Abstract
Background
Venous thromboembolism (VT) is a leading cause of maternal mortality and morbidity worldwide. Catheter-directed thrombolysis (CDT) is an effective and safe treatment modality for VT patients. However, the long-term outcome of CDT in pregnancy-related venous thrombosis are unclear. The aim of this study was to assess long-term results of pregnancy-related VT patients.
Material/Methods
We reviewed 41 pregnancy-related deep venous thrombosis (DVT) patients who underwent CDT from February 2008 to May 2015. Clinical data, including demographic variables, disease location, vascular risk factors, treatment regimen, interventional procedure and complications, were collected retrospectively. Clinical and color-duplex ultrasonography were performed to monitor venous patency during follow-up. Post-thrombotic syndrome (PTS) was assessed with the Villalta scale and quality of life (QOL) was evaluated by the VEINES-QOL/Sym questionnaire.
Results
Twenty-three patients underwent spontaneous abortion or induced abortion within 3 months before DVT, and 18 patients had DVT during the first 3 months after delivery. Technical success was achieved in all patients. Grade III (complete) lysis was obtained in 15 patients and grade II (partial) lysis was obtained in 21 patients. The follow-up period was 3 years. Twenty-eight patients had venous patency at 3-year follow-up; 36.6% of patients developed mild or moderate PTS (Villalta score 5–14) and 4.8% with severe PTS (Villalta score ≥15). VEINES-QOL/Sym scores were 55.24±7.35 and 53.25±6.65, respectively.
Conclusions
Catheter-directed thrombolysis is a reliable and safe treatment modality for postnatal or abortion patients with DVT. CDT can reduce the incidence rate of PTS and increase the quality of life.
MeSH Keywords: Pregnancy Complications, Treatment Outcome, Vascular Access Devices, Venous Thrombosis
Background
Venous thrombosis is the leading cause of pregnancy-related morbidity and mortality around the world. In addition to the mortality and short-term morbidity, women who have experienced venous thrombosis during pregnancy can develop chronic complications such as post-thrombotic syndrome (PTS), ranging from edema and skin changes to recurrent thromboses and ulceration [1]. Standard anticoagulant treatment for venous thrombosis is beneficial for preventing thrombus extension and embolization to the pulmonary arteries; however, it does not directly lyse the acute thrombus, which may be only partially cleared [2]. Previous studies have revealed that although they received adequate anticoagulation, nearly 50% of proximal DVT patients developed some degree of PTS [3].
The absolute incidence of venous thromboembolism in pregnancy is 1 or 2 cases per 1000 pregnancies and this risk is approximately 5 times as high as the risk among women who are not pregnant[4,5]. It is reported that more than 70% of pregnancy-related patients are associated with proximal thrombosis [6]. The prospect of post-thrombotic morbidity in this cohort of young and otherwise healthy women should be taken into account when therapeutic treatment plans are developed. Catheter-directed thrombolysis (CDT) is a modality in which a catheter is directly advanced through the thrombotic segment to deliver a thrombolytic agent into the clots. This method was developed to restore the venous lumen and save the valves [7]. Enden et al. [8] demonstrated that CDT improved the clinically relevant long-term outcomes after iliofemoral DVT by reducing PTS compared to conventional treatment with anticoagulation alone in nonpregnant patients. In clinical practice, vascular surgeons or interventionalists avoid invasive methods with thrombolytic agents for fear of treatment-related pregnancy complications. However, there are a number of studies of invasive options usage such as endovascular thrombolysis for treatment of venous thrombosis in pregnancy and numerous cases series confirming the safety and efficacy in this setting [9,10]. Previous studies showed promising early results, focusing mainly on the feasibility of this method and possible pregnancy-related complications [11]. Very few studies have focused on the long-term results of these invasive method on pregnancy-related venous thrombosis. In this study, we present the long-term outcomes of CDT, a minimally invasive endovascular treatment, in pregnancy-related venous thrombosis.
