Abstract
Pulmonary arterial compliance (PAC), invasively assessed by the ratio of stroke volume to pulmonary arterial (PA) pulse pressure, is a sensitive marker of right ventricular (RV)-PA coupling that differs across the spectrum of pulmonary hypertension (PH) and is predictive of outcomes. We assessed whether the echocardiographically derived ratio of RV outflow tract velocity time integral to PA systolic pressure (RVOT-VTI/PASP) (a) correlates with invasive PAC, (b) discriminates heart failure with preserved ejection-associated PH (HFpEF-PH) from pulmonary arterial hypertension (PAH), and (c) is associated with functional capacity. We performed a retrospective cohort study of patients with PAH (n = 70) and HFpEF-PH (n = 86), which was further dichotomized by diastolic pressure gradient (DPG) into isolated post-capillary PH (DPG < 7 mmHg; Ipc-PH, n = 54), and combined post- and pre-capillary PH (DPG ≥ 7 mm Hg; Cpc-PH, n = 32). Of the 156 patients, 146 had measurable RVOT-VTI or PASP and were included in further analysis. RVOT-VTI/PASP correlated with invasive PAC overall (ρ = 0.61, P < 0.001) and for the PAH (ρ = 0.38, P = 0.002) and HFpEF-PH (ρ = 0.63, P < 0.001) groups individually. RVOT-VTI/PASP differed significantly across the PH spectrum (PAH: 0.13 [0.010–0.25] vs. Cpc-PH: 0.20 [0.12–0.25] vs. Ipc-PH: 0.35 [0.22–0.44]; P < 0.001), distinguished HFpEF-PH from PAH (AUC = 0.72, 95% CI = 0.63–0.81) and Cpc-PH from Ipc-PH (AUC = 0.78, 95% CI = 0.68–0.88), and remained independently predictive of 6-min walk distance after multivariate analysis (standardized β-coefficient = 27.7, 95% CI = 9.2–46.3; P = 0.004). Echocardiographic RVOT-VTI/PASP is a novel non-invasive metric of PAC that differs across the spectrum of PH. It distinguishes the degree of pre-capillary disease within HFpEF-PH and is predictive of functional capacity.
Keywords: pulmonary arterial compliance, pulmonary hypertension, Cpc-PH, non-invasive
Introduction
Pulmonary hypertension (PH) is defined by a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg by right heart catheterization (RHC) and stems from a wide range of etiologies.1 While PH associated with left heart disease (LHD-PH) is common, pulmonary arterial hypertension (PAH) remains a rare and progressive condition associated with a high morbidity and mortality and warrants specific therapeutics.2 Thus, distinguishing those patients with intrinsic abnormalities of the pulmonary vasculature is clinically relevant.
With increasing identification and expanded hemodynamic insight into PH, there has also been an increased awareness of those patients with LHD-PH who also have abnormalities of their pulmonary arterial vasculature, so-called combined post- and pre-capillary disease (Cpc-PH), as compared to those with isolated post-capillary PH (Ipc-PH).1–3 Importantly, Cpc-PH may share features similar to PAH including mortality, histopathologic, genetic, and pathophysiologic mechanisms compared to Ipc-PH.4–8
Pulmonary arterial compliance (PAC), estimated as the ratio of right ventricular (RV) stroke volume to pulmonary arterial (PA) pulse pressure (PP), represents pulsatile RV afterload.9 A reduction in PAC, representing increased PA stiffness, is associated with increased reflected waves during systole, increased PAPP, and increased RV pulsatile load. Studies suggest PAC may be altered earlier in the course of disease compared to pulmonary vascular resistant (PVR).10 PAC is of significant prognostic value in both PAH and LHD-PH and improves with PH therapy.9–13 PAC has also been shown to effectively discriminate Ipc-PH, Cpc-PH, and PAH.4
Given the high prevalence of LHD-PH, and specifically heart failure with preserved ejection fraction-associated PH (HFpEF-PH), and the importance in differentiating it from PAH, identifying readily available markers to help distinguish patients that require more invasive investigation is important clinically.14–17 In this study, we propose the ratio of RV outflow tract velocity time integral (RVOT-VTI), a surrogate of RV SV, and pulmonary artery systolic pressure (PASP; RVOT-VTI/PASP), as a simple, non-invasive estimate of PAC and assess its ability to identify patients across the spectrum of PH. We also sought to assess its association with 6-min walk distance (6MWD) as a marker of RV-PA uncoupling across the spectrum of PH.
Methods
Study population
We performed a retrospective cohort study of consecutive patients referred to the Hospital of the University of Pennsylvania and evaluated in the Pulmonary Hypertension Program who underwent RHC and echocardiography within one year, met criteria for PH defined as mPAP ≥ 25 mmHg, and had a preserved left ventricular ejection fraction (LVEF) on echocardiography. Patients were then stratified into PAH or heart failure with preserved ejection fraction (HFpEF)-associated PH (HFpEF-PH), as defined by current guidelines,1,18 with HFpEF defined by elevated pulmonary artery wedge pressure (PAWP) > 15 mmHg. HFpEF-PH was further subdivided into Cpc-PH and Ipc-PH and defined as PH with a PAWP of > 15 mmHg and diastolic pressure gradient (DPG) ≥ 7 mmHg and/or PVR > 3 WU, or DPG < 7 mmHg and/or PVR ≤ 3 WU, respectively1 (Suppl. Fig. 1). Patients were excluded if they had a reduced LVEF or other known causes of PH including World Health Organization (WHO) groups III–V or congenital heart disease-associated PH. Specifically, patients with significant lung disease per review of pulmonary function tests or chest computed tomography were excluded, as described previously.19,20 Baseline demographic and clinical variables, including age, sex, body mass index (BMI), cause of PAH (classified as idiopathic, connective tissue disease, or other), WHO functional class (FC), and 6MWD, were collected, as previously described.19 The study was approved by the institutional review board of the University of Pennsylvania (Protocol no. 820414).
