Abstract
Medical endoscopy trainees face numerous, often conflicting demands on their time. This can result in suboptimal endoscopy training and in difficulty achieving certification in basic endoscopy within the existing 5-year training programme. Endoscopic management of acute gastrointestinal bleeding and basic polypectomy are integral to basic service provision. Competence in these and other therapeutic procedures, including dealing with complications, is currently acquired opportunistically, or through experiential independent practice. This article proposes several potential solutions that may help with endotherapy training in the current UK training programmes. It also addresses issues relating to speciality training when reduced to 4 years in 2022. Advanced endotherapy training needs to be optimised by understanding how to select individuals with the appropriate skills and how to accelerate therapeutic training at the appropriate time. Training programmes will need to adapt and can learn from countries where the pathway is more developed and established. Future training will include a dedicated subspeciality training programme for advanced therapy with competitive entry. Advanced therapy training will be matched to service needs. Scoring systems for case complexity integrated with regional and supraregional networks, would allow referral of selected cases to the most appropriate specialised units.
Keywords: colonoscopy, diagnostic and therapeutic endoscopy, endoscopic polypectomy, biliary endoscopy, surgical training
Key points.
Basic endoscopy certification is a challenge within existing UK medical endoscopist training programmes.
Gastrointestinal bleed management and basic polypectomy are integral to basic service provision.
Quality standards demand safe, competent endotherapy, irrespective of training received.
Formalised basic endotherapy training will be required within a shortened training programme.
Dedicated subspeciality training for advanced therapy with competitive entry should be linked to service demands.
Endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) training are complementary. It is logical to link ERCP and EUS training in the future wherever possible.
Integrated regional networks servicing referrals to specialised units would help optimise complex endotherapy service provision and training.
Background
UK medical endoscopy trainees currently face numerous, often conflicting demands on their time. One significant concern is the time allocated to provide acute cover in either general surgery or general medicine on the 2016 junior doctor contract. This competes directly with time in speciality training programmes resulting in continual conflict. The competing demands can result in suboptimal endoscopy training.
The UK has driven much of the innovation in endoscopy training and quality assurance. This has resulted in a well-structured pathway which results in Joint Advisory Group on Gastrointestinal Endoscopy (JAG) training certification in the basic endoscopic procedures (gastroscopy, flexible sigmoidoscopy and colonoscopy). The majority of endoscopic training is delivered by senior staff who have received specific training to provide a high-quality training experience. Endoscopy trainees receive 1:1 training on both service and dedicated training lists, until certified for independent practice. Unfortunately, service demands often restrict endoscopy training time both in terms of training list provision and availability of individual trainees and trainers. Nineteen percent of gastroenterology trainees averaged less than one training list per week.1
All trainee endoscopists are assessed to the same standards. Quality markers in endoscopy are being more clearly defined for the service, the individual endoscopists and for individual procedures. For trainees, subsequent independent practice is increasingly monitored and subject to quality assurance, helping to improve the service and protect patients.
With limited endoscopy training time, the transition from trainee to independent endoscopist (endoscopic competence) may become more protracted, and certification in basic endoscopy may not be possible within a 5-year training programme. There is currently limited consideration for the subsequent need to perform safe, effective therapeutic endoscopy. Quality standards will demand that independent endoscopists are safe and competent at endotherapy, irrespective of the training received.
Therapeutic endoscopy
Endoscopic procedures have the potential to be both diagnostic and therapeutic. Although some therapeutic training may accompany basic endoscopy training, there is no specific formal therapeutic training mandated prior to independent practice.
Given the training time issues relating to basic endoscopy training, it is helpful to consider what endotherapy should be included and what would require a more specific or extended training (advanced therapy). There are two main areas that are integral to basic service provision:
Endoscopic management of acute gastrointestinal bleeding.
Basic polypectomy.