Material and Methods
Study population
We enrolled a subgroup of consecutive patients admitted from February 2008 to May 2015 at our institute. Inclusion criteria were: pregnancy-related DVT, underwent catheter-directed thrombolysis, and complete medical records and follow-up. In this study, pregnancy-related DVT referred to patients who developed DVT within 3 months after abortion or delivery (postpartum). All patients were initially treated with systemic anticoagulation but failed owing to persistent pain and edema after 2 to 7 days of treatment. Finally, patients were managed with catheter-directed thrombolysis for thrombus removal. DVT was diagnosed by ultrasound or venography. We excluded patients with nonpregnancy-related DVT, pulmonary embolism, incomplete clinical and follow-up data, or anticoagulant treatment alone. Patient data were collected and retrospectively reviewed to evaluate treatment efficacy and long-term outcomes. The Ethics Committee of our institution approved the study and written informed consent was waived for this study.
Treatment
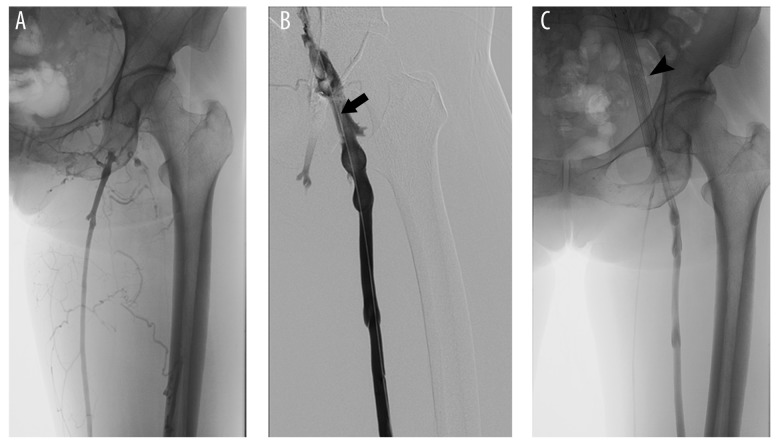
All the included patients underwent systemic anticoagulation with low-molecular-weight heparin (LMWH) 2–7 days prior to CDT. All endovascular procedures were performed by clinicians with expertise in the interventional radiology theatre. At first, lower-limb venography was done to establish the topography of the thrombus and the approach to place an inferior vena cava filter. After applying the local anesthesia, either the contralateral femoral vein or the internal jugular vein was punctured, followed by the placement of a temporary vena cava filter (OptEase, Cordis, USA) in the inferior vena cava. Then, either the ipsilateral popliteal or pretibial vein was catheterized with a 6F micropuncture set. A 4F or 5F infusion catheter with multiple side-holes (20–40 cm in length, AngioDynamics, USA) was then gently placed, covering the thrombosed segments, with the tip embedded in the proximal extent of the thrombus (Figure 1). We administered 2–4×105 U urokinase diluted in 50 ml 0.9% NaCl through the catheter for the first 24 h. From the second day, 6–8×105 U/d was continuously pumped for a maximum of 96 h. LMWH was concomitantly administered at 4000 to 6000 U/12 h according to patient weight. We monitored fibrinogen and activated partial thromboplastin time (APTT) every 6 h and maintained it at >100 mg/dl and between 40 and 60 s. Clot burden during thrombolysis was assessed daily by venography. The infusion catheter and inferior vena cava filter were removed when there was no residual thrombus. Following thrombolytic therapy, patients were anticoagulated with rivaroxaban or warfarin for at least 1 year. As standard adjunctive treatment, the patients were recommended to wear compression stockings (Class II 30–40 mmHg).
Figure 1.
Pre- and post-operative venography for pregnancy-related DVT patient. (A) Preoperative DSA showed the thrombi located in the iliofemoral vein. (B) An infusion catheter was placed into the venous thrombosis segment (arrow). (C) Venography showed the thrombus resolution one week after CDT treatment and the iliac venous stent (arrowhead).
Outcomes assessment and follow-up
Lysis grade was evaluated by daily venography via determining the thrombolysis in each vein segment, including inferior cava vein, common iliac vein, external iliac vein, common femoral vein, proximal and distal segments of superficial femoral veins, and popliteal vein. Each vein segment was given a score, where 0 stands for open vein, 1 for partly occluded vein, and 2 for completely occluded vein. By adding each segmental score, the total pre- and post-operative thrombus scores were calculated. The difference in values between the pre‐ and post‐operative thrombus scores divided by the preoperative score determined the percentage of thrombolysis. Grade I indicated thrombolysis less than 50%, grade II indicated between 50% and 90%, and grade III indicated complete thrombolysis.
