Abstract
Background
Hydroxycitric acid is a potential lithontriptic agent for calcium oxalate (CaOx) stones in the kidneys. This study aimed to evaluate the safety and efficiency of hydroxycitric acid tripotassium (K-HCA) against CaOx crystal formation using Drosophila melanogaster hyperoxaluria models.
Material/Methods
Wild-type D. melanogaster were fed standard medium with ethylene glycol or sodium oxalate added to induce hyperoxaluria. Their Malpighian tubules were dissected and observed under a microscope every 3 days. Crystal deposit score of each Malpighian tubule were evaluated under a magnification of ×200. Using hyperoxaluria Drosophila models, we investigated the inhibitory efficiency of hydroxycitrate acid tripotassium and citric acid tripotassium (K-CA) against CaOx crystal formation. The survival rate of each group was also assessed.
Results
When fed with 0.05% NaOx, the CaOx formation in Malpighian tubules increased significantly, without reduction of life span. Therefore, we selected 0.05% NaOx-induced hyperoxaluria models for the further investigations. After treatment, the stone scores showed that K-CA and K-HCA both significantly inhibit the formation of CaOx crystals in a dose-dependent manner, and with smaller dosage (0.01%), K-HCA was more efficient than K-CA. Moreover, after treatment of K-CA or K-HCA, the life span in different groups did not change, reflecting the safety to life.
Conclusions
The hyperoxaluria Drosophila models fed on 0.05% NaOx diet might be a useful tool to screen novel agents for the management of CaOx stones. K-HCA may be a promising agent for the prevention CaOx stones, with satisfying efficiency and acceptable safety.
MeSH Keywords: Calcium Oxalate, Drosophila, Hyperoxaluria
Background
Urolithiasis is one of the most common benign diseases in the urinary system, with a high prevalence and recurrence [1–3]. In recent years, the incidence of urinary stones has been increasing around the world, resulting in a large economic burden [3]. In humans, calcium oxalate (CaOx) stones are the major component of urolithiasis, constituting more than 80% [2]. Researchers have spent much effort to investigate the mechanism of stone formation; however, the underlying mechanism still remains unclear. Although the surgical techniques for the treatment of stones have improved dramatically in the last decades, effective methods for the prevention of stone diseases are still required.
Hydroxycitric acid (HCA) is a major component of Garcinia cambogia extracts and a derivative of citric acid (Figure 1). The administration of HCA can suppress body weight gain and fat accumulation in animals and humans [4,5]. A recent study showed HCA can induce the dissolution of monohydrate CaOx crystals under specific conditions through adsorption on crystal surfaces [6], and compared with citrate, which is often used for the control of urinary stones, HCA is more efficient [6]. Moreover, a preliminary human trial showed the concentration of HCA in urine increased significantly after oral administration, suggesting that HCA has clinical potential as an alternative treatment to citrate.
Figure 1.
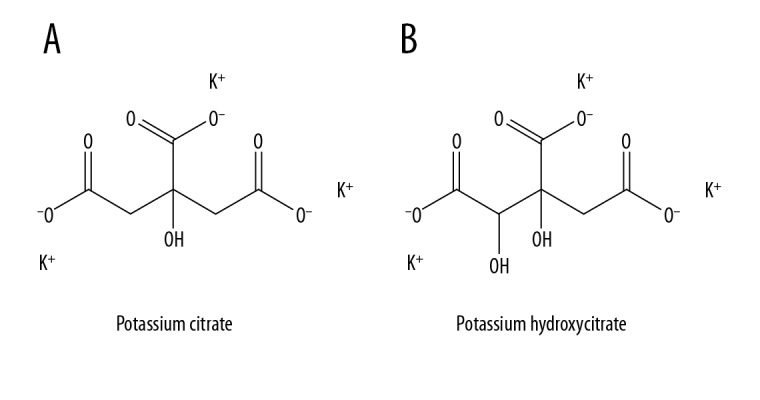
Molecular formula of (A) potassium citrate; (B) potassium hydroxycitrate.