In clinical practice, acute gastrointestinal bleeding relates predominantly to acute upper gastrointestinal bleeding (AUGIB). Current recommendations suggest integration of upper and lower gastrointestinal bleeding management.2 This is potentially challenging as AUGIB is predominantly dealt with by gastroenterology endoscopists and acute lower gastrointestinal bleeding is mainly in the surgical domain. As part of the current Endoscopy Quality Improvement Programme, there is an opportunity to acquire skills in the variety of techniques used to control AUGIB. This initiative, supported by the British Society of Gastroenterology, could benefit other endoscopic therapeutic training, as many of these skills are transferable, particularly in dealing with complications of therapy.
All endoscopic procedures should be considered a potential cancer screening opportunity, irrespective of the indication. In colonoscopy, this screening is associated with cancer prevention by removing precancerous lesions (polyps) by polypectomy. Patient expectation, with limited exceptions, is that pathology is both recognised and dealt with at the time of the procedure. Therefore, basic polypectomy is integral to both training and service provision. JAG is addressing this, supported by the development of dedicated tools for assessing polypectomy (Direct Observation of Polypectomy Skills), with a new certification process necessitating competence at a basic level of polypectomy.3 4
Current UK endoscopy training remains suboptimal. It seems illogical to be assessed for basic diagnostic skills but not for the associated therapy, where potentially the risk is greatest to the patient. Competence in therapeutic procedures is mainly acquired via opportunistic training or experiential practice once independent. The ability to deal with complications is critical and needs to be acquired before commencing independent endotherapy. There are post-Certificate of Completion of Training (CCT) specialist posts evolving. These focus on some of the advanced and therapeutic procedures listed in box 1, but currently post numbers are limited.
Box 1. Common advanced and therapeutic endoscopic procedures.
Endoscopic procedure
Endoscopic management of acute upper and lower gastrointestinal bleeding.
Polypectomy: basic and complex.
Dilatation: upper and lower gastrointestinal.
Stent insertion: upper and lower gastrointestinal.
Endoscopic placement of feeding tubes (nasojejunal, percutaneous endoscopic gastrostomy (PEG), jejunal extension of PEG, etc).
Thermal ablation techniques: upper and lower gastrointestinal.
Endoscopic mucosal resection (EMR)/piecemeal EMR: upper and lower gastrointestinal.
Endoscopic submucosal dissection: upper and lower gastrointestinal.
Third space endoscopic procedures (peroral endoscopic myotomy (POEM), gastric POEM, etc).
Endoscopic retrograde cholangiopancreatography.
Endoscopic ultrasound.
Enteroscopy.
Meeting the current Challenges
The movement of the majority of UK speciality trainees to the 2016 junior doctor contract has had a negative impact on the availability of trainees for speciality training due to more weekend shifts and zero days. Fifty-eight percent of gastroenterology trainees who responded to a survey use their annual leave or zero days to gain additional endoscopy training.1
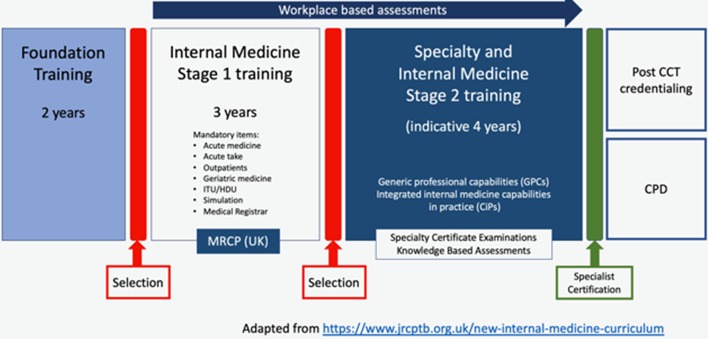
The Shape of Training report recommended changes to the structure of speciality training5 that has resulted in a new internal medicine training (IMT) pathway commencing in August 2019 (figure 1). Time in specialist training is reduced from 5 to 4 years to accommodate more time in ‘broad-based’ general internal medicine (GIM) training in IMT3. This is of huge concern to both trainees and trainers, especially as when surveyed in Spring 2018, less than half (49.0%) of trainees had received full JAG certification for colonoscopy by the last year of their training which is currently 5 years.1
Figure 1.
New internal medicine (IMT) pathway from August 2019.