All the patients were followed up at 1, 3, and 6 months and then semiannually, both clinically and by duplex sonography. Venous patency assessment on each follow-up was based on duplex sonography and defined as a composite outcome measure, in which patients having any of the following were classified as not having regained iliofemoral venous patency: partial or complete incompressibility of the femoral vein, no flow in pelvic or femoral vein, and/or functional venous obstruction [12]. Diagnosis and grading of PTS was performed via Villalta score (Supplementary Table 1). A comprehensive questionnaire including a self-reported VEINES-QOL/Sym measures was finished during follow-up. VEINES-QOL and VEINES-Sym were calculated to assess disease-specific quality of life and symptom severity, respectively [13].
Statistical analysis
Continuous variables are presented as mean and standard deviation and discrete outcomes are presented as percentages. Survival analysis with Kaplan-Meier curves was used for venous patency. Statistical analysis was performed using SPSS 21.0 (SPSS, Inc., IL, USA).
Results
We assessed 41 patients recruited between February 2008 to May 2015, and all were diagnosed with acute proximal DVT of less than 14 days duration. A flow chart for the patients in this study is shown in Supplementary Figure 1. Baseline characteristics of patients are summarized in Table 1.
Table 1.
Baseline characteristics of patients.
| Parameters | |
|---|---|
| Age (years, mean ±SD) | 33.5±8.5 |
| Body weight (kg, mean ±SD) | 71.4±12.6 |
| Marital status, n (%) | |
| Married | 33 (80.5) |
| Single | 8 (19.5) |
| Pregnancy-related status, n (%) | |
| Spontaneous abortion | 8 (19.5) |
| Induced abortion | 15 (36.6) |
| Postpartum | 18 (43.9) |
| Location of DVT, n (%) | |
| Iliac | 5 (12.2) |
| Femoral | 28 (68.3) |
| Iliac femoral | 8 (19.5) |
| Side of DVT, n (%) | |
| Left side | 37 (90.2) |
| Right side | 3 (7.3) |
| Bilateral | 1 (2.5) |
Treatment procedure and lysis grade
The symptom duration before diagnosis was 3.5±2.5 days; 43.9% of these patients had postnatal thrombosis (up to 3 months postpartum), and the remainder had either spontaneous or induced abortion before venous thromboembolism. DVT occurred in the right or both legs in only 10% of patients with, whereas most of the other patients had DVT on the left side. Two-thirds of DVTs were located in the femoral vein. The details of treatment procedure and immediate lysis grade are listed in Table 2. The technique was successful in all patients, and the mean duration of catheter placement was 2.5±1.5 days. Either popliteal or pretibial vein was chosen as puncture access at the discretion of the surgeon. We found that 3 out of 41 patients had iliac vein stenosis and underwent iliac vein stenting; 15 out of 41 patients had lysis grade III and approximately 52% of patients had lysis grade II. The remaining 5 cases had lysis grade I. No major complications, including death and intracranial hemorrhage, were observed during hospitalization.
Table 2.
Details of treatment procedure and lysis grade after CDT.
| Items | |
|---|---|
| Total urokinase dose (U, mean ±SD) | 3.2±1.5×106 |
| Infusion time (days, mean ±SD) | 2.5±1.5 |
| Procedural time (min, mean ±SD) | 21.2±7.6 |
| Infusion catheter length (mm, mean ±SD) | 34.3±10.5 |
| Puncture access, n (%) | |
| Popliteal vein | 32 (78) |
| Pretibial vein | 9 (22) |
| Complication, n (%) | |
| Puncture site bleeding | 2 (4.8) |
| Hematuresis | 1 (2.4) |
| Adjunctive iliac vein stent, n (%) | 3 (7.3) |
| Lysis grade, n (%) | |
| Grade I | 15 (36.6) |
| Grade II | 21 (51.2) |
| Grade III | 5 (12.2) |
CDT – catheter-directed thrombolysis; SD – standard deviation.
DVT – catheter-directed thrombolysis; SD – standard deviation.