Echo-Doppler exam
All patients underwent a clinically indicated echo-Doppler exam using the Philips Sonos 7500, IE33 (Philips Medical Systems, Andover, MA, USA) or GE Vivid 7 Ultrasound (GE, Milwaukee, WI, USA) systems. All studies were analyzed using ProSolv CardioVascular Client software (Fujifilm Medical Systems, Stamford, CT, USA). The echocardiograms were analyzed by two independent PH cardiologists (JAM, AV) in accordance with the American Society of Echocardiography (ASE) guidelines, and blinded to patient clinical characteristics, invasive hemodynamics, or outcomes, as previously described.19 The echo-Doppler exam included standard assessments of right- and left-sided heart chambers and vasculature. Specifically, this included right atrial (RA) size and planimetered RA area1 at end-systole (in the apical four-chamber view), right (RVIDd) and left (LVIDd) ventricular dimensions (obtained at end-diastole in the apical four-chamber view), RV end-diastolic area (RVAd) and RV end-systolic area (RVAs), with right ventricular fractional area of change (RV FAC) calculated in the standard manner ([RVAd- RVAs]/RVAd × 100). Tricuspid annular plane systolic excursion (TAPSE) was measured either from the M-mode at the lateral tricuspid annulus off the apical four-chamber view, or from the two-dimensional (2D) apical four-chamber view. Specifically, if an M-mode TAPSE was not available or of adequate quality, the 2D apical four-chamber view was used, with TAPSE measured by subtracting the distance of displacement of the lateral tricuspid annulus between end-systole and end-diastole, a technique that has been shown to tightly correlate with M-mode TAPSE, as previously described.19,21,22 Both RA area and RV end-diastolic area were indexed to patient height. RVOT VTI (3-beat average), acceleration time (AccT), and systolic notching pattern were obtained from the RVOT pulse wave Doppler profile in the parasternal short- or long-axis views. The maximal trans-tricuspid flow velocity was obtained in the usual manner and PASP was calculated using the sum of the modified Bernoulli equation (4v2) and estimated RA pressure as recommended by ASE guidelines.23 Diastolic function was assessed by trans-mitral Doppler velocity and tissue Doppler using standard techniques.8
Invasive hemodynamics
All patients underwent standard clinical hemodynamic assessment by RHC with all tracings reviewed and values reported at end-expiration, as previously described. Indirect Fick (with an assumed O2 consumption of 125 mL/min/m2 × BSA) was used to estimate cardiac output.1
Statistical analysis
Categorical variables are reported as n (%) and continuous variables are reported with mean ± standard deviation (SD) or median (interquartile range [IQR]) for normally and non-normally distributed variables, respectively. Univariate differences between PH phenotypes, namely Ipc-PH, Cpc-PH, and PAH, were assessed using t-tests/ANOVA or Wilcoxon rank-sum (Mann–Whitney U) test/Kruskal–Wallis test for normally/non-normally distributed data, respectively. Using Kruskal–Wallis tests, we particularly assessed the differences in non-invasively estimated PAC as measured by RVOT-VTI/PASP, between the three groups, along with other echocardiographic and invasive hemodynamic variables. To determine reproducibility of RVOT-VTI, intra-class correlation coefficient (ICC) was calculated to measure variability and agreement between independent PH cardiologists.
Spearman correlation coefficient was used to determine the correlation between invasively measured PAC to the non-invasively measured RVOT-VTI/PASP. ROC analysis was performed to assess the discriminative ability of RVOT-VTI/PASP as a predictive tool to differentiate between PH subgroups, with the Youden index used to determine the optimal cut-off point to differentiate HFpEF-PH phenotypes.
Univariate linear regression was used to determine predictors of functional capacity as measured by 6MWD. Variables with a P value < 0.25 on univariate analysis were entered into a multivariate linear regression model to examine the association between 6MWD and RVOT-VTI/PASP. All analyses were performed in Stata 14.2 (StataCorp, 2017, College Station, TX, USA). A P value < 0.05 was considered significant.
Results
Baseline characteristics
A total of 156 patients comprised the total cohort with 86 having HFpEF-PH and 70 with PAH (idiopathic: n = 36 [51.4%], connective tissue disease: n = 23 [32.9%], other: n = 11 [15.7%]). Of the 156 total patients, 146 had measurable RVOT-VTI and PASP and were included in further analysis (Suppl. Fig. 1). Median time between echocardiogram and RHC was 22.5 days (IQR = 6–43). As noted in Table 1 and Suppl. Tables 1 and 2, there was a significant difference in age (P < 0.001), without differences in sex, BMI, or 6MWD across PH subgroups.
Table 1.