It will be essential to limit acute general medicine/surgery within specialist training programmes in gastroenterology and gastrointestinal surgery to a maximum of 25% of the training programme overall. Ideally, trainees would have at least 1 year (preferably ST4), with no acute general medicine. It remains to be seen if this recommendation is realistic, given the increasing demands of GIM.
It is possible to ensure that current trainees are able to reach basic endoscopy competence within the existing training programmes, but this is becoming increasingly challenging. Without a shift in priorities to enable a focus on endoscopic training, there is the potential for the post-CCT workforce to be composed of mixed abilities, with a risk that some CCT-holders will be unable to provide even the most basic therapy. Endoscopy may not be a key component of service delivery for some individuals and if endoscopy training is not necessary, training resources can be released to those who need it most.
There are a number of potential solutions that could help:
Revise the gastroenterology curriculum to have separate luminal and hepatology training pathways.
Restrict endoscopy training provision to individuals committed to endoscopy service provision.
Facilitate subspeciality training (upper gastrointestinal, lower gastrointestinal or hepatobiliary) early in specialist training programmes.
Prioritise and protect endoscopy training within existing programmes.
Reintroduce colonoscopy as a mandatory competence to be achieved before CCT for the luminal pathway.
Ensure basic gastroscopy and colonoscopy training occurs in parallel rather than sequentially.
Accelerate basic gastroscopy training with simulation and more concentrated blocks of endoscopy training such as the SPRINT Welsh training programme (https://www.walesdeanery.org/specialties/gastroenterology-0) and extend these programmes to colonoscopy, allowing more time to focus on therapeutics.6
Develop dedicated training in basic therapeutic modalities with competency assessment.
Ensure senior trainees receive supervised experience as part of the AUGIB service.
Use Annual Review of Competence Progress (ARCP) outcome 3* to provide additional training time to achieve competence in basic endoscopy if required or time dedicated to therapeutic skills acquisition for those wishing to practice therapeutic endoscopy as part of their core service.
Develop post-CCT credentials to train CCT holders/existing consultants according to the service needs of the local population.
*An Outcome 3 awarded by an Annual Review of Competence Progression (ARCP) panel is defined as: “Inadequate progress - Additional training time required” This is awarded if the panel have identified that a formal additional period of training is required that will extend the duration of training. This will affect the trainee’s CCT date, which will need to be adjusted to reflect the period of additional training
Meeting the future challenges
Acquiring endotherapy skills
There is a need to understand how endoscopic skills are best acquired and the relationship between basic handling skills and therapeutic skills. A concept to consider is serial versus parallel skills acquisition. Frequently, an individual will reach independent practice in a diagnostic procedure before commencing the associated therapeutics related to the procedure (serial skills acquisition). A basic requirement of therapeutic endoscopy is the ability to handle the instrument well and control the tip of the endoscope (the site of therapeutic delivery). Tip control may be sufficiently developed to commence some therapeutic training (parallel skills acquisition) before the individual is deemed independent for the diagnostic procedure. Being able to define this will help trainers to accelerate basic therapeutic training at the appropriate time. This would enable therapeutic training within the existing training programme potentially avoiding additional training beyond this.
There is also a need to more clearly define the key personal attributes necessary for developing an individual into a skilled endoscopist capable of providing safe and effective advanced therapy (table 1). At present, selection of individuals for advanced therapy is based on a variety of factors which do not necessarily relate to their ability to further develop their endoscopic skills. The UK challenge for any advanced therapeutic training programme is to selectively choose appropriate trainees. Trainees should have good basic knowledge, relevant technical skills, the potential for development and the appropriate endoscopic non-technical skills (ENTS). ENTS are recognised as being important for decision-making, improving teamwork and reducing human error and are increasingly integrated into endoscopic training.7 Selection should be competitive and aligned to demand for a particular therapeutic modality. This would result in an appropriately sized workforce appropriate to demand, ensuring maintenance of skills and competencies and providing effective quality assurance.
Table 1.