Long-term outcomes after CDT
The follow-up duration was 3 years. Five out of 41 patients had a recurrent thrombosis; 3 of them in the ipsilateral leg and 2 in the contralateral leg. These 5 patients later had successful endovascular recanalization. Venous patency was assessed via duplex sonography during follow-up. Figure 2 shows the results of patients with patent veins during 3-year follow-up. Data from Kaplan-Meier analysis revealed that cumulative venous patency at 1 year, 2 years, and 3 years was 82.9%, 73.2%, and 68.3%, respectively. We also found that lysis grade was not significantly associated with long-term venous patency. Two of 5 cases with grade I lysis regained venous patency, whereas 11 of 36 cases with grade II and III lysis presented incomplete and partial patency during follow-up. Three patients who underwent iliac vein stenting had patent veins at 3 years after endovascular treatment. Villalta score was obtained during clinical follow-up, which led to the diagnosis and grade of PTS. At 3-year follow-up, severe PTS was detected in 2 patients; 9 out of 41 patients were graded as mild PTS, while 6 had moderate PTS (Figure 3). VEINES-QOL/Sym score was calculated based on the VEINES-QOL/Sym questionnaire, which gradually decreased over time (Figure 4).
Figure 2.
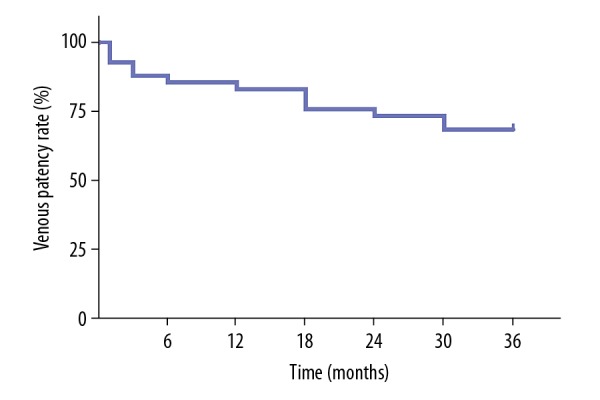
Kaplan-Meier analysis for vein patency during follow-up.
Figure 3.
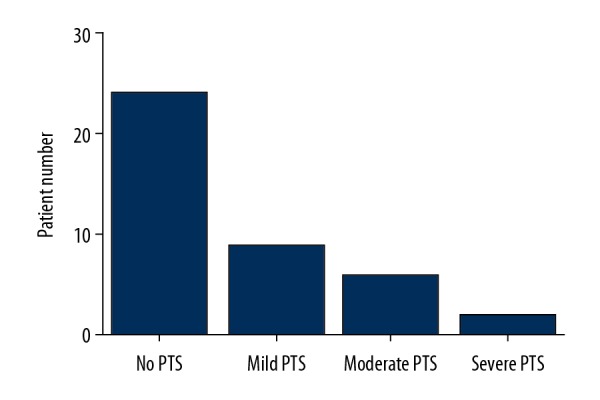
Number of patients with various degrees of PTS or no PTS after 3-year follow-up.
Figure 4.
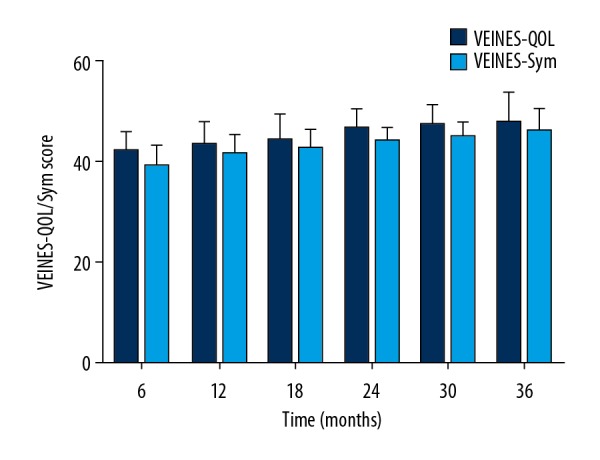
VEINES-QOL/Sym score of recruited patients during the follow-up period.
