Summary
A major translational barrier to the use of stem cell (SC)‐based therapy in patients with myocardial infarction (MI) is the lack of a clear understanding of the mechanism(s) underlying the cardioprotective effect of SCs. Numerous paracrine factors from SCs may account for reduction in infarct size, but myocardial salvage associated with transdifferentiation of SCs into vascular cells as well as cardiomyocyte‐like cells may be involved too. In this study, bone marrow‐derived rat mesenchymal SC (MSCs) were microencapsulated in alginate preventing viable cell release while supporting their secretory phenotype. The hypothesis on the key role of paracrine factors from MSCs in their cardioprotective activity was tested by comparison of the effect of encapsulated vs free MSCs in the rat model of MI. Intramyocardial administration of both free and encapsulated MSCs after MI caused reduction in scar size (12.1 ± 6.83 and 14.7 ± 4.26%, respectively, vs 21.7 ± 6.88% in controls, P = 0.015 and P = 0.03 respectively). Scar size was not different in animals treated with free and encapsulated MSC (P = 0.637). These data provide evidence that MSC‐derived growth factors and cytokines are crucial for cardioprotection elicited by MSC. Administration of either free or encapsulated MSCs was not arrhythmogenic in non‐infarcted rats. The consistency of our data with the results of other studies on the major role of MSC secretome components in cardiac protection further support the theory that the use of live, though encapsulated, cells for MI therapy may be replaced with heart‐targeted‐sustained delivery of growth factors/cytokines.
Keywords: alginate microcapsules, cardiac protection, mesenchymal stem cells, myocardial infarction
1. INTRODUCTION
Despite relevant progress in diagnosis and treatment, myocardial infarction (MI) and postinfarction chronic heart failure (CHF) are still the leading causes of death worldwide.1 Cell‐based therapy of MI is considered a promising strategy for regeneration of functional myocardial tissue, resulting in prevention of postinfarction CHF.2, 3 Because of their ease of isolation, rapid in vitro expansion, multipotency and immunoprivileged state, mesenchymal stem cells (MSCs) are commonly used in both experimental and clinical trials for cell therapy of MI.4, 5, 6 However, recent meta‐analyses demonstrated only a mild clinical improvement in MI patients receiving MSC transplantation, ranging from 3% to 10% increase in systolic left ventricular (LV) function in the treatment group.7, 8 Extremely low cell retention and engraftment in peri‐infarct area have been widely recognized as the major factors limiting the effectiveness of cell‐based MI therapy.2 Animal and human studies have convincingly demonstrated that <10% of injected cells was identified in the heart 24 hours post‐transplantation.9, 10, 11 Moreover, transendocardial delivery of MSCs in a porcine model of MI resulted in retention of only approximately 3% of cells at day 14 post‐treatment.12 There are two principal mechanisms of cell loss from the target tissue: (a) biological cell loss caused by massive cell death induced by hypoxia, inflammatory mediators and immune attack and (b) mechanical cell loss caused by rapid cardiac contraction‐enhanced washout of the cells from the myocardium through the injured microvessels and/or injection channel.13 The problem of cell loss cannot be solved by simply elevating the dose of transplanted cells, as the dose‐effect curve reaches a plateau at a relatively low dose.14 Alternatively, the transplanted cells can be genetically modified or conditioned with certain physical and chemical factors in order to increase their survival, engraftment, integration, proliferation and differentiation in the recipient heart (for review, see 15 and references therein). The introduction of cell‐seeded hydrogel scaffolds or cell‐containing patches apposed on to the infarcted area of the heart represents a technological advance in the prevention of cell loss.16, 17 Micro‐encapsulation of stem cells is another approach to prevent cell loss. The cells are embedded in semi‐permeable biodegradable matrix, which is freely permeable to oxygen, nutrients and signalling molecules, and helps maintain cell survival and function.13, 14 Encapsulation effectively attenuates cell injury by physically separating the cells from antibodies and phagocytes, and decreasing biological losses of cells, especially after allo‐ or xenogeneic transplantation.18 In addition, encapsulation decreases early vascular washout of the encased cells, because the capsule diameter typically lies in the range of 150‐300 μm and exceeds the diameter of microvessels.14 It is not surprising that the administration of encapsulated stem cells results in improved LV function and reduced postinfarct scar size compared to bare cells.19, 20 As shown in these studies, the enhanced cardioreparative effect of encapsulated stem cells could be attributed to the decreased mechanical cell loss associated with increased cell survival during the early postinjection period, with subsequent release, migration and integration of viable cells in the host myocardium.
