Summary
Schwann cells (SCs) critically maintain the plasticity of the peripheral nervous system. Peripheral nerve injuries and infections stimulate SCs in order to retrieve homeostasis in neural tissues. Previous studies indicate that Mycobacterium leprae (ML) regulates the expression of key factors related to SC identity, suggesting that alterations in cell phenotype may be involved in the pathogenesis of neural damage in leprosy. To better understand whether ML restricts the plasticity of peripheral nerves, the present study sought to determine the expression of Krox‐20, Sox‐10, c‐Jun and p75NTR in SC culture and mice sciatic nerves, both infected by ML Thai‐53 strain. Primary SC cultures were stimulated with two different multiplicities of infection (MOI 100:1; MOI 50:1) and assessed after 7 and 14 days. Sciatic nerves of nude mice (NU ‐Foxn1 nu) infected with ML were evaluated after 6 and 9 months. In vitro results demonstrate downregulation of Krox‐20 and Sox‐10 along with the increase in p75NTR‐immunolabelled cells. Concurrently, sciatic nerves of infected mice showed a significant decrease in Krox‐20 and increase in p75NTR. Our results corroborate previous findings on the interference of ML in the expression of factors involved in cell maturation, favouring the maintenance of a non‐myelinating phenotype in SCs, with possible implications for the repair of adult peripheral nerves.
Keywords: leprosy, schwann cells, sciatic nerve, transcription factors
1. INTRODUCTION
Mycobacterium leprae (ML) is a low‐virulent pathogen responsible for the development of leprosy.1 The evolutionary reduction has limited ML to intracellular parasitism, rendering it dependent on the host cell to survive and propagate.2 In spite of being generated by a genetically conserved aetiological agent, leprosy exhibits a heterogeneous spectrum of cutaneous and peripheral neural manifestations.1, 3 Neural involvement occurs in all clinical forms of the disease, ranging from acute and focal manifestations to insidious and widespread forms.3 Leprosy affects sensory, motor and autonomic functions, but sensitive nerve fibres are compromised earlier.4, 5 Neural damage in leprosy leads to physical disabilities leading to stigma and high morbidity in the individuals affected by the disease.6, 7
The glial cells of the peripheral nervous system (PNS) constitute distinct classes of Schwann cells (SCs) closely associated with different types of neurons.8, 9 The development of the glial lineage encompasses a rich sequence of molecular and cellular events in which neural crest stem cells differentiate into precursor SCs, followed by immature cells that generate non‐myelinating and myelinating SCs.8, 10 The myelinating phenotype requires the inactivation of a number of genes linked to the production of immature SC markers, such as GFAP (glial fibrillary acidic protein), L1‐CAM (L1 cell adhesion molecule), NCAM‐1 (neural cell adhesion molecule) and p75NTR (nerve growth factor receptor).10, 11 Concurrently, the appropriate maturation of SCs is ensured by factors such as Sox‐10 (SRY‐box 10) that acts synergistically with Oct‐6 (POU class 3 homeobox 1), sustaining the expression of Krox‐20 (early growth response 2).12 Krox‐20 is a key inducer of myelin genes, such as MBP (myelin basic protein), MPZ (myelin protein zero; P0), MAG (myelin‐associated glycoprotein) and PRX (periaxin).11, 12 The maintenance of myelinating phenotype requires the continuous expression of Krox‐20 and Sox‐10.13, 14
Adult SCs exhibit remarkable plasticity and aggression to the peripheral nerves induce in these cells the process recently characterized as adaptive cellular reprogramming.15, 16 In this process, the cells undergo dedifferentiation, assume reparative properties and turn into Büngner cells.16 After supporting the regeneration of injured axons, Büngner cells redistribute and remyelinate the peripheral nerves.17 The downregulation of genes that define the myelinating profile is a key feature of the reparative phenotype, as well as the elevation of molecular patterns similar to early stages of development.15, 18
Büngner cells actively participate in the immune response, in addition to degrading myelin debris that could potentially inhibit axonal regeneration.19 The proto‐oncogene c‐Jun controls the conversion of Büngner cells after neural injury by mechanisms not yet fully clarified.20 C‐Jun is present at high levels in SCs prior to the establishment of the myelinating profile, and its expression is suppressed by Krox‐20 during cell maturation.17, 21 Additionally, the restriction of c‐Jun interferes in the expression of important neurotrophic factors and cell surface proteins, limiting the potential of peripheral nerve to repair.22, 23
Myelinating and non‐myelinating SCs respond distinctly to ML infection,24 which induces the re‐entry of quiescent cells into the cell division cycle in order to enhance the availability of bacillary proliferation niches.25 Compact myelin seems to confer to SCs some resistance to ML invasion. Nevertheless, the impairment of cellular integrity and subsequent demyelination in the early stages of infection favour the spread of the pathogen.26 Masaki et al27 recapitulated the early molecular events in ML‐infected adult peripheral glia and reported that ML explores the plasticity of adult SCs by subverting their differentiation programme. The authors demonstrated that SCs infected in vitro undergo reprogramming to the point of converting into neural crest‐like cells, termed stem‐like progenitor cells (pSLC). According to these authors, such conversion occurs when the cell undergoes removal of Sox‐10 along with maintenance of the stem cell marker Sox‐2, thus characterizing a phenotype Sox‐2+/Sox‐10−/p75NTR− in ML‐reprogrammed cells.27
The effective redifferentiation of SCs after neural aggression depends on critical markers in the glial cell lineage.28 Since previous studies indicated that ML infection induces in vitro alterations in some of these markers,27 this study aimed to address the expression in vivo of Krox‐20, Sox‐10, c‐Jun and p75NTR. Accordingly, we infected indirectly the sciatic nerves of athymic nude mice (NU‐Foxn1 nu) with ML, for a duration of six and nine months, alongside studies of in vitro infection of primary SC cultures.