Discussion
Deep vein thrombosis is a serious disease worldwide, and it is estimated that over 10 million cases occur annually [14]. Although several molecular targets could be used for diagnosis of VT in acute phase, most VT is diagnosed according to the symptoms, such as leg swelling and pain, and image examination [15]. Previous studies have identified a series of risk factors for DVT, including immobilization, thrombophilia, oestrogen therapy, pregnancy, and [16,17]. The absolute incidence of DVT in pregnancy is 5 times higher than that in nonpregnant women [5]. This increased risk is due to the procoagulant changes and venous stasis as part of the physiological alteration in the hemostatic challenge of delivery. Although there has been significant improvement in prophylactic measures and treatment modalities, venous thromboembolism remains a leading cause of maternal morbidity and mortality [18]. PTS, a common chronic complication after DVT, develops in more than one-third of women with DVT and is associated with considerable morbidity, poor quality of life, and huge economic burden [19]. Wik et al. [20] reported that the prevalence of PTS after pregnancy-related DVT in the lower limbs is 42%. In addition, women with PTS derived from previous pregnancy-related DVT have much lower VEINES-QOL scores, indicating reduced quality of life and higher costs.
The conventional treatment for DVT is anticoagulation, involving application of unfractionated heparin and use of low-molecular-weight heparin [21,22]. Vitamin K antagonists are a mainstay treatment for nonpregnant venous thromboembolism during an extended treatment phase. However, pregnant women should avoid vitamin K antagonists and direct oral anticoagulant due to the ability to cross the placental barrier, which can cause fetal harm [23], and their use is associated with embryopathy, central nervous system abnormalities, pregnancy loss, and fetal anticoagulation with possible bleeding [24]. LMWH has largely replaced unfractionated heparin for the management of venous thromboembolism in pregnancy. In addition, oral direct thrombin inhibitors such as dabigatran and anti-factor Xa inhibitors such as rivaroxaban should generally be avoided during pregnancy due to possible adverse fetal effects [25]. Although anticoagulant treatment for venous thrombosis is effective in preventing thrombus extension and embolization to the pulmonary arteries, it does not contribute to thrombus lysis and does little to minimize the post-thrombotic complications. It is intuitive to assume that early thrombus removal might prevent recurrence of the thrombosis and the deleterious effects of vein wall inflammation [26]. Catheter-directed thrombolysis refers to the infusion of a fibrinolytic drug directly into the thrombus via a catheter embedded to cover the thrombus segment. Several studies have demonstrated that patients with iliofemoral DVT treated with CDT have better functioning and well-being and lower rate of PTS incidence when compared to patients who underwent standard anticoagulation alone [8,27]. In general, peripartum are contraindicated for CDT due to the high risk of placental abruption, the potential radiation hazard associated with teratogenesis, and other complications, including bleeding. However, we applied this therapy for DVT in our study for the following reasons. First, the recruited patients either underwent abortion or had already terminated the pregnancy. Second, during the postpartum period there is increased risk of uterine bleeding, but no data are available on the relative risk of major hemorrhage associated with thrombolytic therapy during pregnancy or the puerperium. In addition, the risk of major complications reported in pregnant women treated with anticoagulation for DVT is similar to that reported in nonpregnant women [28]. Third, the benefits of reducing PTS and improving quality of life in the long term is well established. Therefore, we applied CDT with low-dosage thrombolytic agent to minimize the risk of major bleeding. A temporary inferior vena cava filter was placed in case of floating thrombus from the iliofemoral vein to the pulmonary artery, which can lead to pulmonary embolism. All the filters were removed as soon as protection from pulmonary embolism was no longer needed. In our study, post-thrombolytic therapy included anticoagulant treatment with rivaroxaban or warfarin for at least 1 year and use of compression stocking as adjunctive treatment. Anticoagulant therapy should be continued for at least 3 months to prevent early recurrences after thrombolytic treatment [24]. Previous studies have demonstrated that anticoagulants can reduce the risk of recurrent venous thromboembolism by 80% to 90% [29,30]. Graduated elastic compression stockings have been reported as an integral part of venous thrombosis treatment because of a proven lower risk of post-thrombotic syndrome with their use [3,31].