Apart from low cell retention, the lack of a clear understanding of the mechanism(s) underlying the cardioprotective effect of MSCs is another translational barrier for their routine clinical use. Paracrine mechanisms of MSC‐mediated cardiac protection are considered responsible for the cardioreparative properties and reduction in the myocardial damage of MSC.21 However, myocardial salvage associated with transdifferentiation of MSCs into vascular cells as well as cardiomyocyte‐like cells plays a role too.22, 23, 24 New experimental data unequivocally demonstrating that paracrine signalling is a principal mechanism of cardiac protection after MSC transplantation may have important practical applications, providing a rationale for heart‐targeted‐sustained delivery of specific MSC secretome components instead of using living cells. Cell encapsulation technique represents a valuable but underused tool for dissection of the mechanisms of MSC‐mediated cardiac protection. In particular, mechanical isolation of encapsulated cells from the host myocardium during the entire period of postinfarct scar formation would nullify putative protective effects related to cell migration and differentiation, maintaining the secretion of paracrine factors by the encased cells. To achieve this goal, the cells with limited lifespan should be encapsulated in poorly biodegradable material and transplanted in the peri‐infarct area to ensure minimal, if any, viable cell release upon capsule dissolution. In this study, bone marrow‐derived rat MSCs were encapsulated in alginate capsules to compare the cardioprotective effects of encapsulated and free MSCs in the rat model of MI. Both functional and histological criteria were used to evaluate the effect of encapsulated/free MSCs on postinfarct LV remodelling. In addition, we specifically addressed the issue of arrhythmia incidence in non‐infarcted animals treated with either encapsulated or bare MSCs. The data obtained indicate that secretion of soluble molecules is the only mechanism involved in the protective effect exerted by MSCs in the setting of MI.
2. MATERIALS AND METHODS
2.1. Animals
Male Wistar rats (Pushchino, Russia) weighing 200‐250 g were used for the experiments. The animals were fed regular chow, and water was available to them ad libitum. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2. Ethical approval
This study was approved by the local Ethics Committee of Almazov National Medical Research Centre.
2.3. Isolation and culture of rat MSC
Mesenchymal stem cells were isolated from the bone marrow of male Wistar rats. The bone marrow was extruded from tibia, humerus and femur bones by flushing the cavity of the bones with phosphate‐buffered saline. The bone marrow was dissociated in cell culture media consisting of α Eagle's minimal essential medium (α‐MEM) (PanEco, St. Petersburg, Russia) supplemented with 10% foetal bovine serum (HyClone, Waltham, MA), L‐glutamine and antibiotics, and cultured in T75 flasks in CO2 incubator in standard conditions. Non‐adherent haemopoietic cells were removed. Culture medium was changed every 5 days.
2.4. Characterization of MSCs
The immunophenotype of rat MSCs was evaluated by flow cytometry analysis performed on CytoFlex (Beckman Coulter). Cells were resuspended in 100 μL of PBS containing 1% of bovine serum albumin (Sigma‐Aldrich, Saint Luis, MO), incubated for 20 minutes at 20°C in the darkness with the following monoclonal antibodies: anti‐CD90, anti‐CD44, anti‐CD31, anti‐CD34, anti‐CD45 and anti‐vimentin (Bioss). Unstained cells of corresponding control isotype antibodies were used as a negative control. A threshold was set to a forward‐scatter (FSC) parameter to exclude cell debris. The SSC (side‐scatter) and FSC settings were done with logarithmic amplification scale as well as fluorescence channels and dot plot analysis. 10 000 of target events were analysed. For data analysis, Kaluza 2.0 software (Beckman Coulter) was used.