Demographic, clinical, echocardiographic, and hemodynamic characteristics across PH subgroups.
| Ipc-PH DPG < 7 mmHg (n = 54) | Cpc-PH DPG ≥ 7 mmHg (n = 32) | PAH (n = 70) | P value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Mean age (years) | 65.3 ± 13 | 66.7 ± 14 | 54.9 ± 15 | < 0.001 |
| Male (n (%)) | 20 (37) | 12 (38) | 15 (21) | 0.24 |
| Race (n (% White)) | 35 (65) | 25 (78) | 49 (70) | 0.78 |
| BMI (kg/m2) | 30.5 (25–38) | 29.2 (26–36) | 28 (24–33) | 0.12 |
| 6MWD (m) | 278 ± 114 | 225 ± 117 | 284 ± 117 | 0.42 |
| Echocardiographic variables | ||||
| LVEF (%) | 64 ± 7.7 | 66 ± 10.5 | 66 ± 11.3 | 0.36 |
| IVS (cm) | 1.2 (1.1–1.5) | 1.2 (1–1.4) | 1 (0.9–1.2) | < 0.001 |
| LVIDd (cm) | 4.72 ± 0.64 | 4.4 ± 0.97 | 3.8 ± 0.57 | 0.001 |
| LA d (cm) | 4.7 ± 0.78 | 4.7 ± 0.82 | 3.4 ± 0.61 | 0.09 |
| PASP (est TTE) (mmHg) | 53.0 ± 20.7 | 72.2 ± 24 | 72.7 ± 28.8 | < 0.001 |
| TR grade ≥ 3 (n (%)) | 11 (20.4) | 13 (40.6) | 19 (27) | 0.29 |
| RV:LV ratio | 0.97 ± 0.25 | 1 ± 0.23 | 1.3 ± 0.35 | 0.004 |
| RV FAC (%) | 31.3 (27–41) | 33.5 (24–38) | 24.4 (16–30) | < 0.001 |
| RVOT-VTI (cm) | 16.5 ± 5 | 12.7 ± 4 | 11 ± 3 | 0.003 |
| RVOT-VTI/PASP | 0.35 (0.22–0.44) | 0.20 (0.12–0.25) | 0.13 (0.10–0.25) | < 0.001 |
| Acceleration time (ms) | 99 ± 29 | 77.5 ± 17.8 | 69 ± 18 | < 0.001 |
| TAPSE (mm) | 17 (15–23) | 15 (11–19) | 16 (13–19) | < 0.001 |
| Notch (n (%)) | 29 (54) | 24 (75) | 66 (96) | 0.0003 |
| Invasive hemodynamics | ||||
| Heart rate (bpm) | 69.5 ± 12.8 | 77 ± 10.9 | 82.2 ± 15.7 | 0.05 |
| Mean right atrial pressure (mmHg) | 13 (11–17) | 16 (12–21) | 9 (6–14) | < 0.001 |
| PASP (mmHg) | 63.35 ± 16.6 | 80.22 ± 19.2 | 86.53 ± 21.1 | 0.193 |
| PADP (mmHg) | 24.65 ± 4.8 | 34.88 ± 5.9 | 34.27 ± 8.2 | < 0.001 |
| mPAP (mmHg) | 37.55 ± 7.7 | 49.99 ± 9.2 | 52.62 ± 12.1 | 0.002 |
| PCWP (mmHg) | 23 (20–27) | 22 (19.5–24) | 10.5 (8–13) | < 0.001 |
| TPG (mmHg) | 14.3 ± 5.6 | 27.7 ± 8.5 | 42.0 ± 12 | < 0.001 |
| DPG (mmHg) | 1.4 ± 2.7 | 12.6 ± 5 | 23.7 ± 8 | < 0.001 |
| CO (L/min) | 6.2 (4.7–7.9) | 4.9 (3.7–6) | 4.1 (3.3–4.7) | < 0.001 |
| CI (L/min/m2) | 3.1 (2.6–3.8) | 2.5 (2–2.8) | 2.2 (1.9–2.6) | < 0.001 |
| PVR (WU) | 2.2 (1.7–3) | 6.2 (3.6–7.8) | 11 (6.7–14.5) | < 0.001 |
| PAC (L/mmHg) | 2.66 (1.7–3.4) | 1.35 (0.97–2.15) | 0.95 (0.64–1.41) | < 0.001 |
| SVR (WU) | 14 (10.3–17) | 16 (12.7–21.3) | 17 (14–20) | < 0.001 |
Echocardiographic data
Echo-Doppler data revealed similarities in LVEF across groups, but with differences in parameters of left heart structure, including IVS and LVIDd, which were more prominent in the HFpEF-PH subgroups compared to the PAH group (Table 1, Suppl. Table 1). Furthermore, several markers of RV dysfunction and disrupted RV-PA interaction were more pronounced in moving across the PH subgroups (Ipc-PH, Cpc-PH, and PAH). For example, RV:LV ratio and presence of a notched RVOT pulsed-wave Doppler profile increased across groups along with reductions in AccT, RVOT-VTI, and TAPSE. Additionally, RV fractional area change was lower in PAH patients compared to HFpEF-PH patients (31% vs. 24%, P < 0.001; Suppl. Table 1). Similar findings were seen comparing the Ipc-PH and Cpc-PH groups (Suppl. Table 2). Finally, ICC between readers for RVOT-VTI was 0.97 (95% confidence interval [CI] = 0.96–0.98) and 0.98 (95% CI = 0.97–0.99) for PASP.
Invasive hemodynamics
Invasive hemodynamic data revealed significant differences across groups including increased mPAP, TPG, DPG, and PVR with a reduction in CO and PAC in in PAH compared to the other groups (Table 1 and Suppl. Tables 1 and 2). Of note, RAP was significantly lower in PAH compared to HFpEF-PH subgroups, with PAWP similarly and significantly increased in the HFpEF-PH subgroups compared to PAH (Suppl. Tables 1 and 2).
