on MRI examinations did not demonstrate any significant correlation with clinical outcomes. A larger sample size and more sophisticated imaging evaluations, particularly focusing on subchondral bone, are warranted.
Although 53% of the patients in this study required a mean of 1.4 subsequent surgical procedures, the very high patient satisfaction rate reported is encouraging. This high satisfaction rate may be related to the fact that most of the subsequent procedures were performed arthroscopically, which allowed for quick recovery after the procedures. The most common subsequent surgical procedures performed in this study were for adhesions and graft hypertrophy. The rate of graft hypertrophy from the membrane decreased after we began using a collagen membrane instead of a periosteum.15 Most of the study patients (12/15; 80%) had undergone multiple surgical procedures before the index surgery; of these patients, 7 required subsequent surgical procedures. Among the 3 patients who had not undergone previous surgical procedures, 1 patient required a subsequent surgical procedure. A previous study also showed a relatively high rate of subsequent surgical procedures (49%) in patients who had complex knee problems and had undergone previous failed surgical procedures.49 Nevertheless, the requirement for subsequent surgical procedures did not lead to treatment failure in that study49 or in our study.
All of the procedures in our study were performed by a single surgeon, and all of the study patients had the same indication for surgery and were instructed to follow the same rehabilitation schedule. Nevertheless, this study did have several limitations. First, there was no empty defect group to use as a control; however, because the lesions in the study patients were resistant to nonoperative treatment and were leading to disabling symptoms, it would have been difficult and unethical to create a control group. Second, the small sample size of the study made subanalyses of clinical outcomes per baseline demographics difficult. Further studies with larger sample sizes might be able to identify factors predictive of better clinical outcomes.
Conclusion
This study showed that the ACI segmental-sandwich technique provides excellent survival rates with good functional and radiological results at midterm follow-up. This unique treatment may be an option that provides native joint preservation by restoring congruence and the osteochondral unit for a condition that naturally will progress to OA.
Acknowledgment
The authors thank scientific medical writer Megan Griffiths, MA, for her editorial assistance.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: C.S.W. has received consulting fees and educational support from Aastrom Biosciences (contested) and has stock/stock options in Pfizer. T.M. has received consulting fees from Aastrom Biosciences, Conformis, and Vericel and receives royalties from Conformis. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Partners Human Research Committee (No. 2007P000470/PHS).
References
- 1. Aglietti P, Ciardullo A, Giron F, Ponteggia F. Results of arthroscopic excision of the fragment in the treatment of osteochondritis dissecans of the knee. Arthroscopy. 2001;17(7):741–746. [DOI] [PubMed] [Google Scholar]
- 2. Anderson AF, Pagnani MJ. Osteochondritis dissecans of the femoral condyles: long-term results of excision of the fragment. Am J Sports Med. 1997;25(6):830–834. [DOI] [PubMed] [Google Scholar]
- 3. Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32(10):2160–2168. [DOI] [PubMed] [Google Scholar]
- 4. Aurich M, Anders J, Trommer T, Liesaus E, Wagner A, Venbrocks R. Autologous chondrocyte transplantation by the sandwich technique. Unfallchirurg. 2007;110(2):176–179. [DOI] [PubMed] [Google Scholar]
- 5. Bartlett W, Gooding CR, Carrington RW, Skinner JA, Briggs TW, Bentley G. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft: a preliminary report. J Bone Joint Surg Br. 2005;87(3):330–332. [DOI] [PubMed] [Google Scholar]
- 6. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 7. Bhattacharjee A, McCarthy HS, Tins B, et al. Autologous bone plug supplemented with autologous chondrocyte implantation in osteochondral defects of the knee. Am J Sports Med. 2016;44(5):1249–1259. [DOI] [PubMed] [Google Scholar]
- 8. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Browne JE, Anderson AF, Arciero R, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;436:237–245. [DOI] [PubMed] [Google Scholar]
- 10. Chambers HG, Shea KG, Carey JL. AAOS Clinical Practice Guideline: diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg. 2011;19(5):307–309. [DOI] [PubMed] [Google Scholar]
- 11. de Windt TS, Welsch GH, Brittberg M, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695–1702. [DOI] [PubMed] [Google Scholar]
- 12. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176–181. [PubMed] [Google Scholar]
- 13. Garrett J. Fresh osteochondral allografts for treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Relat Res. 1994;303:33–37. [PubMed] [Google Scholar]
- 14. Gomoll AH, Madry H, Knutsen G, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomoll AH, Probst C, Farr J, Cole BJ, Minas T. Use of a type I/III bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;37(suppl 1):20S–23S. [DOI] [PubMed] [Google Scholar]
- 16. Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468(5):1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87. [DOI] [PubMed] [Google Scholar]
- 18. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25–32. [DOI] [PubMed] [Google Scholar]