Steps involved in safe and effective therapeutic practice
| Action | Action | Skill/attribute |
| 1 | Reaching the area needing therapy | Intubation skills |
| 2 | Recognising the pathology needing the therapy | Lesion recognition |
| 3 | Deciding on the most appropriate therapy and assessment of capability to deliver this safely and effectively | Decision-making and judgement, self-awareness |
| 4 | Delivering the therapy | Therapeutic skills |
| 5 | Managing complications | Therapeutic skills |
Templates for therapeutic training exist in other countries. One of the most successful emanates from Japan, where advanced therapeutic innovation and excellence are widely recognised around the world. Training is based on an apprenticeship model focused almost exclusively on the skills acquisition and associated competencies involved. During training, there is a clear pathway split between ‘resectional’ training (endoscopic mucosal resection, endoscopic submucosal dissection, per-oral endoscopic myotomy) and Hepatobiliary (HPB) training (endoscopic ultrasound (EUS)-endoscopic retrograde cholangiopancreatography (ERCP)).
Expert therapeutic endoscopy mentors guide trainees through a number of stages:
Observation (blue print for trainee for optimal therapy technique).
Assisting (familiarity with accessories and procedure).
Practice (animal models).
Supervised cases (staged case selection based on complexity).
Independent practice.
This is supplemented with intense exposure to case review, image analysis and research. This includes 1:1 training in ENTS, critical to decision-making. Progression is dictated by the mentor rather than a formal competency or number-based progression.
To replicate this in the UK would require a significant shift back to a 1:1 mentorship style of training and investment in animal model training. This is a potential solution, but with improvements in on-line learning and non-animal models, there may be other alternative options.
The rate of achieving competence in advanced endoscopic therapy varies between individuals. Relevant factors affecting the acquisition rate include, trainer skill, case volume exposure, procedure complexity and existing transferable skills of the individual. A more individualised bespoke approach to therapeutic training is preferable. In sport and other complex motor skills training, intense block training leads to more rapid skills acquisition.8 9 Therefore, protected time and high-volume units would be an optimal environment for this.
The option of advanced skills training occurring within a definitive consultant post (as is currently often the case) is not sustainable. It is neither ideal for training nor in the long term, likely to be tolerated or acceptable to organisations responsible for service provision. In the future, it is likely that there will be dedicated subspeciality training programme for advanced therapy after CCT (post-CCT credential). The proposed advanced skills training would be restricted to a cohort of individuals who wish to pursue a career where regular advanced therapeutic endoscopy is integral to subsequent service provision. It would necessitate individuals being trained in specialised and dedicated high-volume units for an extended, as yet undefined, training period (figure 2).
Figure 2.
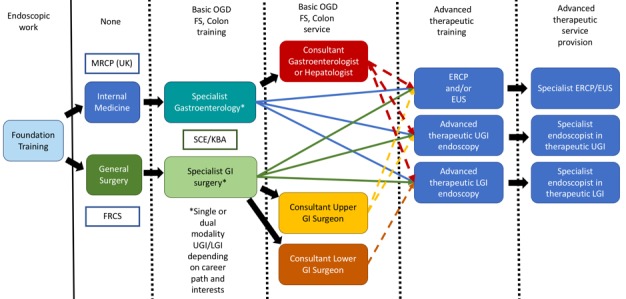
Potential future pathway for advanced therapeutic endoscopy training. ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; FS, flexible sigmoidoscopy; GI, gastrointestinal; LGI, lower GI; UGI, upper GI.
ERCP and EUS
ERCP is becoming increasingly subspecialised. The technical challenges involved in ERCP skills acquisition are matched by the critical decision-making which occurs throughout the procedure. Both technical and non-technical skills need to be developed simultaneously, with a trainer who is competent at both ERCP and the ability to train. ERCP is purely therapeutic. There are not only some transferable skills from generic endoscopic practice but also the need to acquire several new ones. Despite existing guidelines and quality standards in ERCP,10 key skills have not been defined for effective and safe ERCP practice, and there is currently no regulation of individuals delivering ERCP in the UK.
The need for adequate case volume exposure limits both the number of ERCP endoscopists and the number of trainees who can realistically expect training in ERCP. EUS has a role in other areas outside of hepatobiliary disease, including cancer staging and tissue acquisition. EUS could potentially remain independent of ERCP as indications for diagnostic and therapeutic EUS grow. However, it is more logical that future ERCP training will be linked to simultaneous EUS training. In hepatobiliary disease, ERCP and EUS are very complimentary and EUS affords the benefit of enabling therapeutic access in cases where conventional ERCP may be unsuccessful.