Non-invasive assessment of PAC
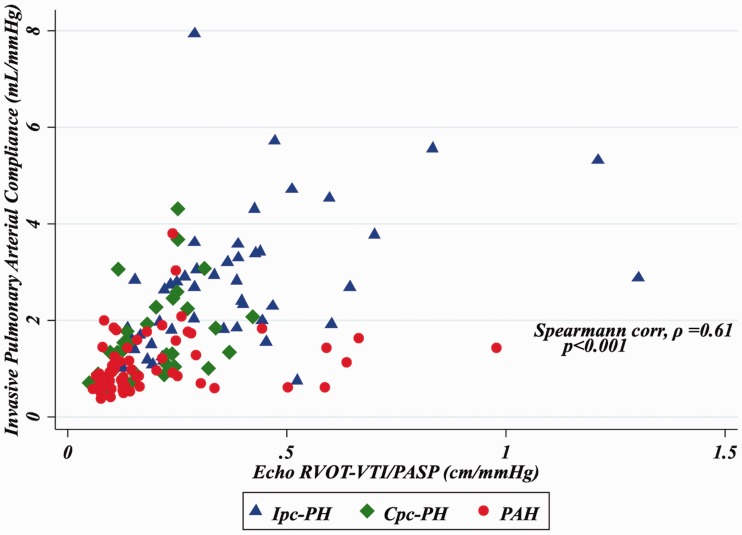
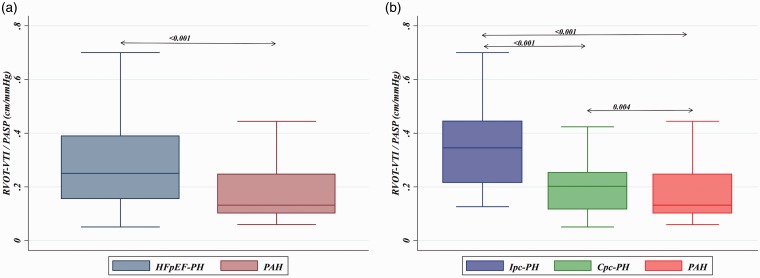
As seen in Fig. 1, RVOT-VTI/PASP correlated with invasively measured PAC for the group overall (ρ = 0.61, P < 0.001) and remained significantly correlated for the PAH (ρ = 0.38, P = 0.002) and HFpEF-PH (ρ = 0.63, P < 0.001) groups individually. Furthermore, non-invasive PAC as determined by RVOT-VTI/PASP was significantly lower in the PAH group (median = 0.13, IQR = 0.010–0.25) compared to the HFpEF-PH group (median = 0.25, IQR = 0.16–0.39; P < 0.001; Suppl. Table 1 and Fig. 2a) and differed across the spectrum of PH with the lowest ratio in those with PAH, intermediate in Cpc-PH, and the highest in those with Ipc-PH (P < 0.001; Table 1 and Fig. 2b).
Fig. 1.
Correlation of RVOT-VTI/PASP to invasive PAC.
Fig. 2.
Box plots of RVOT-VTI/PASP across PH subgroup. (a) HFpEF-PH vs. PAH. (b) Ipc-PH vs. Cpc-PH vs. PAH.
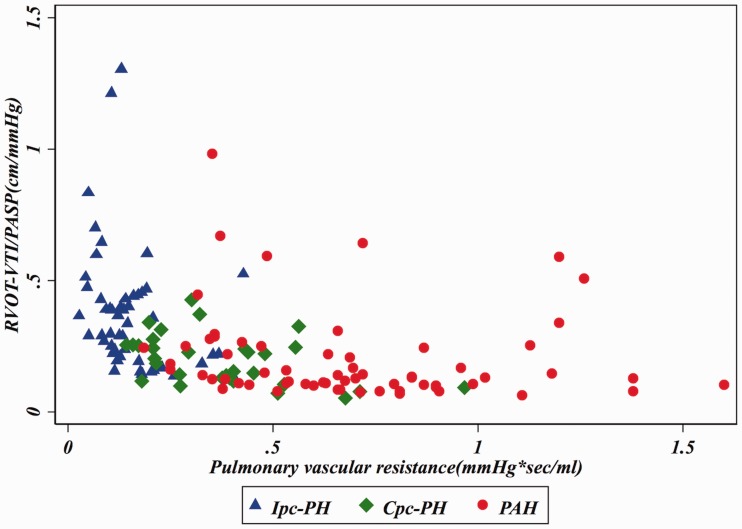
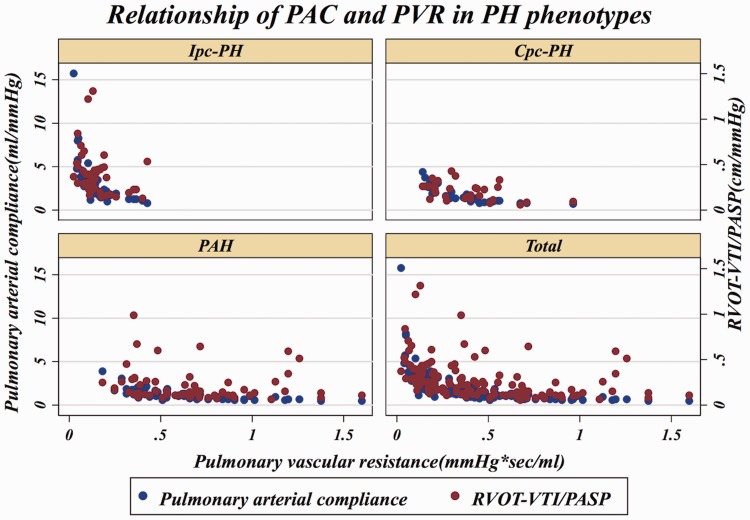
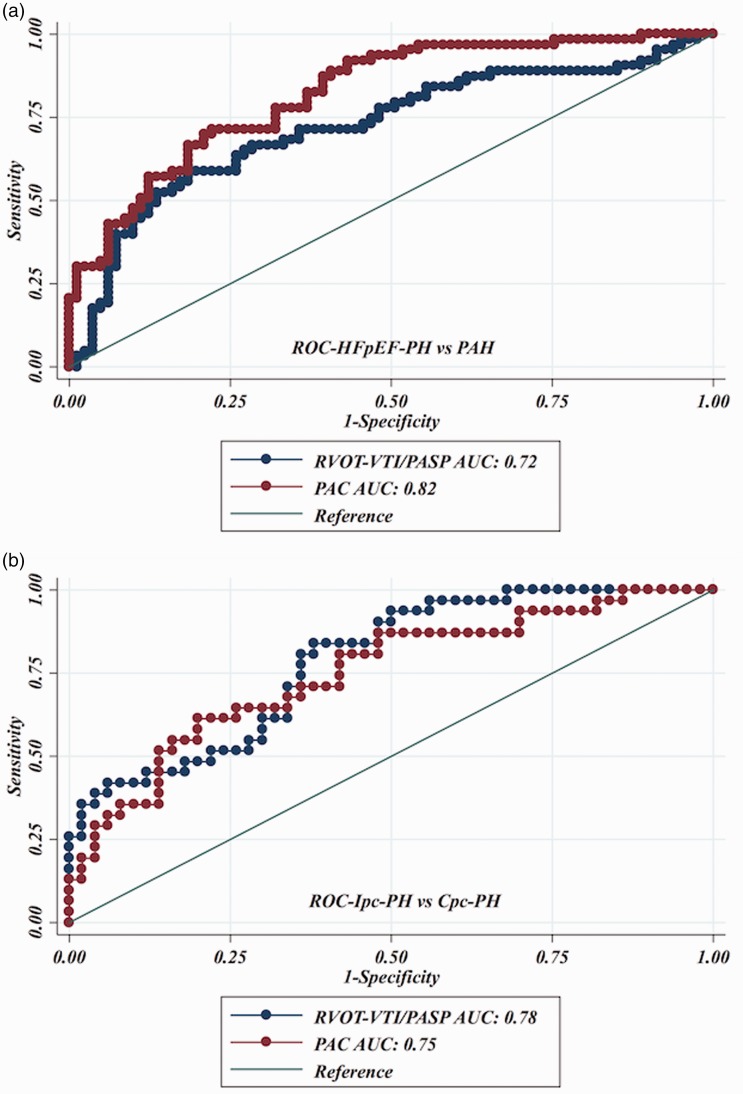
In an effort to reproduce the invasively derived PAC-PVR product (the ‘RC’ time), as previously described,4,13 and assess if RVOT-VTI/PASP would maintain that relationship, we plotted RVOT-VTI/PASP versus PVR as seen in Figs. 3 and 4. As noted in these figures, for a given PVR, the PAC in the Ipc-PH group, whether assessed invasively or non-invasively using RVOT-VTI/PASP, was shifted downward and to the left compared to the Cpc-PH and PAH groups. There was a significant difference in the RVOT-VTI/PASP to PVR product across groups with Ipc-PH displaying the lowest and most leftward-shifted (median = 0.039, IQR = 0.027–0.059), followed by Cpc-PH (median = 0.053, IQR = 0.040–0.088) with the PAH group the highest and shifted most rightward (median = 0.088, IQR = 0.059–0.139; P < 0.001). Specifically, this relationship is significantly