The animal model of infection we have adopted is of popliteal fossa inoculation with viable ML since this anatomical site is considered an appropriate reservoir for the release of drugs towards the sciatic nerve.29 From this premise, we aimed to reproduce experimentally the ascending route of infection and the development of leprosy neuropathy.
In this descriptive study, our central motivation was to fulfil possible model gaps detected in previous studies and maybe contribute to improving the experimental design of nerve infection by ML. We also sought herein to contribute to the understanding of glial alterations after ML infection that could participate in the pathogenesis of leprosy nerve damage.
2. MATERIAL AND METHODS
2.1. Animals
The experiment was conducted in 20 Swiss white mice (SW) and 36 athymic nude mice (NU‐Foxn1 nu), with 30 to 60 days old, from the Lauro de Souza Lima Institute/SES, Bauru/SP. The death of the animals was induced by an overdose of ketamine 200 mg/kg (Vetnil, Brazil) and Rompun® 30 mg/kg (Bayer, Germany) intraperitoneally.
2.2. Ethical approval
The handling of the experimental animals was in accordance with the Commission for Ethics in the Use of Animals of the Lauro de Souza Lima Institute rules (CEUA‐ILSL, protocol n° 001/16), based on ethical principles of animal experimentation elaborated by the Brazilian Society of Science in Laboratory Animals (SBCAL).
2.3. Tissue isolation and cell culture
Tissue and cell procedures were conducted as previously described.30, 31 Briefly, sciatic nerves were carefully detached from each epineurium and fragmented. Specimens were transferred to a conical tube and washed with phosphate‐buffered saline, for 5 minutes at 4°C. The pellet was resuspended in Dulbecco's modified Eagle's medium with 4500 mg/L glucose (DMEM‐Hg, Gibco®) containing 10% foetal bovine serum (FBS, Sigma‐Aldrich), 10 ng/mL heregulin β1 (HRG, Sigma‐Aldrich), 2 μmol/L forskolin (Sigma‐Aldrich) and 1% penicillin/streptomycin (Gibco®). Nerve fragments were explanted for 7 days, in 5% CO2 incubator with 95% humidity, at 37°C. The specimens were then centrifuged at 161 g for 5 minutes at 4°C, resuspended in DMEM‐Hg containing 1% penicillin/streptomycin, 0.5 mg/mL collagenase type I and 2.5 mg/mL dispase II (Gibco®), and maintained in a CO2 incubator for 24 hours. Following enzymatic digestion, nerve tissue was mechanically homogenized on graduated hypodermic needles (18G and 21G). The cell suspension was filtered at 40‐μm membrane, transferred to DMEM‐Hg/10% FBS and centrifuged at 252 g for 10 minutes at 4°C. Cell counting and trypan blue viability exclusion were conducted and the cell density was adjusted to 2.5 x 104 cells/cm2. Cells were then seeded in polystyrene 24‐well plates, coated with 20 μg/mL laminin (Sigma‐Aldrich). The culture proceeded with supplemented medium changes every 48 hours.
2.4. Mycobacterium leprae
The inoculum was obtained from serial passages in athymic nude mice (NU‐Foxn1 nu) footpads, according to the previously described technique.32, 33 Briefly, the animals were inoculated into the plantar surface of both hind footpads with 30 μl of a suspension containing 1 x 107 bacilli/mL. After 4 months, the animals were killed and the bacillary suspension was prepared.
2.5. In vitro infection
Schwann cells dissociated from sciatic nerves of SW mice were divided into two experimental groups and a control group. The first experimental group (ML50) received live ML at the multiplicity of infection (MOI) of 50 ML/SC. The second group (ML100) received ML at MOI 100:1. The third group (CTRL) did not receive ML. All groups were cultivated under the same incubation conditions and maintained for 7 and 14 days. Samples were then stored appropriately for in situ determination of Krox‐20, Sox‐10, c‐Jun and p75NTR by scanning laser confocal microscopy.
2.6. In vivo infection
Nude mice inoculation was performed by intradermal injection with 100 μL of bacillary suspension at 1 x 106 bacilli/mL, near to popliteal lymph nodes of each hindlimb. The Weizhong acupuncture point, in the popliteal fossa, was selected based on the notion that it is an adequate access point for anaesthetic blockade of muscle spasms below the knee.34 Additionally, 30 μL of the same bacillary suspension were inoculated into both hind footpads of each mouse. Mice were maintained for six and nine months (n = 06/period). The first time point was chosen based on previous studies of mouse footpad inoculation, in which some of them consider six months a peak of bacillary growth rate.35 At the end of each period, mice were killed and their sciatic nerves collected to the same evaluations conducted in the in vitro assays. Nerves of non‐infected nude mice (n = 03/period) were used as controls.