2.5. Microencapsulation of MSCs
Mesenchymal stem cells were encapsulated in ultrapure sodium alginate (Sigma‐Aldrich, St. Louis, MO), using commercially available electrostatic encapsulator (B‐390; Buchi Labortechnik AG, Flawil, Switzerland). The suspension containing 2 × 106 MSCs in 1 mL of 1% sodium alginate solution was loaded in the encapsulator cuvette. Encapsulation was performed using the following parameters: frequency of vibration—3000 Hz, voltage—2 kV, pressure—450‐500 mBar and nozzle diameter—120 μm. Microencapsulated cells were sprayed in a solution containing 50 mmol/L CaCl2 and 50 mmol/L BaCl2 under continuous stirring. Encapsulated MSCs were incubated in the solution for 10 min, followed by washing in 0.9% saline. Prior to encapsulation, MSCs were stained with 4’, 6‐diamidino‐2‐phenylindole (Sigma‐Aldrich, St. Louis, MO). The number of MSC per capsule was calculated by confocal fluorescence microscopy (Leica TCS SP5; Leica Microsystems, Wetzlar, Germany) at λ = 405 nm. Leica Application Suite Advanced Fluorescence (LAS AF; Leica Microsystems) software was used to analyse images.
2.6. Assessment of MSC viability
Viability of encapsulated MSCs was measured using LIVE/DEAD Cell Imaging Kit (Thermo Fisher Scientific, Waltham, MA) according to manufacturer's instruction. The numbers of calcein‐AM‐stained viable cells and ethidium homodimer‐stained dead cells were calculated 30 minutes, 5 and 21 days after encapsulation using serial z‐stacked images obtained by confocal fluorescent microscopy (Leica TCS SP5, Leica Microsystems, Germany) at 25‐μm intervals.
2.7. In vitro capsule degradation testing
Degradation of alginate microcapsules was assessed on days 5, 15 and 21 of their incubation in phosphate‐buffered saline. Their diameter and density, and sharpness of the margins, were determined under a light microscope (Axiostar Plus; Carl Zeiss, Oberkochen, Germany) at 40× and 100× magnification. Twenty capsules were analysed per time point.
2.8. Analysis of transforming growth factor‐β1 (TGF‐β1) secretion by encapsulated MSC
To ascertain that the encapsulation procedure did not interfere with the secretory ability of MSCs, the concentration of TGF‐β1 was determined using the TGF‐β1 ELISA kit (Life Technologies), in the media collected from encapsulated and free MSC. Optical density readings were recorded using a microplate reader (Model 680; Bio‐Rad Laboratories, Hercules, CA).