Drosophila is a currently widely used model for human diseases such as cancer, neurological disorders, diabetes, and drug addiction, as well as for hyperoxaluria or CaOx stones [7]. Compared with traditional animal models in rats or mice, Drosophila are easy and cost-effective to breed, and easily undergo genetic manipulations [8,9]. The Malpighian tubule of Drosophila has a function similar to the human kidney and is a site of rapid calcium excretion and oxalate transportation. The CaOx crystal formation in the tubules exactly mimics stone formation in human kidneys. In addition, the formation of birefringent CaOx crystals can be observed in Malpighian tubules by scanning electron microscopy or energy-dispersive X-ray spectroscopy after the administration of hyperoxaluria-causing agents [8,9]. With rapid stone formation, this versatile invertebrate is now emerging as a new model for pre-clinical induction and treatment of CaOx nephrolithiasis. In this study, we attempted to establish an efficient method to conduct Drosophila hyperoxaluria models and evaluate the safety and efficiency of HCA against CaOx crystal formation.
Material and Methods
Drosophila and rearing conditions
Wild-type D. melanogaster (Canton-S) flies were obtained from Tsinghua University, Beijing. Because the prevalence of urolithiasis in female Drosophila is much higher than in males [9], we only used female Drosophila to make stone models in this study. These flies were reared in plastic vials containing standard growth medium at 25°C and 40–60% humidity with 12-h light–dark cycles. The media was replaced every 3 days, which consisted of water, agar, brewer’s yeast, glucose, sugar, and corn meal. The solution was heated to boiling until a homogenous mixture was attained, and after cooling to below 60°C the extra drugs were added.
Induction and observation of CaOx crystal formation
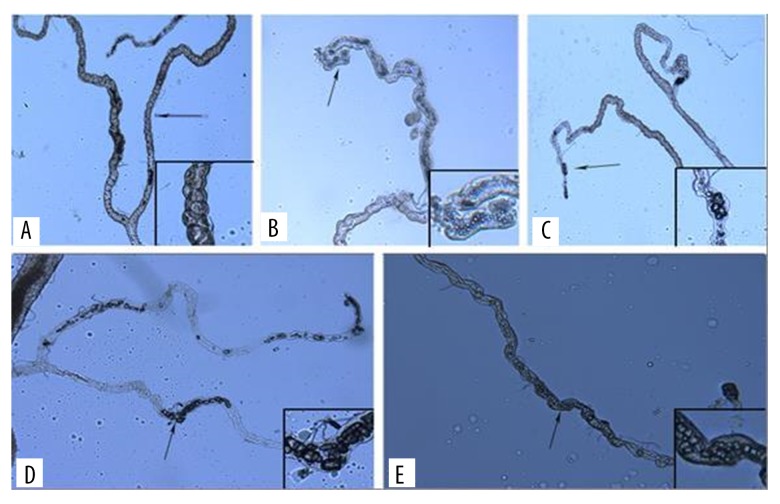
700 flies were randomly divided to 7 groups (100 flies in each group). Each group was fed with: (1) control group standard medium; (2) standard media with extra 0.1% ethylene glycol (EG); (3) standard media with extra 0.5% EG; (4) standard media with extra 1.0% EG; (5) standard media with extra 0.01% sodium oxalate (NaOx); (6) standard media with extra 0.05% NaOx; and (7) standard media with extra 0.1% NaOx. Every 3 days, 5 flies from each group were randomly extracted and anaesthetized by CO2. Their Malpighian tubules were carefully dissected out and observed under a microscope (OLYMPUS BX41). Under a magnification of ×200, we observed each freshly-dissected Malpighian tubule and evaluated the crystal formation in it. To describe the stone deposit more precisely, we developed a stone formation score by observing the number of stones in each Malpighian tubule under a ×200 magnification of each individual fly (Figure 2). The scores were defined as follows: 0 (none): No crystal formation in the lumen of Malpighian tubules; 0.5 (suspected): granular or fragments can be seen in the lumen with no bulk crystals; 1 (weak): there are large transparent bulk crystals (crystal length greater than half the lumen diameter), and the number of them less than 10; 2 (moderate): the number of large bulk crystals more than 10 but less than 30; and 3 (strong): the number of large bulk crystals is more than 30. Using this scoring method, we assessed the crystal deposit score of each group by combining the scores from each fly in the same group together.