different within HFpEF-PH (Ipc-PH vs. Cpc-PH: P = 0.009). ROC analysis revealed modest ability of RVOT-VTI/PASP to differentiate HFpEF-PH from PAH groups (area under the curve [AUC] = 0.72, 95% CI = 0.63–0.81) and Ipc-PH from Cpc-PH (AUC = 0.78, 95% CI = 0.68–0.88), at a Youden index cut-off point of 0.27 (AUC = 0.73; sensitivity = 62%, specificity = 84%) to distinguish Ipc-PH from Cpc-PH, with a similar performance to invasively measured PAC (Fig. 5).
Fig. 3.
Relationship of non-invasive compliance (RVOT-VTI/PASP) and invasively measured pulmonary vascular resistance (PVR) across PH subgroups. Median RC Time: Ipc-PH: 0.039 (IQR = 0.027–0.059) vs. Cpc-PH: 0.053 (IQR = 0.040–0.088) vs. PAH: 0.088 (IQR = 0.059–0.139); P < 0.001.
Fig. 4.
Dual scatter plot with invasive PAC (on left y-axis) and RVOT-VTI/PASP on right y-axis plotted against invasive PVR.
Fig. 5.
Receiver operating characteristic curves for RVOT-VTI/PASP to distinguish: (a) HFpEF-PH and PAH; and (b) Ipc-PH and Cpc-PH.
RVOT-VTI/PASP and 6MWD
We also assessed predictors of functional capacity as assessed by 6MWD in using linear regression analysis. RVOT-VTI/PASP was an independent predictor of 6MWD on multivariate analysis after adjustment for age and BMI (Table 2).
Table 2.
Univariate and multivariate linear regression analysis of predictors 6MWD.
| β-coefficient* | 95% CI | P value | |
|---|---|---|---|
| Univariate | |||
| Age | −49.4 | −68.5–-30.2 | < 0.001 |
| Sex (male) | 25.9 | −18.3–70.0 | 0.249 |
| Race (White) | −5.5 | −36.7–25.7 | 0.729 |
| HR | −11.6 | −31.9–8.6 | 0.257 |
| BMI | −48.5 | −67.2–-29.9 | < 0.001 |
| RVOT-VTI/PASP | 16.8 | −3.63–37.22 | 0.106 |
| TAPSE/PASP | 0.22 | −20.2–20.7 | 0.983 |
| Multivariate | |||
| Sex (male) | 8.5 | −31.8–48.9 | 0.676 |
| BMI | −62.0 | −82.4–-41.5 | < 0.001 |
| RVOT-VTI/PASP | 27.7 | 9.2–46.3 | 0.004 |
All continuous variables in standardized units.