2.7. Cell morphology and bacillary index determination
Part of the SC samples and neural fragments were destined for bacillary index evaluation by Fite‐Faraco staining. The bacillary index (IB) was determined by ML count at 100‐200 infected cells per microscopic field. Briefly, the specimens were kept in mineral oil for 30 minutes at 60°C. Following this procedure, tap water was used to remove the excess of oil and the specimens were gently dried. Subsequently, specimens were coated with filtered fuchsine for 30 minutes and washed with tap water. Sections were then dehydrated through alcohol series and mounted with Permount Mounting Media (Fisher Scientific).
2.8. Immunofluorescence
Schwann cells and neural fragments were fixed in 4% paraformaldehyde (Sigma‐Aldrich) for 15 minutes at room temperature and washed in PBS. Part of the samples was permeabilized with PBS/0.25% Triton X‐100, for 10 minutes. After washing in PBS, non‐specific binding sites were blocked with 1% bovine serum albumin (BSA, Sigma‐Aldrich) and 22.5 mg/mL glycine, in PBST (PBS/0.1% Triton X‐100). After 30 minutes, samples were incubated overnight with target‐specific primary antibodies, c‐Jun (100 μg/mL. 1:125. clone E254. Abcam, UK), p75NTR (200 μg/mL. 1:225. clone ME20.4. Santa Cruz Biotechnology), Krox‐20 (100 μg/mL. 1:100. PRB‐236P. Covance) and Sox‐10 (200 μg/mL. 1:225. clone G‐11. Santa Cruz Biotechnology). Following the incubation period, the samples were washed in PBS and incubated with secondary antibodies conjugated to AlexaFluor® 488 and AlexaFluor® 594 (1 mg/mL. 1:400. Molecular Probes®) for 60 minutes, at RT and protected from light. Cell nuclei were stained with DAPI (10 mg/mL. 1:150. Molecular Probes®). After 10 minutes, the specimens were washed, dried and mounted on glass microscopic slides. The image capture was performed in confocal laser scanning microscope TCS SP5 (Leica, Germany) with image processing software LAS AF (Leica, Germany). In vivo experiments used five animals per group and evaluated 14 microscopic fields per group, on average. In vitro experiments employed 20 animals in two independent assays, and evaluated 12 microscopic fields per group, on average. Results were achieved by the relative frequency of each target of interest, calculated by the ratio of positive cells over the total of DAPI+ nuclei per field.
2.9. Statistical analysis
The analysis of the results was performed on GraphPad Prism 5.01® software (GraphPad Inc.). Data were submitted to the Kruskal‐Wallis test, with Dunn post‐test for comparison in control and experimental groups. The results are expressed by the median. Values of P < 0.05 were considered statistically significant.
3. RESULTS
3.1. Cell morphology and identification of ML
Phase‐contrast microscopy and haematoxylin‐eosin staining confirmed the tripolar or fusiform morphology of SCs, whereas flat or polygonal cells were recognized as fibroblasts (Figure 1). Part of the cells was stained by Fite‐Faraco for identification and enumeration of intracellular bacilli (Figure 2). After seven days, the group ML50 showed an average of 14.63 ML/SC (± 7.13). In the group ML100, an average of 20.99 ML/SC (± 12.65) has been calculated after 7 days. After 14 days, the group ML50 showdd 5.51 ML/SC (± 1.85), while in ML100 group the average was 8.66 of ML/SC (± 0.51). Nude mice‐infected sciatic nerves were also stained by haematoxylin‐eosin and Fite‐Faraco for investigation of ML within the neural structure (Figure 2). After six months of infection, no bacilli or any signs of degeneration have been shown inside the nerve fibres. After nine months, the perineurial bacillary invasion was observed in infected nerves, although no signs of neural degeneration were found. In each period, one sciatic nerve per animal was collected for haematoxylin‐eosin and Fite‐Faraco staining.
Figure 1.