Figure 2.
Scoring of CaOx crystal deposition in the Malpighian tubules. A – 0 (none); B – 0.5 (suspected); C – 1 (weak); D – 2 (moderate); E – 3 (strong).
Life-span assay
Newly hatched Drosophila flies were anaesthetized by CO2 and collected into vials with medium. The vials were located horizontally instead of vertically to avoid weaker flies accidentally being stuck to medium or cotton plugs. Survivors in each vial were counted and transferred into a new vial containing fresh medium every 3 days until all flies died. Based on the number of flies alive, the survival curves were made.
Medications in vivo
Hydroxycitrate acid tripotassium/K-HCA (Toronto Research Chemicals, Toronto, Canada) and citrate acid tripotassium/K-CA (Sigma-Aldrich Chemicals Co, CA, USA) were tested as inhibitors to prevent the formation of stones. We randomly divided 600 flies into 6 groups (100 flies in each group) and fed them high-oxalate (0.05% NaOx) medium. Different concentrations (0.01%, 0.1%, and 1%) of K-HCA and K-CA were added in the medium of corresponding groups. Stone formation and life span were assessed in the same way as above.
Statistical analysis
To compare the stone scores among different groups during the experimental period, we used Kruskal-Wallis tests. Log-rank tests were applied to compare the life span. Post hoc analyses were used to compare groups. All statistical analyses were performed with SAS version 9.4 and GraphPad Prism version 6.01 software. Statistical significance was considered at p<0.05.
Results
0.05% NaOx efficiently induced CaOx crystals without lethal risk
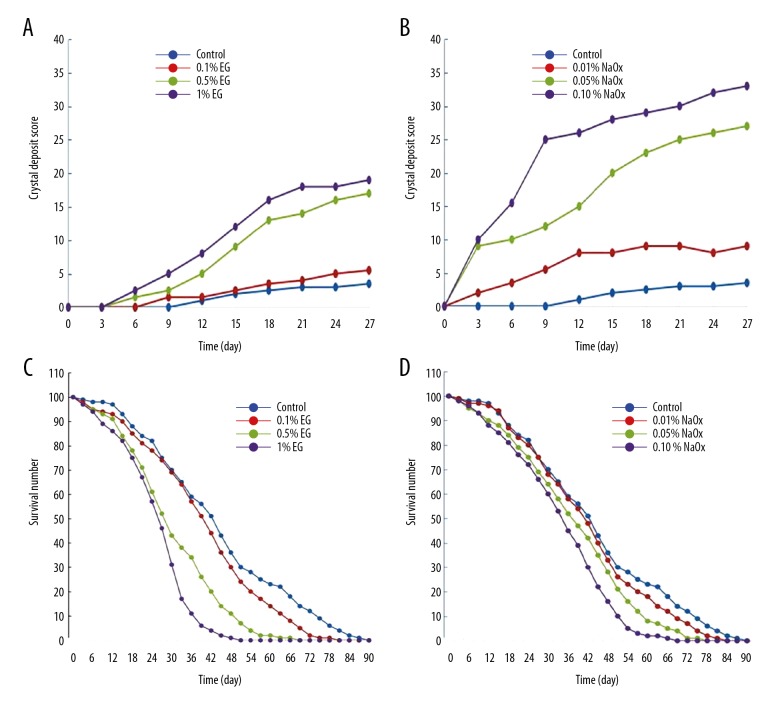
We calculated crystal formation scores of each group by using the scoring methods described above and made the formation curves shown in Figure 3. Administration of either EG or NaOx induced stone formation in a dose-dependent manner and appeared to be safe. Compared with the control group, both 0.05% and 0.1% NaOx significantly promoted the formation of CaOx crystals. However, only higher concentration up to 1% EG significantly raised the stone scores. Furthermore, 0.5% or 1% EG significantly reduced the life span, while 0.05% NaOx diet did not. Therefore, we employed the 0.05% NaOx-induced hyperoxaluria model as the candidate to evaluate the safety and efficiency of HCA.
Figure 3.