As with other therapeutic procedures, ERCP/EUS training numbers must match the opportunity for high volume independent practice. This is unlikely to happen by chance and needs to be actively managed. Additional ERCP/EUS training will be required similar to the advanced training posts which currently exist within speciality training. The training pathway will include dedicated simultaneous training in both EUS and ERCP, within a networked region of service provision.
Appropriate case selection
Physiological and Operative Severity Score for the enumeration of Mortality and morbidity (POSSUM) and Portsmouth POSSUM (P-POSSUM) are routinely used in the assessment of outcomes in surgical patients.11 12 These are well-validated scoring systems which can inform both the suitability of a patient for training and serve as a comparative measure for outcomes from individual surgical units. Using scoring systems or some other measure for case complexity would be useful for all forms of advanced endotherapy. Integrated with regional and supraregional networks, this would allow units, if required, to refer selected cases to the most appropriate specialised units. The main driver for this would be to improve quality and patient outcomes but would have the benefit of ensuring individuals have sufficient case load to maintain their skills.
In colonoscopy, the complexity of a polypectomy can be scored using the size, morphology, site and access system.13 A modified scoring system is proposed in table 2 and may function to help services ensure that the appropriate individual deals with more complex polyps further developing existing guidelines.14 15 For flexible sigmoidoscopy screening, it has been suggested that Level 1 polypectomy competence would be adequate given that lesions of >10 mm are referred for colonoscopy. The majority of polyps removed at colonoscopy are Level 2 or less, suggesting this should be the minimum competence expectation for individuals independent in colonoscopy.
Table 2.
Modified SMSA scoring to help management of colonic polyps
| Level | Type of polyp | SMSA | Setting/endoscopist |
| 1 | Lesions <10 mm in diameter | 4–5 | Flexible sigmoidoscopy screening |
| 2 | Polypoid and sessile lesions <20 mm providing there is good access. | 6–8 | All colonoscopists |
| 3 | Flat lesions (<20 mm) that are suitable for endoscopic therapy, larger sessile and polypoid lesions and smaller lesions with more difficult access | 9–12 | Faecal immunochemical testing screening positive colonoscopy |
| 4 | Larger flat lesions (>20 mm) or other challenging polypoid lesions (site, size or access) | 12–17 | Regionally based colonoscopists Polyp MDT |
| 5 | Flat lesions >40 mm Levels 2, 3 or 4 lesions with:
|
NA | Supraregional endoscopists Polyp MDT Endoscopic submucosal dissection option Surgical option |
LST, laterally spreading tumour; LST-G, granular LST; LST-NG, non-granular LST; MDT, multidisciplinary team; SMSA, size, morphology, site and access.
There is evidence from the surgical literature that reducing the number of individuals performing a procedure, to concentrate expertise and exposure, results in better outcomes.16–19 An integrated regional network would overcome many of the issues which relate to low-volume and limited-service provision. The concept of centralised specialist care is not new. Surgeons accept and use regional and supraregional centres for certain surgical cancer procedures,20 21 following the two key principles in cancer management:
Care should be delivered locally wherever possible to maximise patient convenience.
Services should be centralised where necessary to improve outcomes.
At present, it is unclear how many endoscopists are required within the existing service or needed for a future with rapidly expanding therapeutic developments and applications. However, it should be possible to map this out and develop the metrics to calculate case complexity and number of centres/endoscopists required to ensure optimal care is available and provided for those in need of it. This in turn will enable concentration of expertise and resource, driving better outcomes and training.
Future balance
The geography and number of specialist units providing advanced therapeutic practice will be mapped against the demand. Those selected for advanced therapeutic endoscopy training, will be responsible for ensuring that a significant part of their workload involves advanced therapy provision. This ensures appropriate utilisation of advanced therapeutic training and facilitates robust quality assurance of practice.
Footnotes
Patient consent for publication: Not required.