Primary Schwann cells (SCs) from sciatic nerves of Swiss white mice (SW). (A‐J) SC characterization by phase contrast and immunofluorescence. Phase contrast of SCs cultivated for 7 d (A‐C) and 14 d (D‐F). Control (A, D) and experimental groups (B, C, E, F) exposed to ML in multiplicities of infection (MOI) of 50 bacilli/cell (B, E) and 100 bacilli/cell (C, F). Confocal microscopy showing immunodetection of S100β protein (green: AlexaFluor 488®) and nuclear staining by DAPI (blue) in SC culture for 7 (G, H) and 14 d (I, J). Bar graphs indicate medians of fluorescence density (K) and relative frequency (L) of SCs labelled by S100β. Mann‐Whitney test with P > 0.05. Displayed results represent two independent assays and 12 microscopic fields/group, on average. Magnification: 400x (A‐F) and 630x (G‐J). CTRL, control; ML50, ML/SC MOI 50:1; ML100, ML/SC MOI 100:1; 7D, 7 d; 14D, 14 d [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
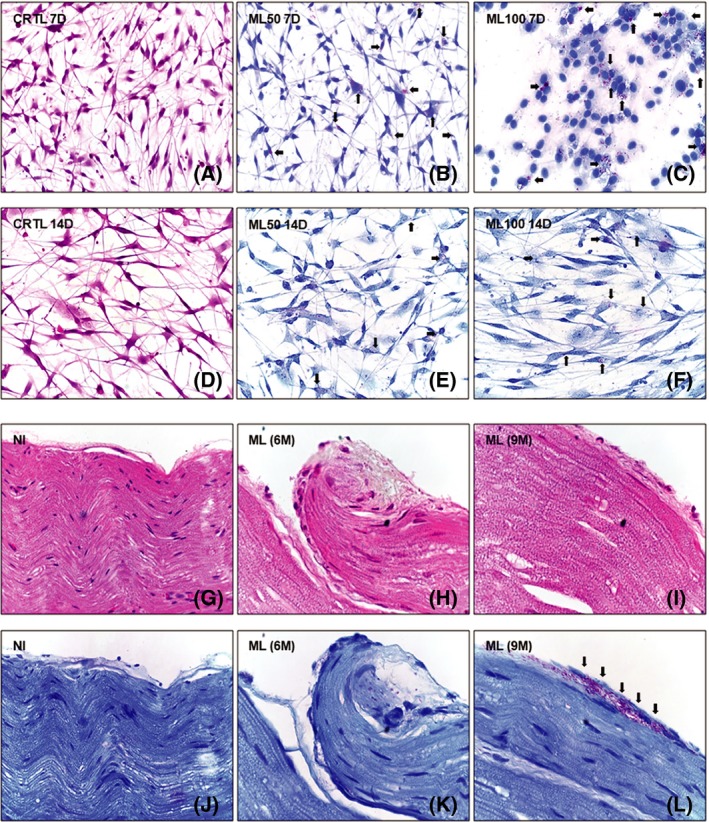
Schwann cells (SC) and mice sciatic nerves infected by ML. Morphological evaluation by haematoxylin‐eosin (A, D; G‐I) and identification of intracellular bacilli by Fite‐Faraco (B, C, E, F; J‐L). SC maintained for 7(A‐C) to 14 d (D‐F) and stimulated by ML (arrows) under multiplicities of infection (MOI) of 50 bacilli/cell (B, E) and 100 bacilli/cell (C, F). Longitudinal sections of peripheral nerve fibres in non‐infected (G, J) and ML‐infected sciatic nerves (arrows) (1 x 106 bacilli/mL), over 6 (H, K) and 9 mo (I, L), all of them displaying inner (endoneurium and perineurium) and outer compartments (epineurium). Microscopic fields selected from two independent assays/groups. Images of sciatic nerves selected from five specimens/group. Magnification: 400x. CTRL, control; ML50, ML/SC MOI 50:1; ML100, ML/SC MOI 100:1; 7D, 7 d; 14D, 14 d; NI = uninfected; ML (6mo), ML (6 mo); ML (9M), ML (9 mo) [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Modulation of Krox‐20 and Sox‐10 in murine model of ML infection
SC culture stimulated by ML demonstrated elevation of Krox‐20 in the CTRL group compared to ML100 (P < 0.001) after 14 days, whereas after 7 days there were no statistically significant changes among the groups (Figure 3). In the sciatic nerves of nude mice, the expression of Krox‐20 was higher in uninfected mice than experimental groups, after six and nine months (P < 0.01 and P < 0.001, respectively) (Figure 4). The determination of Sox‐10 in SC culture exposed to ML showed a decrease in Sox‐10+ cells in ML100 in relation to CTRL, after seven days (P < 0.05). At 14 days of cell culture, Sox‐10 did not show statistically significant changes among the groups (Figure 5). During ML infection of mouse sciatic nerves, statistically significant alterations of Sox‐10 among the groups were not observed (Figure 6).
Figure 3.

Krox‐20 in Schwann cells (SC) culture. Confocal microscopy demonstrates immunodetection of Krox‐20 (green: AlexaFluor 488®) and nuclear labelling by DAPI (blue) in SCs after 7 and 14 d. Uninfected controls (A‐F) and experimental groups exposed to ML, 50 bacilli/cell (G‐L) and 100 bacilli/cell (M‐R). Krox‐20‐negative nuclei are identified by asterisks (*) (I, O). Graphics illustrate the relative frequency of Krox‐20 in SCs (S, T). Statistics bars identify significant differences between CTRL and ML100 after 14 d of infection. Kruskal‐Wallis test indicates P < 0.001 (***) at 14 d. Data expressed as medians of two independent assays, in which an average of 12 microscopic fields per group were recorded. Scale bar: 30 μm. CTRL, control; ML50, ML/SC MOI 50:1; ML100, ML/SC MOI 100:1; 7D = seven days; 14D = fourteen days [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.

Krox‐20 in sciatic nerves of nude mice infected by ML. Immunodetection of Krox‐20 (green: AlexaFluor 488®) in uninfected sciatic nerve fragments (A‐C) and infected by ML (1 x 106 bacilli/mL) over 6 (D‐F) and 9 mo (G‐I). Graphic of the relative frequency of Krox‐20 (J) indicates higher expression in uninfected animals compared to experimental groups. Kruskal‐Wallis test shows P < 0.01 (**) at NI x 6M and P < 0.001 (***) at NI x 9M. Images selected from five specimens/group. Bar: 30 μm. NI, uninfected; ML (6M), ML (6 mo); ML (9M), ML (9 mo) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 5.