Our data showed that cumulative venous patency at 1 year, 2 years, and 3 years was 82.9%, 73.2%, and 68.3%, respectively, for patients with pregnancy-related DVT who underwent CDT. AbuRahma et al. [32] reported that DVT patients who underwent CDT had patent veins after 5 years in 69% of the limbs, which is similar to the patency rate found in the present study. A study by Bækgaard found the 6-year patency rate for patients with DVT who received CDT was 82%. This discrepancy might reflect the different population. We did not observe as much severe PTS as was reported in previous studies. Our results elucidate the role of CDT in pregnancy-related DVT by demonstrating the long-term outcomes after interventional procedures. Our study has certain limitations, including the small number of subjects, and because it was a retrospective study, we could not obtain laboratory measurements such as FV Leiden (G1691A) and the prothrombin (G20210A) mutation, which might affect the outcomes for VT patients.
Conclusions
We found that CDT is a reliable and safe treatment modality for pregnancy-related DVT individuals. However, larger prospective and randomized studies are needed to further delineate the role of CDT in pregnancy-related DVT patients.
Supplementary Files
Supplementary Table 1.
Villalta score for the assessment of PTS.
| Venous symptoms | Signs |
|---|---|
| Pain | Pretibial edema |
| Cramps | Skin induration |
| Heaviness | Hyperpigmentation |
| Paraesthesia | Pain on calf compression |
| Itching | Venous ectasia |
| Redness |
Scoring: Each sign or symptom is rated as 0 (none), 1 (mild), 2 (moderate), or 3 (severe), and total scores are summed. Total score less than 5 excludes PTS; a score of 5–14 indicates mild or moderate PTS; a score greater than 15 or venous ulcer indicates severe PTS.
Flow chart of the patients included in this study.
Footnotes
Source of support: Departmental sources
References
- 1.Stain M, Schönauer V, Minar E, et al. The post-thrombotic syndrome: Risk factors and impact on the course of thrombotic disease. J Thromb Haemost. 2010;3(12):2671–76. doi: 10.1111/j.1538-7836.2005.01648.x. [DOI] [PubMed] [Google Scholar]
- 2.Hull RD, Liang J, Townshend G. Long-term low-molecular-weight heparin and the post-thrombotic syndrome: A systematic review. Am J Med. 2011;124(8):756–65. doi: 10.1016/j.amjmed.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: A randomized, controlled trial. J Vasc Surg. 2005;41(1):249–56. doi: 10.7326/0003-4819-141-4-200408170-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kane EV, Calderwood C, Dobbie R, et al. A population-based study of venous thrombosis in pregnancy in Scotland 1980–2005. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):223–29. doi: 10.1016/j.ejogrb.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann Intern Med. 2005;143(10):697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chan WS, Spencer FA, Ginsberg JS, et al. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ. 2010;182(7):657–60. doi: 10.1503/cmaj.091692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho JS, Martelli E, Mozes G, et al. Effects of thrombolysis and venous thrombectomy on valvular competence, thrombogenicity, venous wall morphology, and function. J Vasc Surg. 1998;28(5):787–99. doi: 10.1016/s0741-5214(98)70053-9. [DOI] [PubMed] [Google Scholar]
- 8.Enden T, Haig Y, Kløw N, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): A randomised controlled trial. J Vasc Surg. 2012;379(9810):31–38. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 9.Herrera S, Comerota AJ, Thakur S, et al. Managing iliofemoral deep venous thrombosis of pregnancy with a strategy of thrombus removal is safe and avoids post-thrombotic morbidity. J Vasc Surg. 2014;59(2):456–64. doi: 10.1016/j.jvs.2013.07.108. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen M, Broholm R, Bækgaard N. Pregnancy after catheter-directed thrombolysis for acute iliofemoral deep venous thrombosis. Phlebology. 2013;28(Suppl 1):34–38. doi: 10.1177/0268355513477286. [DOI] [PubMed] [Google Scholar]
- 11.Coutinho JM, Stam J. How to treat cerebral venous and sinus thrombosis. J Thromb Haemost. 2010;8(5):877–83. doi: 10.1111/j.1538-7836.2010.03799.x. [DOI] [PubMed] [Google Scholar]