Contributors: JTA prepared and wrote the manuscript and revision. MJL contributed to the first draft and assisted with editing and further revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Biswas S, Alrubaiy L, China L, et al. Trends in UK endoscopy training in the BSG trainees' national survey and strategic planning for the future. Frontline Gastroenterol 2018;9:200–7. 10.1136/flgastro-2017-100848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NCEPOD Time to Get Control?. A review of the care received by patients who had a severe gastrointestinal haemorrhage. 2015. https://www.ncepod.org.uk/2015report1/downloads/TimeToGetControlFullReport.pdf
- 3. Direct Observation of Polypectomy Skills (DOPyS). https://www.thejag.org.uk/Downloads/DOPS%20forms%20(international%20and%20reference%20use%20only)/Formative%20DOPyS_Colonoscopy%20and%20Flexible%20sigmoidoscopy.pdf.
- 4. Gupta S, Bassett P, Man R, et al. Validation of a novel method for assessing competency in polypectomy. Gastrointest Endosc 2012;75:568–75. 10.1016/j.gie.2011.09.028 [DOI] [PubMed] [Google Scholar]
- 5. Shape of training – securing the future of patient care; led by professor David Greenaway. 2013. https://www.shapeoftraining.co.uk/static/documents/content/Shape_of_training_FINAL_Report.pdf_53977887.pdf.
- 6. Turner J, Hawkes N, Hurley J, et al. Accelerated training in upper GI endoscopy – an analysis of SPRINT programme outcomes. United European Gastroenterol J 2015;2(suppl 1). [Google Scholar]
- 7. Ravindran S, Thomas-Gibson S, Murray S, et al. Improving safety & reducing error in endoscopy: simulation training in human factors. Frontline Gastroenterol, 2019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donovan JJ, Radosevich DJ. A meta-analytic review of the distribution of practice effect: now you see it, now you don’t. J Appl Psychol 1999;84:795–805. [Google Scholar]
- 9. Rahmaninia F. Principles and application of motor learning. 1st edition Tehran: Bamdad Ketab, 2003. [Google Scholar]
- 10. BSG ERCP Working Party. ERCP – the way forward. A standards framework. 2014. https://www.bsg.org.uk/resource/ercp-the-way-forward-a-standards-framework-pdf.html
- 11. Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg 1991;78:355–60. [DOI] [PubMed] [Google Scholar]
- 12. Prytherch DR, Whiteley MS, Higgins B, et al. POSSUM and Portsmouth POSSUM for predicting mortality. Br J Surg 1998;85:1217–20. [DOI] [PubMed] [Google Scholar]
- 13. Gupta S, Miskovic D, Bhandari P, et al. A novel method for determining the difficulty of colonoscopic polypectomy. Frontline Gastroenterol 2013;4:244–8. 10.1136/flgastro-2013-100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rutter MD, Chattree A, Barbour JA, et al. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut 2015;64 12:1847–73. 10.1136/gutjnl-2015-309576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valori R, Rey JF, Atkin WS, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition-Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy 2012;44 Suppl 3:SE88–SE105. 10.1055/s-0032-1309795 [DOI] [PubMed] [Google Scholar]
- 16. Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364–9. 10.1056/NEJM197912203012503 [DOI] [PubMed] [Google Scholar]
- 17. Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–37. 10.1056/NEJMsa012337 [DOI] [PubMed] [Google Scholar]
- 18. Lemmens VE, Bosscha K, van der Schelling G, et al. Improving outcome for patients with pancreatic cancer through centralization. Br J Surg 2011;98:1455–62. 10.1002/bjs.7581 [DOI] [PubMed] [Google Scholar]
- 19. Funk LM, Gawande AA, Semel ME, et al. Esophagectomy outcomes at low-volume hospitals: the association between systems characteristics and mortality. Ann Surg 2011;253:912–7. 10.1097/SLA.0b013e318213862f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Department of Health. A policy framework for commissioning cancer services: a report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales, 1995. [Google Scholar]
- 21. Department of Health. The NHS Cancer plan: a plan for investment, a plan for reform, 2000. [Google Scholar]