Detection of Sox‐10 in mice Schwann cells (SC) culture. Immunodetection of Sox‐10 (red: AlexaFluor 594®) and nuclear labelling by DAPI (blue) in SCs after 7 and 14 d. Control (A‐F) and experimental groups exposed to ML, 50 bacilli/cell (G‐L) and 100 bacilli/cell (M‐R). Graphics illustrate the relative frequency of Sox‐10 in SCs (S, T). Statistics bars identify significant differences between CTRL and ML100 after 7 d of infection. Kruskal‐Wallis test shows P < 0.05 (*) at day seven. Results are shown as medians of two independent assays, in which an average of 12 microscopic fields per group were recorded. Scale bar: 30 μm. CTRL, control; ML50, ML/SC MOI 50:1; ML100, ML/SC MOI 100:1; 7D, 7 d; 14D, 14 d [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 6.

Sox‐10 in sciatic nerves of nude mice infected by ML. Immunodetection of Sox‐10 (green: AlexaFluor 488®) in fragments of uninfected sciatic nerves (A‐C) and infected with ML (1 x 106 bacilli/mL) over 6 (D‐F) and 9 mo (G‐I). Relative frequency of Sox‐10 (J). Kruskal‐Wallis test indicates P > 0.05. Images selected from five specimens/group. Scale bar: 30 μm. NI, uninfected; ML (6M), ML (6 mo); ML (9M), ML (9 mo) [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Increase in p75NTR in cells and nerves infected by ML
Primary SC culture exposed to ML for seven and 14 days demonstrated lower frequency of p75NTR+ cells in CTRL compared to ML100 (7 days, P < 0.001; 14 days,P < 0, 05) (Figure 7). Similarly, in the sciatic nerves of nude mice the expression of p75NTR was significantly lower in uninfected animals than in those infected for six (P < 0.001) and nine months (P < 0.001) (Figure 8). The evaluation of the expression of c‐Jun in SCs exposed to ML did not indicate significant differences between control and experimental groups (Figure S1). It was not possible to identify c‐Jun+ cells in the sciatic nerves of nude mice, whether or not infected by ML.
Figure 7.

Detection of p75NTR in Schwann cells culture (SCs). Immunodetection of p75NTR (red: AlexaFluor 594®) and nuclear labelling by DAPI (blue) in SCs after 7 and 14 d. Control (A‐F) and experimental groups exposed to ML 50 bacilli/cell (G‐L) and 100 bacilli/cell (M‐R). Graphics illustrate the relative frequency of p75NTR in SCs (S‐T). Statistics bars identify significant differences between CTRL and ML100 in both experimental periods. Kruskal‐Wallis test indicates P < 0.001 (***) at 7 d and P < 0.05 (*) at 14 d. Data expressed as medians of two independent assays, in which an average of 12 microscopic fields per group was recorded. Scale bar: 30 μm. CTRL, control; ML50, ML/SC MOI 50:1; ML100, ML/SC MOI 100:1; 7D, 7 d 14D, 14 d [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 8.

P75NTR on sciatic nerve of nude mice after experimental infection by ML. Immunodetection of p75NTR (green: AlexaFluor 488®) in fragments of the uninfected sciatic nerve (A‐C), and infected by ML (1 x 106 bacilli/mL) over 6 (D‐F) and 9 mo (G‐I). The graphic indicates lower expression of p75NTR (J) in the uninfected animals in relation to the experimental groups. Kruskal‐Wallis test with P < 0.001 (***) at NI x 6M and NI x 9M. Images selected from five specimens/group. Scale bar: 30 μm. NI, not infected; ML (6M), ML (6 mo); ML (9M),ML (9 mo) [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This study sought to contribute to the understanding of changes in Schwann cells after ML infection, describing the expression of critical markers related to glial differentiation and maturation. The transcription factors Krox‐20, Sox‐10, c‐Jun and the receptor p75NTR were evaluated in vitro in primary culture of SCs obtained from sciatic nerves of Swiss white (SW) mice stimulated by ML for seven and 14 days. The same factors were evaluated in vivo on sciatic nerves of athymic nude mice (NU‐Foxn1 nu), after six and nine months of ML infection.
The previous study by Masaki and colleagues (2013), as well as ours, approached the early molecular events resulting from peripheral glia infection by ML.27 However, some differences must be highlighted. Firstly, in both studies, SC cultures from different mouse strains were adopted. Masaki et al27 have isolated SCs directly from CD‐1 and C57BL/6 mice, while we dissociated primary SCs from nerve explants of SW mice for the in vitro assays. Such choice was based on the ML infection susceptibility of SW strain, which was previously reported.36 Secondly, Masaki et al27 have generated and characterized FACS‐derived single clones of SCs, which were posteriorly infected by ML, allowing the selection of a reprogrammed cell population (progenitor stem‐like cells, pSLC). In our study, prior to SC isolation, we performed an ex vivo degeneration step (nerve explant), allowing glial cells phenotype shift to a more immature population prior to infection. In a temporal window of in vitro infection (7‐14 days), we believe we reproduced the dynamic interplay between non‐myelinating SCs and the pathogen, without necessarily pushing them into reprogramming.