- 19. Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts: results in the patellofemoral joint. Clin Orthop Relat Res. 2005;437:176–185. [PubMed] [Google Scholar]
- 20. Johnson L. Arthroscopic abrasion arthroplasty In: McGinty J, ed. Operative Arthroscopy. New York: Raven Press; 1991:341–360. [Google Scholar]
- 21. Johnson LL, Delano M, Spector M, Pittsley A, Gottschalk A. The long-term clinical outcomes following autogenous bone grafting for large-volume defects of the knee: 12- to 21-year follow-up. Cartilage. 2014;5(2):86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones D, Peterson L. Autologous chondrocyte implantation. Instr Course Lect. 2007;56:429–445. [PubMed] [Google Scholar]
- 23. Jones MH, Williams AM. Osteochondritis dissecans of the knee: a practical guide for surgeons. Bone Joint J. 2016;98(6):723–729. [DOI] [PubMed] [Google Scholar]
- 24. Jurgensen I, Bachmann G, Schleicher I, Haas H. Arthroscopic versus conservative treatment of osteochondritis dissecans of the knee: value of magnetic resonance imaging in therapy planning and follow-up. Arthroscopy. 2002;18(4):378–386. [DOI] [PubMed] [Google Scholar]
- 25. Kawasaki K, Uchio Y, Adachi N, Iwasa J, Ochi M. Drilling from the intercondylar area for treatment of osteochondritis dissecans of the knee joint. Knee. 2003;10(3):257–263. [DOI] [PubMed] [Google Scholar]
- 26. Kellgren JH, Lawrence JS. Radiological assessment of rheumatoid arthritis. Ann Rheum Dis. 1957;16(4):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linden B. Osteochondritis dissecans of the femoral condyles: a long-term follow-up study. J Bone Joint Surg Am. 1977;59(6):769–776. [PubMed] [Google Scholar]
- 28. Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zaffagnini S. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med. 2007;35(12):2014–2021. [DOI] [PubMed] [Google Scholar]
- 29. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. [DOI] [PubMed] [Google Scholar]
- 30. Micheli LJ, Browne JE, Erggelet C, et al. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med. 2001;11(4):223–228. [DOI] [PubMed] [Google Scholar]
- 31. Minas T. A Primer in Cartilage Repair and Joint Preservation of the Knee. Philadelphia: Elsevier; 2011. [Google Scholar]
- 32. Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005;436:30–39. [DOI] [PubMed] [Google Scholar]
- 33. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902–908. [DOI] [PubMed] [Google Scholar]
- 34. Minas T, Ogura T, Headrick J, Bryant T. Autologous chondrocyte implantation “sandwich” technique compared with autologous bone grafting for deep osteochondral lesions in the knee. Am J Sports Med. 2018;46(2):322–332. [DOI] [PubMed] [Google Scholar]
- 35. Murray JR, Chitnavis J, Dixon P, et al. Osteochondritis dissecans of the knee: long-term clinical outcome following arthroscopic debridement. Knee. 2007;14(2):94–98. [DOI] [PubMed] [Google Scholar]
- 36. Nehrer S, Spector M, Minas T. Tissue retrieved from revised articular cartilage repair procedures reflects mechanisms of failure. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 12-15, 1997; San Francisco, California, USA. [Google Scholar]
- 37. Niemeyer P, Salzmann G, Steinwachs M, et al. Presence of subchondral bone marrow edema at the time of treatment represents a negative prognostic factor for early outcome after autologous chondrocyte implantation. Arch Orthop Trauma Surg. 2010;130(8):977–983. [DOI] [PubMed] [Google Scholar]
- 38. Niethammer TR, Valentin S, Ficklscherer A, Gulecyuz MF, Pietschmann MF, Muller PE. Revision surgery after third generation autologous chondrocyte implantation in the knee. Int Orthop. 2015;39(8):1615–1622. [DOI] [PubMed] [Google Scholar]
- 39. Pestka JM, Bode G, Salzmann G, Südkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325–331. [DOI] [PubMed] [Google Scholar]
- 40. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17–24. [DOI] [PubMed] [Google Scholar]
- 41. Sanders TL, Pareek A, Obey MR, et al. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-year follow-up. Am J Sports Med. 2017;45(8):1799–1805. [DOI] [PubMed] [Google Scholar]
- 42. Sharpe JR, Ahmed SU, Fleetcroft JP, Martin R. The treatment of osteochondral lesions using a combination of autologous chondrocyte implantation and autograft: three-year follow-up. J Bone Joint Surg Br. 2005;87(5):730–735. [DOI] [PubMed] [Google Scholar]
- 43. Steadman J, Rodkey W, Singleton S, Briggs K. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7(4):300–304. [Google Scholar]
- 44. Tetta C, Busacca M, Moio A, et al. Knee osteochondral autologous transplantation: long-term MR findings and clinical correlations. Eur J Radiol. 2010;76(1):117–123. [DOI] [PubMed] [Google Scholar]
- 45. Twyman RS, Desai K, Aichroth PM. Osteochondritis dissecans of the knee: a long-term study. J Bone Joint Surg Br. 1991;73(3):461–464. [DOI] [PubMed] [Google Scholar]
- 46. van Dyk GE, Dejardin LM, Flo G, Johnson LL. Cancellous bone grafting of large osteochondral defects: an experimental study in dogs. Arthroscopy. 1998;14(3):311–320. [DOI] [PubMed] [Google Scholar]
- 47. von Keudell A, Gomoll AH, Bryant T, Minas T. Spontaneous osteonecrosis of the knee treated with autologous chondrocyte implantation, autologous bone-grafting, and osteotomy: a report of two cases with follow-up of seven and nine years. J Bone Joint Surg Am. 2011;93(24):e149. [DOI] [PubMed] [Google Scholar]
- 48. Wright RW, McLean M, Matava MJ, Shively RA. Osteochondritis dissecans of the knee: long-term results of excision of the fragment. Clin Orthop Relat Res. 2004;424:239–243. [PubMed] [Google Scholar]
- 49. Zaslav K, Cole B, Brewster R, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42–55. [DOI] [PubMed] [Google Scholar]