Crystal deposit scores and survival curves of EG and NaOx groups. (A, B) The crystal deposit scores of different groups. (Kruskal-Wallis test: 0.1% EG vs. control: p>0.99, 0.5% EG vs. control: p=0.139, 1% EG vs. control: p=0.019; 0.01% NaOx vs. control: p=0.626, 0.05% NaOx vs. control: p=0.001, 0.1% NaOx vs. control: p<0.001). (C, D) The life span curves of different groups. (Log-rank test: 0.1% EG vs. control: p=0.4458, 0.5% EG vs. control: p=0.0177, 1% EG vs. control: p=0.0019; 0.01% NaOx vs. control: p=0.5855, 0.05% NaOx vs. control: p=0.2914, 0.1% NaOx vs. control: p=0.0865).
K-HCA was more efficient in inhibition of CaOx formation than K-CA, with no reduction of life span to flies
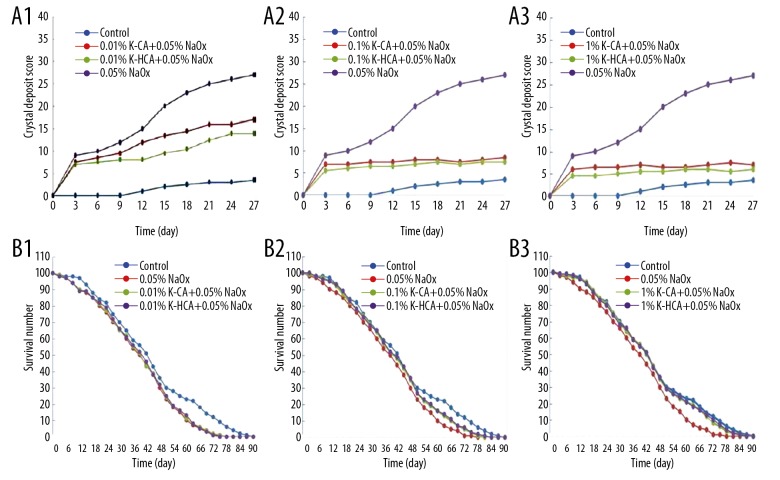
The crystal deposit scores (Figure 4A1–4A3) showed that both K-CA and K-HCA significantly inhibited the formation of CaOx crystals in flies fed a high-oxalate diet, in a dose-dependent manner. At the same concentration, the stone scores in K-HCA groups decreased more than in K-CA groups. Especially in the 0.01% concentration, K-HCA decreased the stone scores significantly more compared with the NaOx group (p=0.026), while K-CA failed to do so (p=0.124). Meanwhile, there was no significant difference in the life span curves between the control (healthy) group and K-HCA groups at any concentration (Figure 4B1–4B3), reflecting the safety of K-HCA.
Figure 4.
(A1–A3) The crystal deposit scores of different groups(Kruskal-Wallis test). (A1) 0.01% K-CA+0.05% NaOx vs. 0.05% NaOx: p=0.124, 0.01% K-HCA+0.05% NaOx vs. 0.05% NaOx: p=0.026; (A2) 0.1% K-CA+0.05% NaOx vs. 0.05% NaOx: p=0.008, 0.1% K-HCA+0.05% NaOx vs. 0.05% NaOx: p=0.005; (A3) 1% K-CA+0.05% NaOx vs. 0.05% NaOx: p=0.006, 1% K-HCA+0.05% NaOx vs. 0.05%NaOx: p=0.002. (B1–B3) The life span curves of different groups (Log-rank test). (B1) 0.01% K-CA+0.05% NaOx vs. control: p=0.3683, 0.01% K-HCA+0.05% NaOx vs. control: p=0.4927; (B2) 0.1%K-CA+0.05% NaOx vs. control: p=0.6217, 0.1% K-HCA+0.05% NaOx vs. control:p=0.6173; (B3) 1% K-CA+0.05% NaOx vs. control: p=0.7632; 1% K-HCA+0.05% NaOx vs. control: p=0.8436.