The development of glial lineage and myelination of peripheral nerves are centrally regulated by transcription factors like Krox‐20 and Sox‐10.12, 13 In adult peripheral nerves, myelinating SCs may dedifferentiate when removed from axonal contact or soon after nerve injury.37 In these cells, the re‐induction of immature SC markers is observed, including c‐Jun and p75NTR.38 The relevance of Krox‐20 to SC maturation is unequivocally illustrated in studies on mutations in the EGR2 gene associated with peripheral myelinopathies, including Charcot‐Marie‐Tooth syndrome.39 Inhibition of Krox‐20 is also observed in non‐hereditary demyelinating neuropathies, such as Guillain‐Barré syndrome.40 In line, our results demonstrate that ML decreases the frequency of murine SCs expressing Krox‐20 in vivo and in vitro, suggesting that the bacillus may hinder the myelinating phenotype in these cells. Krox‐20 is poorly explored in the context of glial alterations due to infection by ML. As far as we know, there are no other non‐leprosy‐related intracellular pathogens capable to downregulate Krox‐20 in SCs; nevertheless, it is an issue that should be taken into account and we are addressing it in our ongoing studies.
Although Sox‐10 is required at all stages of glial cell line development, it is believed that its expression does not change following peripheral nerve injury, when SCs turn into Büngner cells.41, 42 Previous data point to a lack of Sox‐10 nuclear translocation in mice SCs after 10 days of ML infection, at MOI < 50.27 In our study, we could not determine such difference in fluorescent signal of Sox‐10. However, our results indicate that after seven days of infection, there is a decline in the frequency of Sox‐10‐positive cells in ML100, compared to control.
In adult life and homeostatic conditions, p75NTR is downregulated in different neurons and other cell types of nervous system, namely astrocytes, oligodendrocytes, SCs and microglia.43 Accordingly, adult mice sciatic nerves keep p75NTR downregulated when compared to newborns.10 However, adult peripheral nerves submitted to injury demonstrate a drastic elevation of p75NTR.42 In parallel, our data indicate that ML significantly increases the frequency of p75NTR+ cells in primary SC culture and adult mice sciatic nerves.
Although we could not find significant signs of neural degeneration in our in vivo experiment, the elevation of p75NTR in mice sciatic nerves infected by ML suggests an increase in non‐myelinating cell population in the adult neural environment. Such alteration could chronically interfere with the homeostasis of the sciatic nerves. The concept that p75NTR plays an essential role in neural protection and repair is corroborated by pieces of evidence showing that in sciatic nerves of p75NTR‐knockout mice, remyelination is deficient after nerve injury.44 Further evidence indicates that in the animal model of amyotrophic lateral sclerosis (ALS), the expression of p75NTR is correlated with the extension of the degenerative process.45
The plasticity of adult SCs is among the prerequisites for the return to homeostasis after nerve damage.37, 46 In this context, c‐Jun plays a crucial role in the tissue repair programme, providing support for axon regeneration. On the other hand, the deficiency of c‐Jun induction after neural aggression may lead to dysfunctional repair patterns, resulting in neuronal death.22 Weiss et al20 elegantly demonstrated that among the key factors associated with the repair programme, c‐Jun was strongly expressed in human SC cultures and explants from peripheral nerve fascicles.20 In the present study, we sought to determine the expression of this same transcription factor in the murine model.
However, our in vitro results did not indicate significant changes in c‐Jun expression in SCs dissociated from sciatic nerves and exposed to ML preparations. Nevertheless, we believe that additional studies may contribute to a better understanding of the interplay between ML and the expression of c‐Jun in its target cells. In sciatic nerves of nude mice inoculated by ML for six and nine months, the deficient frequency of c‐Jun did not allow us to perform data analysis. The transcription factor c‐Jun is not expressed in myelinating cells of normal adult nerves in vivo,21, 22 whereas it is upregulated at the onset of nerve injury.
In vitro and in vivo systems carry some particularities, mainly regarding the microenvironments, that naturally generate some differences in levels of specific proteins. Some discrepancies in protein expression should be highlighted, especially regarding Krox‐20 and p75NTR. The average percentage of SCs positive to Krox‐20 was far lower in cell culture exposed to ML at the MOI of 100 bacilli/SC than in the controls (ML100 = 80.26% to CTRL = 19.08%), after 14 days. Such differences were also comparatively observed in vivo were Krox‐20 fell on average, from 49.69% of frequency in non‐infected animals to 11.08% after 9 months of infection. Even without endoneurial infection, it seems that the persistence of ML on epineurial structure has led to alterations in Krox‐20 behaviour. Concurrently, the frequency of p75NTR showed discrepancies in the animal model, in which the non‐infected nerves jumped from 14.65% to 93.63% after 9 months.
It is important to note that temperature is an environmental factor recognized as harmful to ML.47 Consequently, some discrepancies among our in vitro and in vivo data may result from ML inactivation by incubation at 37°C in vitro. According to Hagge et al,47 viable ML infecting rat Schwann cells in vitro at 33°C retained 56% of viability after three weeks, compared to 3.6% viability at 37°C. In spite of this concurring evidence and taking into account a probable drop in the viability of ML in vitro, the presence of the pathogen (viable or not) seemed to be sufficient to induce alterations in our targeted factors. Another issue that led us to retain 37°C in cell culture is that we previously tested in our laboratory primary mouse SCs infected by ML at 33°C and detected a decrease in SC viability and proliferation rate (unpublished data). For this reason, we decided here to maintain 37°C in favour of SCs.