- 12.Enden T, Kløw NE, Sandvik L, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2010;7(8):1268–75. doi: 10.1111/j.1538-7836.2009.03464.x. [DOI] [PubMed] [Google Scholar]
- 13.Lamping DL, Schroter S, Kurz X, et al. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg. 2003;37(2):410–19. doi: 10.1067/mva.2003.152. [DOI] [PubMed] [Google Scholar]
- 14.ISTH Steering Committee for World Thrombosis Day. Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: A major contributor to global disease burden. Thromb Haemost. 2014;111(05):843–52. doi: 10.1160/TH14-08-0671. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z, Ma J, Wang Q, et al. Combination of circulating miRNA-320a/b and D-dimer improves diagnostic accuracy in deep vein thrombosis patients. Med Sci Monit. 2018;24:2031–37. doi: 10.12659/MSM.906596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 17.Engbers MJ, Blom JW, Cushman M, et al. The contribution of immobility risk factors to the incidence of venous thrombosis in an older population. J Thromb Haemost. 2014;12(3):290–96. doi: 10.1111/jth.12480. [DOI] [PubMed] [Google Scholar]
- 18.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 19.Guanella R, Ducruet T, Johri M, et al. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: A prospective evaluation. J Thromb Haemost. 2011;9(12):2397–405. doi: 10.1111/j.1538-7836.2011.04516.x. [DOI] [PubMed] [Google Scholar]
- 20.Wik HS, Jacobsen AF, Sandvik L, Sandset PM. Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: A population-based, cross-sectional, case-control study. J Thromb Haemost. 2012;10(5):840–47. doi: 10.1111/j.1538-7836.2012.04690.x. [DOI] [PubMed] [Google Scholar]
- 21.Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA. 2014;311(7):717–28. doi: 10.1001/jama.2014.65. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Fu Q, Zhao Y, et al. Short-term anticoagulant therapy and thrombus location are independent risk factors for delayed recanalization of deep vein thrombosis. Med Sci Monit. 2016;22:219–25. doi: 10.12659/MSM.895228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyer-Westendorf J, Ageno W. Benefit-risk profile of non-vitamin K antagonist oral anticoagulants in the management of venous thromboembolism. Thromb Haemost. 2015;113(02):231–46. doi: 10.1160/TH14-06-0484. [DOI] [PubMed] [Google Scholar]
- 24.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang AW, Greer I. A systematic review on the use of new anticoagulants in pregnancy. Obstet Med. 2013;6(2):64–71. doi: 10.1177/1753495X12472642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: Long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239(1):118–26. doi: 10.1097/01.sla.0000103067.10695.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popuri RK, Vedantham S. The role of thrombolysis in the clinical management of deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2011;31(3):479–84. doi: 10.1161/ATVBAHA.110.213413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginsberg JS, Kowalchuk G, Hirsh J, et al. Heparin therapy during pregnancy. Risks to the fetus and mother. Arch Intern Med. 1989;149(10):2233–36. [PubMed] [Google Scholar]
- 29.Ost D, Tepper J, Mihara H, et al. Duration of anticoagulation following venous thromboembolism. JAMA. 2005;294(6):706–15. doi: 10.1001/jama.294.6.706. [DOI] [PubMed] [Google Scholar]
- 30.Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340(12):901–7. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 31.Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349(9054):759–62. doi: 10.1016/S0140-6736(96)12215-7. [DOI] [PubMed] [Google Scholar]
- 32.AbuRahma AF, Perkins SE, Wulu JT, Ng HK. Iliofemoral deep vein thrombosis: Conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233(6):752–60. doi: 10.1097/00000658-200106000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Villalta score for the assessment of PTS.
| Venous symptoms | Signs |
|---|---|
| Pain | Pretibial edema |
| Cramps | Skin induration |
| Heaviness | Hyperpigmentation |
| Paraesthesia | Pain on calf compression |
| Itching | Venous ectasia |
| Redness |
Scoring: Each sign or symptom is rated as 0 (none), 1 (mild), 2 (moderate), or 3 (severe), and total scores are summed. Total score less than 5 excludes PTS; a score of 5–14 indicates mild or moderate PTS; a score greater than 15 or venous ulcer indicates severe PTS.
Flow chart of the patients included in this study.