Discussion
Invasively derived PAC has emerged as an increasingly important marker of RV pulsatile load and RV-PA uncoupling and is prognostically important across the spectrum of PH.4,9 In addition, abnormalities in PAC may occur earlier compared to PVR and thus may be a more sensitive marker of PH disease severity. This study is the first to describe RVOT-VTI/PASP as a simplified, non-invasively derived estimation of PAC. This study highlights the differences in echocardiographic and hemodynamic parameters, including significant differences in RVOT-VTI/PASP, across the spectrum of PH. We show for the first time the ability of RVOT-VTI/PASP to distinguish PAH from HFpEF-PH as well as between Ipc-PH and Cpc-PH. Lastly, RVOT-VTI/PASP remains an independent predictor of functional capacity as assessed by 6MWD after multivariate analysis.
Previously, Mahapatra et al. reported on an echo-Doppler estimate of PAC, calculated as echo-derived SV/PAPP, and showed its prognostic value in patients with PAH.24 Unfortunately, this approach is more time-intensive and requires several mathematical assumptions to echocardiographically estimate SV and PAPP, while some parameters may not be available or interpretable in some patients.25 The currently proposed metric serves to simplify the estimate by Mahapatra et al. and allow for routine clinical use.
Guazzi et al.26 as well as others have more recently reported on the use of TAPSE/PASP as a surrogate of PAC in several heart failure populations with strong correlations with invasively measured PAC, specific markers on cardiopulmonary exercise testing (CPET), and outcomes.3,27–29 Interestingly, TAPSE/ PASP was not sensitive for distinguishing Ipc-PH in a most recent analysis of HFpEF-PH.26 Limitations of TAPSE including load dependence, a reduction in TAPSE in the setting of atrial fibrillation independent of PH,21,30 as well as disruption of longitudinal fibers (and thus “falsely” reduced RV function by TAPSE) after cardiac surgery31 may suggest RVOT-VTI/PASP is more reflective of RV-PA uncoupling as it incorporates a surrogate of RV stroke volume relative to a given pressure. Furthermore, the RVOT-VTI, as a stroke volume surrogate, incorporates full RV ejection (RV basal and free wall motion) as opposed to TAPSE which reflects RV basal motion only. In fact, in our study, while RVOT-VTI/PASP independently predicted functional capacity as determined by 6MWD, TAPSE/PASP did not.
RVOT-VTI/PASP in distinguishing PH subgroups
Identifying those with combined post- and pre-capillary PH, termed Cpc-PH, and distinguishing them from PAH has potential clinical and prognostic implications. We and others have proposed use of echo-Doppler parameters in stratifying these populations which can then more appropriately identify patients who should proceed with invasive assessment, trial of PH therapies, and/or enrollment in clinical trials.7,15,16,32 As reported in this study, RVOT-VTI/PASP displays discriminatory ability of PAH from HFpEF-PH and perhaps more importantly within HFpEF-PH in distinguishing Cpc-PH and Ipc-PH. Importantly, and in contradistinction to our prior work including RVOT PW Doppler notching and the “echo score,” this parameter serves as a non-invasive surrogate of an invasively derived hemodynamic metric, namely, PAC. Indeed, both notching alone and the echo score (which includes the presence of RVOT notching and/or acceleration time < 80 ms) address the issue of hemodynamic phenotyping, but do not specifically give a hemodynamic value that can be integrated into understanding a given patient's hemodynamic condition. Moreover, notching is a categorical variable that can sometimes be challenging to correctly define, especially in non-expert hands, while the tracing of a VTI and TR velocity may be less challenging. Unlike the echo score or notching, the RVOT-VTI/PASP is a continuous value and may serve as a non-invasive metric gauging response to PH therapy in patients with PAH.33 Lastly, the echo score, while comprehensive, is a bit more cumbersome to generate and integrates left-sided parameters that do not specifically speak to RV-PA interaction. In summary, this metric does not negate the role of either notching alone or the echo score but serves as another tool in the non-invasive tool box for clinicians to phenotype patients.
Recently, studies have assessed the ability of the resistance-compliance (RC) time, defined as the product of invasively derived PVR and PAC thereby integrating mean and pulsatile components of RV afterload, to differentiate Cpc-PH from Ipc-PH.4 Assad et al.4 and Gerges et al.3 have showed that the RC time in Cpc-PH is elevated in a similar fashion to that of PAH as compared to Ipc-PH, despite similar PAWP elevations in both Cpc-PH and Ipc-PH.4 In the study by Assad et al., PAC was highest in Ipc-PH, intermediate in Cpc-PH, and most reduced in PAH, consistent with the presumed increase in PA stiffness across these subgroups.4 Within this context, we have reproduced the relationship between invasively determined PAC and PVR which is altered in patients with Ipc-PH compared to Cpc-PH and PAH using RVOT-VTI/PASP as the non-invasive estimate of PAC.