In summary, our findings demonstrate the downregulation of Krox‐20 and Sox‐10, along with the increase in p75NTR in SCs and sciatic nerves from nude mice infected by ML. Such alterations may be associated with possible remyelination deficits in nerves affected by leprosy and therefore shall be addressed further in our future studies.
CONFLICT OF INTERESTS
The authors have no conflict of interests to declare related to this study.
AUTHORS’ CONTRIBUTIONS
MBC contributed to in vitro and in vivo experiments, data analysis, interpretation of results and manuscript writing. PSR involved in ML isolation and inoculum preparation. DFFB contributed to ML isolation and inoculum preparation. DCN involved in harvesting of sciatic nerves. ASAAB contributed to immunofluorescence assays. VNBS involved in formal evaluation of the study. MRSN contributed to research design, in vitro and in vivo experiments, data analysis, results interpretation and manuscript writing.
Supporting information
ACKNOWLEDGEMENTS
We thank Dr. Yugi Miyamoto for kindly providing the ML strain Thai‐53 (Leprosy Research Center, National Institute of Infectious Diseases, Higashimurayama, Tokyo, Japan). We thank Ana Elisa Fusaro, Nelci Ana Vieira and Osmar de Abreu Francisco for the technical support (Laboratories of Microbiology and Pathology, Lauro de Souza Lima Institute, Bauru, SP, Brazil). We also thank Shelly Favorito de Carvalho for confocal images acquisition (Center of Electronic Microscopy, UNESP, Botucatu, Brazil).
Casalenovo MB, Rosa PS, Bertoluci DFDF, et al. Myelination key factor krox‐20 is downregulated in Schwann cells and murine sciatic nerves infected by Mycobacterium leprae . Int J Exp Path. 2019;100:83–93. 10.1111/iep.12309
Funding information
This work was supported by São Paulo State Foundation Against Leprosy (grant: 159/2013) and by São Paulo Research Foundation (grant: 2010/00146‐7).
REFERENCES
- 1. Singh P, Cole ST. Mycobacterium leprae: genes, pseudogenes and genetic diversity. Future Microbiol. 2011;6:57‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cole ST, Eiglmeier K, Parkhill J, et al. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007‐1011. [DOI] [PubMed] [Google Scholar]
- 3. Franco‐Paredes C, Rodriguez‐Morales AJ. Unsolved matters in leprosy: a descriptive review and call for further research. Ann Clin Microbiol Antimicrob. 2016;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: a new diagnostic and predictive tool for peripheral neuropathies. J Neuropathol Exp Neurol. 2007;66:1059‐1073. [DOI] [PubMed] [Google Scholar]
- 5. Garbino JA, Heise CO, Marques W Jr. Assessing nerves in leprosy. Clin Dermatol. 2016;34:51‐58. [DOI] [PubMed] [Google Scholar]
- 6. Boku N, Lockwood DN, Balagon MV, et al. Impacts of the diagnosis of leprosy and of visible impairments amongst people affected by leprosy in Cebu, the Philippines. Lepr Rev. 2010;81:111‐120. [PubMed] [Google Scholar]
- 7. Monteiro LD, Alencar CH, Barbosa JC, Novaes CC, da Silva RC, Heukelbach J. Limited activity and social participation after hospital discharge from leprosy treatment in a hyperendemic area in North Brazil. Rev Bras Epidemiol. 2014;17:91‐104. [DOI] [PubMed] [Google Scholar]
- 8. Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon Schwann cell interactions. J Neurosci. 2004;24:9250‐9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salzer JL. Axonal regulation of Schwann cell ensheathment and myelination. J Peripher Nerv Syst. 2012;17(Suppl 3):14‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Jin YQ, Chen L, et al. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS One. 2015;10:e0123278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salzer JL. Schwann cell myelination. Cold Spring Harb Perspect Biol. 2015;7:a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Decker L, Desmarquet‐Trin‐Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci. 2006;26:9771‐9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bremer M, Fröb F, Kichko T, et al. Sox10 is required for Schwann‐cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59:1022‐1032. [DOI] [PubMed] [Google Scholar]
- 14. Jagalur NB, Ghazvini M, Mandemakers W, et al. Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding. J Neurosci. 2011;31:8585‐8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521‐3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinen A, Lehmann HC, Küry P. Negative regulators of Schwann cell differentiation‐novel targets for peripheral nerve therapies? J Clin Immunol. 2013;33(Suppl 1):S18‐S26. [DOI] [PubMed] [Google Scholar]
- 18. Yang DP, Kim J, Syed N, et al. p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J Neurosci. 2012;32:7158‐7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vidal PM, Lemmens E, Dooley D, Hendrix S. The role of “anti‐inflammatory” cytokines in axon regeneration. Cytokine Growth Factor Rev. 2013;24:83‐12. [DOI] [PubMed] [Google Scholar]
- 20. Weiss T, Taschner‐Mandl S, Bileck A, et al. Proteomics and transcriptomics of peripheral nerve tissue and cells unravel new aspects of the human Schwann cell repair phenotype. Glia. 2016;64:2133‐2153. [DOI] [PubMed] [Google Scholar]