Discussion
Hyperoxaluria plays a key role in the formation of CaOx stones because the secretion of calcium is much higher than oxalate in urine [10]. The main source of oxalate in urine consists of endogenous oxalate from liver and dietary oxalate from food. In animals, no enzymes that metabolize oxalate exist; the oxalate can be secreted in vivo only through urine. In this study, we attempted to establish an efficient and safe method to construct hyperoxaluria models by administration of EG as a simulation of endogenous hyperoxaluria and NaOx as a simulation of dietary hyperoxaluria. The commonly used mammalian models of urolithiasis have inherent limitations, such as high financial costs, ethical problems, and genetic and physiologic complexities [9–12]. Drosophila, with their transparent and genetically conserved Malpighian tubules, provide a simple and convenient candidate model for human CaOx stone diseases in vivo [12]. It facilitates efficient and large-scale screening of contributory or preventive compounds and helps better understand common mechanisms of renal stone formation [8,13]. In our previous study, we used Drosophila melanogaster models to evaluate the efficiency of Hydroxy-L-proline analogs in the treatment of primary hyperoxaluria [14]. The dAGXT gene was successfully knocked down in fruit flies, and the CaOx crystal formation was significantly increased in Malpighian tubules [14]. In the present study, we confirmed that 0.05% NaOx efficiently induced CaOx stones and did not significantly reduce the life span of Drosophila, which would be an ideal model to evaluate the effect and safety of a novel medication for the management of CaOx stone diseases.
Recent decades have witnessed great advancement in technologies and methods for the surgical treatment of nephrolithiasis, but little progress has been made in the development of effective medications for prevention [12]. Although dietary and medical regimens for stone prevention are currently available, there is no valid method to decrease the incidence or recurrence rate. Potassium citrate has long been employed to control kidney stones derived from uric acid, cystine, or CaOx. HCA is a novel ramification of citrate, which was proved to adsorb on CaOx crystal surfaces and cause monohydrate crystal surfaces to dissolve [6]. However, whether it can inhibit crystal formation in vivo has never been investigated before. To function in vivo, it needs to keep the primary structure in the urine. Prior studies have documented detectable HCA in circulation after ingestion [15,16], but the metabolism of HCA in human remains unclear.
In this study, we used a NaOx-induced D. melanogaster hyperoxaluria model to evaluate the safety and inhibition efficacy of K-HCA against CaOx crystal formation in vivo. Results demonstrated that administration of K-CA and K-HCA both significantly decreased the formation of CaOx stones. Moreover, we found that K-HCA showed an even more significant inhibitory effect compared with K-CA at the lower concentration. On the other hand, although K-HCA did not improve the life span, it is confirmed to be harmless compared with healthy flies. Our findings support that K-HCA has potential to be an alternative to K-CA. Furthermore, given the lack of efficient methods to reduce hyperoxaluria, K-HCA may provide a new way to prevent CaOx stones, as it just blocks crystallization, regardless of endogenous or dietary hyperoxaluria.
Our study has limitations. Firstly, the experiments were only performed in Drosophila melanogaster models. The similarity in the HCA metabolism in humans and Drosophila requires further investigations. In clinical practice, analyses of HCA levels in serum and urine is needed, as well as the biochemical parameters to estimate the inference of HCA on the mini-environment of stone formation and renal functions [17]. However, it is difficult to collected fluid samples from Drosophila models [18]. Secondly, to investigate the effect of HCA, only dietary hyperoxaluria models were used. Other kinds of hyperoxaluria pathological models, such as primary hyperoxaluria and idiopathic hyperoxaluria, should be applied to provide comprehensive evaluation. Finally, with little knowledge about K-HCA metabolism in Drosophila, it makes our findings largely phenomenological [18], which means clinical trials are still needed.
Conclusions
Our study suggests that administration of 0.05% NaOx is an efficient method to induce CaOx Drosophila models without significant risk to life. K-HCA is a promising therapy for the control of CaOx stones in hyperoxaluria models. It was as safe as K-CA, but was more efficient in preventing stone formation at smaller dosages. Further exploration of the clinical potential of K-HCA as an alternative treatment to K-CA for kidney stones is still needed, including the metabolism in vivo, long-term safety and tolerability, and optimal dosing regimens.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81470935, 81500534, 81602236, 81670645) and the National Major Scientific and Technological Special Project for “Significant New Drugs Development” 2017ZX09304022
Conflict of interest
None.