Limitations
This study is limited by its modest sample size and single-center design, which limited more robust multivariable assessment as well as the generalizability of our findings to other PH cohorts. Specifically, the prevalence of PAH, Cpc-PH, and distribution compared to Ipc-PH may differ in other centers. In addition, recently, and well after the conclusion of this analysis, changes to the definition of PH overall (mPAP ≥ 20 mmHg) and Cpc-PH (defined by PVR ≥ 3 WU as opposed to DPG), have been proposed.34 The trend of RVOT-VTI/PASP persisted across the three subgroups (Ipc-PH, Cpc-PH, and PAH) using the new PVR definition (Cpc-PH defined by PVR ≥ 3WU and Ipc-PH < 3 WU) with similar findings (P < 0.001). Furthermore, while the RVOT PW Doppler profile is a standard assessment in our echocardiographic laboratory, it may not be routinely interpreted in clinical echocardiography laboratories. While this is true, there are now significant data showing the importance of this profile in the interpretation and prognostication of PH and pulmonary vascular disease,17,35 and it is a recommended view in the latest PH guidelines.1 Additionally, while echocardiographic variables may not always be present/measurable, thus limiting generalizability across populations, we were able to measure RVOT-VTI/PASP in 146/156 (94%) of the overall study cohort. Similarly, as mentioned above, this parameter is a non-invasive surrogate of PAC and not a non-invasive calculation of PAC. While this may in part explain modest correlation with invasive PAC, its simplicity allows for ease of calculation, with less measurement assumptions and error, as well as limitation of views needed for its assessment. Importantly, while there was an allowance for a time period of up to one year between echocardiogram and invasive hemodynamics, the majority of patients had an echocardiogram and RHC within 30 days, which represents clinical practice and thus suggests “real-world” applicability of this parameter. Lastly, 6MWD was the outcome used to assess functional capacity in this study; while there are known limitations to this metric,36 it is routinely assessed in clinical practice and is a clinically available parameter of submaximal functional capacity.
Conclusion
This study describes RVOT-VTI/PASP as a non-invasive estimate of PAC, which distinguishes between PAH and HFpEF-PH as well as within HFpEF-PH and is independently predictive of functional capacity as assessed by 6MWD. Further study is needed to validate this metric in larger populations along with the assessment of its prognostic ability, correlation with parameters on CPET, and its change in response to PH therapy.
Supplemental Material
Supplemental material, Supplemental Material1 for Right ventricular outflow tract velocity time integral-to-pulmonary artery systolic pressure ratio: a non-invasive metric of pulmonary arterial compliance differs across the spectrum of pulmonary hypertension by Priyanka T. Bhattacharya, Gregory S. Troutman, Frances Mao, Arieh L. Fox, Monique S. Tanna, Payman Zamani, E. Wilson Grandin, Jonathan N. Menachem, Edo Y. Birati, Julio A. Chirinos, Sula Mazimba, Kerri Akaya Smith, Steven M. Kawut, Paul R. Forfia, Anjali Vaidya and Jeremy A. Mazurek in Pulmonary Circulation
Supplemental Material
Supplemental material, Supplemental Material2 for Right ventricular outflow tract velocity time integral-to-pulmonary artery systolic pressure ratio: a non-invasive metric of pulmonary arterial compliance differs across the spectrum of pulmonary hypertension by Priyanka T. Bhattacharya, Gregory S. Troutman, Frances Mao, Arieh L. Fox, Monique S. Tanna, Payman Zamani, E. Wilson Grandin, Jonathan N. Menachem, Edo Y. Birati, Julio A. Chirinos, Sula Mazimba, Kerri Akaya Smith, Steven M. Kawut, Paul R. Forfia, Anjali Vaidya and Jeremy A. Mazurek in Pulmonary Circulation
Supplemental Material
Supplemental material, Supplemental Material3 for Right ventricular outflow tract velocity time integral-to-pulmonary artery systolic pressure ratio: a non-invasive metric of pulmonary arterial compliance differs across the spectrum of pulmonary hypertension by Priyanka T. Bhattacharya, Gregory S. Troutman, Frances Mao, Arieh L. Fox, Monique S. Tanna, Payman Zamani, E. Wilson Grandin, Jonathan N. Menachem, Edo Y. Birati, Julio A. Chirinos, Sula Mazimba, Kerri Akaya Smith, Steven M. Kawut, Paul R. Forfia, Anjali Vaidya and Jeremy A. Mazurek in Pulmonary Circulation
Conflict of interest
The author(s) declare the following potential conflicts of interest: SMK reports non-financial support from the American Thoracic Society, and grants from Actelion, United Therapeutics, Gilead, Lung Biotech, Bayer, grants and non-financial support from Cardiovascular Medical Research and Education Fund, and non-financial support from Pulmonary Hypertension Association, outside the submitted work. PZ is supported by NIH grant 5K23HL13055102. EYB has received grants from HeartWare Ltd., and personal fees from Luitpold Pharmaceuticals, Inc., outside the submitted work. JAC has received consulting honoraria from Sanifit, Microsoft, Fukuda-Denshi, Bristol-Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck and Bayer. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb, and Microsoft. JAC is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. The other authors do not have a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
Funding
This work was supported in part by NIH grant K24-HL103844 (SMK).