2.9. Arrhythmia registration and quantification in the animals treated with encapsulated or free MSCs
Male Wistar rats were used for evaluation of arrhythmic risk occurring after intramyocardial injection of encapsulated or free MSCs. The animals were anesthetized with sodium pentobarbital (60 mg/kg), followed by implantation of radiotelemetry probe (ADInstruments Ltd., Australia) in the abdominal cavity, with one electrode fixed to the abdominal muscles and another one to the subcutaneous fascia of the right side of the thorax. The animals were allowed to recover for 7 days, re‐anesthetized, mechanically ventilated and randomly allocated into one of the following three groups: (i) sham‐operated (Sham, n = 3): after left thoracotomy, a total 0.1 mL of 0.9% saline was injected into three sites of anterior LV wall; (ii) MSC (n = 3): free MSCs at a dose of 2 × 106 and a volume of 0.1 mL were administered at three sites of anterior LV wall; (iii) encapsulated MSC (eMSC, n = 3): encapsulated MSCs at a dose of 2 × 106 and a volume of 0.1 mL were administered in the same way as in the previous group. The wound was closed in layers, and the animals were allowed to recover. Electrocardiogram (ECG) was regularly registered for 1 hours on the 1st, 7th and 21st postoperative day, using the LabChart software (ADInstruments Ltd.). Arrhythmias were quantified according to the Lambeth Conventions guidelines.25
2.10. Rat model of MI
Male Wistar rats (n = 39) were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneally), intubated and ventilated (SAR‐830P; CWE, Inc., Ardmore, PA), using room air, with a tidal volume of 2 mL/100 g and a rate of 60 breaths per minutes. The core body temperature was maintained at 37.0 ± 0.5°C by a feedback‐controlled heating pad (TCAT‐2LV controller; Physitemp Instruments Inc., Clifton, NJ). ECG was monitored for the registration of heart rate (HR) and arrhythmias. After 10 minutes of stabilization, a left thoracotomy was performed. A 6‐0 polypropylene thread was placed around a prominent branch of the left coronary artery and ligated. Myocardial ischaemia was verified by visual inspection of the anterior surface of the heart and ST‐segment elevation on ECG.
2.11. Experimental protocol
The animals were randomized into the following five groups (Figure 1):
Figure 1.
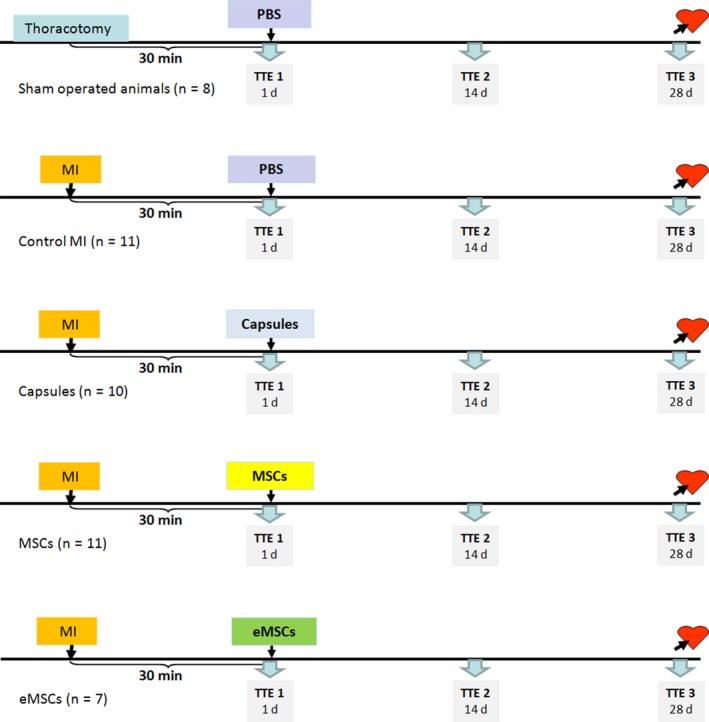
Experimental protocol of the main experimental series aimed at comparison of the cardioreparative effect of encapsulated and free mesenchymal stem cells in the rat model of myocardial infarction. See text for details. Sham, sham‐operated animals; MI, myocardial infarction; MSC, mesenchymal stem cells; eMSC, encapsulated mesenchymal stem cells; TTE, transthoracic echocardiography; PBS, phosphate‐buffered saline [Colour figure can be viewed at wileyonlinelibrary.com]
Sham‐operated animals (Sham, n = 8): thoracotomy was performed without coronary ligation. Phosphate‐buffered saline was injected in the myocardium. The inner diameter of injection needle was 0.3 mm (30G). The needle was inserted at an acute angle to the myocardial surface, which prevented microcapsules from entering the LV cavity. The total volume of injection was 0.1 mL for each animal regardless of the group.