References
- 1.Gambaro G, Croppi E, Coe F, et al. Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: A consensus statement. J Nephrol. 2016;29:715–34. doi: 10.1007/s40620-016-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung H, Andonian S, Assimos D, et al. Urolithiasis: Evaluation, dietary factors, and medical management: An update of the 2014 SIU-ICUD international consultation on stone disease. World J Urol. 2017;35:1331–40. doi: 10.1007/s00345-017-2000-1. [DOI] [PubMed] [Google Scholar]
- 3.Sorokin I, Mamoulakis C, Miyazawa K, et al. Epidemiology of stone disease across the world. World J Urol. 2017;35:1301–20. doi: 10.1007/s00345-017-2008-6. [DOI] [PubMed] [Google Scholar]
- 4.Chuah LO, Ho WY, Beh BK, Yeap SK. Updates on antiobesity effect of garcinia origin (−)-HCA. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/751658. 751658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng M, Han J, Li L, Ma H. Suppression of fat deposition in broiler chickens by (−)-hydroxycitric acid supplementation: A proteomics perspective. Sci Rep. 2016;6:32580. doi: 10.1038/srep32580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J, Granja I, Taylor MG, et al. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature. 2016;536:446–50. doi: 10.1038/nature19062. [DOI] [PubMed] [Google Scholar]
- 7.Dow JA, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol. 2010;299:F1237–44. doi: 10.1152/ajprenal.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirata T, Cabrero P, Berkholz DS, et al. In vivo Drosophilia genetic model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. 2012;303:F1555–62. doi: 10.1152/ajprenal.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YH, Liu HP, Chen HY, et al. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: A Drosophila model for nephrolithiasis/urolithiasis. Kidney Int. 2011;80:369–77. doi: 10.1038/ki.2011.80. [DOI] [PubMed] [Google Scholar]
- 10.Siener R, Ebert D, Nicolay C, Hesse A. Dietary risk factors for hyperoxaluria in calcium oxalate stone formers. Kidney Int. 2003;63:1037–43. doi: 10.1046/j.1523-1755.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 11.Tzou DT, Taguchi K, Chi T, Stoller ML. Animal models of urinary stone disease. Int J Surg. 2016;36:596–606. doi: 10.1016/j.ijsu.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Miller J, Chi T, Kapahi P, et al. Drosophila melanogaster as an emerging translational model of human nephrolithiasis. J Urol. 2013;190:1648–56. doi: 10.1016/j.juro.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SY, Shen JL, Man KM, et al. An emerging translational model to screen potential medicinal plants for nephrolithiasis, an independent risk factor for chronic kidney disease. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/972958. 972958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Male M, Li Y, et al. Efficacy of hydroxy-L-proline (HYP) analogs in the treatment of primary hyperoxaluria in Drosophila melanogaster. BMC Nephrol. 2018;19:167. doi: 10.1186/s12882-018-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Loon LJ, van Rooijen JJ, Niesen B, et al. Effects of acute (−)-hydroxycitrate supplementation on substrate metabolism at rest and during exercise in humans. Am J Clin Nutr. 2000;72:1445–50. doi: 10.1093/ajcn/72.6.1445. [DOI] [PubMed] [Google Scholar]
- 16.Loe YC, Bergeron N, Rodriguez N, Schwarz JM. Gas chromatography/mass spectrometry method to quantify blood hydroxycitrate concentration. Anal Biochem. 2001;292:148–54. doi: 10.1006/abio.2001.5046. [DOI] [PubMed] [Google Scholar]
- 17.Morgan MS, Pearle MS. Medical management of renal stones. BMJ. 2016;352:i52. doi: 10.1136/bmj.i52. [DOI] [PubMed] [Google Scholar]
- 18.Knauf F, Preisig PA. Drosophila: A fruitful model for calcium oxalate nephrolithiasis? Kidney Int. 2011;80:327–29. doi: 10.1038/ki.2011.166. [DOI] [PubMed] [Google Scholar]