References
- 1.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2.Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013; 62: D100–108. [DOI] [PubMed] [Google Scholar]
- 3.Gerges M, Gerges C, Pistritto AM, et al. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med 2015; 192: 1234–1246. [DOI] [PubMed] [Google Scholar]
- 4.Assad TR, Brittain EL, Wells QS, et al. Hemodynamic evidence of vascular remodeling in combined post- and precapillary pulmonary hypertension. Pulm Circ 2016; 6: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assad TR, Hemnes AR, Larkin EK, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol 2016; 68: 2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143: 758–766. [DOI] [PubMed] [Google Scholar]
- 7.Naeije R, Gerges M, Vachiery JL, et al. Hemodynamic phenotyping of pulmonary hypertension in left heart failure. Circ Heart Fail 2017; 10: 004082. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 9.Thenappan T, Prins KW, Pritzker MR, et al. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc 2016; 13: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medrek SK, Kloefkorn C, Nguyen DTM, et al. Longitudinal change in pulmonary arterial capacitance as an indicator of prognosis and response to therapy and in pulmonary arterial hypertension. Pulm Circ 2017; 7: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Naamani N, Preston IR, Paulus JK, et al. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 2015; 3: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandin EW, Zamani P, Mazurek JA, et al. Right ventricular response to pulsatile load is associated with early right heart failure and mortality after left ventricular assist device. J Heart Lung Transplant 2017; 36: 97–105. [DOI] [PubMed] [Google Scholar]
- 13.Tedford RJ, Hassoun PM, Mathai SC, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012; 125: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alto M, Romeo E, Argiento P, et al. A simple echocardiographic score for the diagnosis of pulmonary vascular disease in heart failure. J Cardiovasc Med (Hagerstown) 2017; 18: 237–243. [DOI] [PubMed] [Google Scholar]
- 15.Opotowsky AR, Ojeda J, Rogers F, et al. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging 2012; 5: 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazurek JA, Forfia PR. Enhancing the accuracy of echocardiography in the diagnosis of pulmonary arterial hypertension: looking at the heart to learn about the lungs. Curr Opin Pulm Med 2013; 19: 437–445. [DOI] [PubMed] [Google Scholar]
- 17.Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med 2011; 183: 268–276. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 19.Mazurek JA, Vaidya A, Mathai SC, et al. Follow-up tricuspid annular plane systolic excursion predicts survival in pulmonary arterial hypertension. Pulm Circ 2017; 7: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–116. [DOI] [PubMed] [Google Scholar]
- 21.Mohammed SF, Hussain I, AbouEzzeddine OF, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014; 130: 2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercer-Rosa L, Parnell A, Forfia PR, et al. Tricuspid annular plane systolic excursion in the assessment of right ventricular function in children and adolescents after repair of tetralogy of Fallot. J Am Soc Echocardiogr 2013; 26: 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. quiz 786–688. [DOI] [PubMed] [Google Scholar]
- 24.Mahapatra S, Nishimura RA, Oh JK, et al. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr 2006; 19: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 25.Opotowsky AR, Clair M, Afilalo J, et al. A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol 2013; 112: 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guazzi M, Dixon D, Labate V, et al. RV Contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 2017; 10: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 27.Gorter TM, van Veldhuisen DJ, Voors AA, et al. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2018; 19: 425–432. [DOI] [PubMed] [Google Scholar]
- 28.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013; 305: H1373–1381. [DOI] [PubMed] [Google Scholar]
- 29.Guazzi M, Naeije R, Arena R, et al. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest 2015; 148: 226–234. [DOI] [PubMed] [Google Scholar]
- 30.Bosch L, Lam CSP, Gong L, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2017; 19: 1664–1671. [DOI] [PubMed] [Google Scholar]
- 31.Raina A, Vaidya A, Gertz ZM, et al. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post-surgical assessment of right ventricular function. J Heart Lung Transplant 2013; 32: 777–783. [DOI] [PubMed] [Google Scholar]
- 32.Mazurek JA, Vaidya A, Grandin EW, et al. RVOT doppler notching predicts diastolic-to-wedge gradient In left heart disease- associated pulmonary hypertension. JACC 2015; 65: a1537. [Google Scholar]
- 33.Tanna MS, Fox AL, Troutman GS, et al. RVOT-VTI/PASP is a novel noninvasive parameter of pulmonary artery compliance and improves after treatment with pulmonary hypertension-specific therapy. The Journal of Heart and Lung Transplantation 2017; 36: S363. [Google Scholar]
- 34.Vachiery JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019; 53: 1801897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahama H, McCully RB, Frantz RP, et al. Unraveling the RV ejection Doppler envelope: insight into pulmonary artery hemodynamics and disease severity. JACC Cardiovasc Imaging 2017; 10: 1268–1277. [DOI] [PubMed] [Google Scholar]
- 36.Gabler NB, French B, Strom BL, et al. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation 2012; 126: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Right ventricular outflow tract velocity time integral-to-pulmonary artery systolic pressure ratio: a non-invasive metric of pulmonary arterial compliance differs across the spectrum of pulmonary hypertension by Priyanka T. Bhattacharya, Gregory S. Troutman, Frances Mao, Arieh L. Fox, Monique S. Tanna, Payman Zamani, E. Wilson Grandin, Jonathan N. Menachem, Edo Y. Birati, Julio A. Chirinos, Sula Mazimba, Kerri Akaya Smith, Steven M. Kawut, Paul R. Forfia, Anjali Vaidya and Jeremy A. Mazurek in Pulmonary Circulation
Supplemental material, Supplemental Material2 for Right ventricular outflow tract velocity time integral-to-pulmonary artery systolic pressure ratio: a non-invasive metric of pulmonary arterial compliance differs across the spectrum of pulmonary hypertension by Priyanka T. Bhattacharya, Gregory S. Troutman, Frances Mao, Arieh L. Fox, Monique S. Tanna, Payman Zamani, E. Wilson Grandin, Jonathan N. Menachem, Edo Y. Birati, Julio A. Chirinos, Sula Mazimba, Kerri Akaya Smith, Steven M. Kawut, Paul R. Forfia, Anjali Vaidya and Jeremy A. Mazurek in Pulmonary Circulation
Supplemental material, Supplemental Material3 for Right ventricular outflow tract velocity time integral-to-pulmonary artery systolic pressure ratio: a non-invasive metric of pulmonary arterial compliance differs across the spectrum of pulmonary hypertension by Priyanka T. Bhattacharya, Gregory S. Troutman, Frances Mao, Arieh L. Fox, Monique S. Tanna, Payman Zamani, E. Wilson Grandin, Jonathan N. Menachem, Edo Y. Birati, Julio A. Chirinos, Sula Mazimba, Kerri Akaya Smith, Steven M. Kawut, Paul R. Forfia, Anjali Vaidya and Jeremy A. Mazurek in Pulmonary Circulation