- 21. Parkinson DB, Bhaskaran A, Arthur‐Farraj P, et al. c‐Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arthur‐Farraj PJ, Latouche M, Wilton DK, et al. c‐Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Painter MW, Brosius Lutz A, Cheng YC, et al. Diminished Schwann cell repair responses underlie age‐associated impaired axonal regeneration. Neuron. 2014;83:331‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tapinos N, Rambukkana A. Insights into regulation of human Schwann cell proliferation by Erk1/2 via a MEK‐independent and p56Lck‐dependent pathway from leprosy bacilli. Proc Natl Acad Sci U S A. 2005;102:9188‐9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rambukkana A. Usage of signaling in neurodegeneration and regeneration of peripheral nerves by leprosy bacteria. Prog Neurobiol. 2010;91:102‐107. [DOI] [PubMed] [Google Scholar]
- 27. Masaki T, Qu J, Cholewa‐Waclaw J, Burr K, Raaum R, Rambukkana A. Reprogramming adult Schwann cells to stem cell‐like cells by leprosy bacilli promotes dissemination of infection. Cell. 2013;152:51‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finzsch M, Schreiner S, Kichko T, et al. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melemedjian OK, Tillu DV, Moy JK, et al. Local translation and retrograde axonal transport of CREB regulates IL‐6‐induced nociceptive plasticity. Mol Pain. 2014;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Päiväläinen S, Nissinen M, Honkanen H, et al. Myelination in mouse dorsal root ganglion/Schwann cell cocultures. Mol Cell Neurosci. 2008;37:568‐578. [DOI] [PubMed] [Google Scholar]
- 31. Tao Y. Isolation and culture of Schwann cells. Methods Mol Biol. 2013;1018:93‐104. [DOI] [PubMed] [Google Scholar]
- 32. Truman RW, Krahenbuhl JL. Viable ML as a research reagent. Int J Lepr Other Mycobact Dis. 2001;69:83‐12. [PubMed] [Google Scholar]
- 33. Trombone AP, Pedrini SC, Diório SM, et al. Optimized protocols for Mycobacterium leprae strain management: frozen stock preservation and maintenance in athymic nude mice. J Vis Exp. 2014;85:83‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hurt JK, Zylka MJ. PAPupuncture has localized and long‐lasting antinociceptive effects in mouse models of acute and chronic pain. Mol Pain. 2012;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hagge DA, Scollard DM, Ray NA, et al. IL‐10 and NOS2 modulate antigen‐specific reactivity and nerve infiltration by T cells in experimental leprosy. PLoS Negl Trop Dis. 2014;8(9):e3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Birdi TJ, Shetty VP, Antia NH. Differences in M. leprae‐induced nerve damage in Swiss white and C57Bl/6 mice. Int J Lepr Other Mycobact Dis. 1995;63:573‐574. [PubMed] [Google Scholar]
- 37. Webber C, Zochodne D. The nerve regenerative microenvironment: early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51‐59. [DOI] [PubMed] [Google Scholar]
- 38. Bellone E, Di Maria E, Soriani S, et al. A novel mutation (D305V) in the early growth response 2 gene is associated with severe Charcot‐Marie‐Tooth type 1 disease. Hum Mutat. 1999;14:353‐354. [DOI] [PubMed] [Google Scholar]
- 39. Szigeti K, Wiszniewski W, Saifi GM, et al. Functional, histopathologic and natural history study of neuropathy associated with EGR2 mutations. Neurogenetics. 2007;8:257‐262. [DOI] [PubMed] [Google Scholar]
- 40. Hyung S, Yoon Lee B, Park J‐C, Kim J, Hur E‐M, Francis Suh J‐K. Coculture of primary motor neurons and Schwann cells as a model for in vitro myelination. Sci Rep. 2015;5:15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balakrishnan A, Stykel MG, Touahri Y, Stratton JA, Biernaskie J, Schuurmans C. Temporal analysis of gene expression in the murine Schwann cell lineage and the acutely injured postnatal nerve. PLoS One. 2016;11:e0153256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meeker RB, Williams KS. The p75 neurotrophin receptor: at the crossroad of neural repair and death. Neural Regen Res. 2015;10:721‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramírez‐García L, Cevallos R, Gazarian K. Unveiling and initial characterization of neural crest‐like cells in mesenchymal populations from the human periodontal ligament. J Periodontal Res. 2017;52:609‐616. [DOI] [PubMed] [Google Scholar]
- 44. Fontana X, Hristova M, Da Costa C, et al. c‐Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol. 2012;198:127‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shepheard SR, Chataway T, Schultz DW, Rush RA, Rogers ML. The extracellular domain of neurotrophin receptor p75 as a candidate biomarker for amyotrophic lateral sclerosis. PLoS One. 2014;9:e87398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim HA, Mindos T, Parkinson DB. Plastic fantastic: schwann cells and repair of the peripheral nervous system. Stem Cells Transl Med. 2013;2:553‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hagge DA, Oby Robinson S, Scollard D, McCormick G, Williams DL. A new model for studying the effects of Mycobacterium leprae on Schwann cell and neuron interactions. J Infect Dis. 2002;186(9):1283‐1296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