Control MI (n = 11): phosphate‐buffered saline was injected in the myocardium 30 minutes after coronary ligation;
Empty capsule (Capsule, n = 10): alginate capsule without cells suspended in phosphate‐buffered saline were administered 30 minutes postischaemia;
Mesenchymal stem cells (n = 11): free 2 × 106 MCSs in buffer were administered in the peri‐infarct area 30 minutes after coronary ligation;
Encapsulated MSC (eMSC, n = 7): 2 × 106 of encapsulated MCSs suspended in phosphate‐buffered saline were administered in the peri‐infarct area 30 minutes after coronary ligation.
After intramyocardial injections, the wound was closed, and the animals were allowed to recover for 28 days.
2.12. Assessment of LV function by transthoracic echocardiography (TTE)
Transthoracic echocardiography was performed in all animals immediately after intramyocardial injections, 14 and 28 days after surgery. The procedure was performed by an experienced person who was blinded to the experimental groups. Echocardiograms were obtained with a commercially available system (Vevo 2100; Fujifilm VisualSonics, Toronto, Canada). After sedating the animals, LV end‐diastolic (LVEDD) and end‐systolic (LVESD) diameters were measured with M‐mode tracings between the anterior and posterior walls from the short‐axis view. Fractional shortening (FS) was calculated as [LVEDD − LVESD/LVEDD] × 100 (%).
2.13. Histological analysis
On day 28 after surgery, the animals were euthanized by asphyxiation in a CO2 chamber, and heart samples were collected for histological examination. Briefly, the hearts were fixed in 10% buffered formaldehyde solution. Subsequently, the hearts were embedded in paraffin, cut into three transverse slices from apex to base and stained with haematoxylin and eosin and Picro‐Sirius red. The slides were analysed under a light microscope (Axiostar Plus) by a pathologist blinded to the treatments used in the groups, and the following parameters were calculated using a commercially available software (VideoTest Morphology; VideoTest, St. Petersburg, Russia): scar size expressed as a percentage of scar area to the total LV wall area (%); scar thickness (mm); scar area (mm2); total LV wall area (mm2); interventricular septum thickness (mm); LV hypertrophy index expressed as a ratio of interventricular septum thickness to scar thickness; and index of dilatation expressed as a ratio of LV cavity area to the total LV wall area (%). In sham‐operated animals, the thickness of LV anterior wall was measured instead of scar thickness.
2.14. Statistics
Statistical analysis was performed using the SPSS 11.0 (SPSS Inc. Software, Chicago, IL). Myocardial scar size (%) on day 28 post‐MI has been selected as primary endpoint of the study. The sample size per group was determined using the following parameters: SD value (±2%) on the basis of previous similar study,26 desired confidence level (95%), statistical power (0.9) and effect size (3.5) calculated according to J. Cohen formula (free hMSC vs encapsulated hMSC) (Power & Sample Size Program, version 3.1.6). According to the results of sample size calculation, n = 6 per group has been found to be sufficient for making conclusions. The Kruskal‐Wallis test was performed to determine differences in outcomes, followed by pairwise inter‐group comparisons by non‐parametric Mann‐Whitney U test. The differences in the number of arrhythmia episodes per animal and total duration of arrhythmias were analysed non‐parametrically by Kruskal‐Wallis test. P values ≤ 0.05 were considered to indicate statistical significance. All data are expressed as mean ± standard deviation. FS values are presented as box plots with median and quartiles as well as whiskers to express minimum and maximum values.
3. RESULTS
3.1. Characterization of MSCs
Primary cultures of rat MSCs were established and characterized as described in the Materials and Methods section. FACS analysis had confirmed a characteristic immunophenotype of MSC populations (CD45‐/CD31low/Vimentin+/CD44+/CD90+) (Figure 2).
Figure 2.
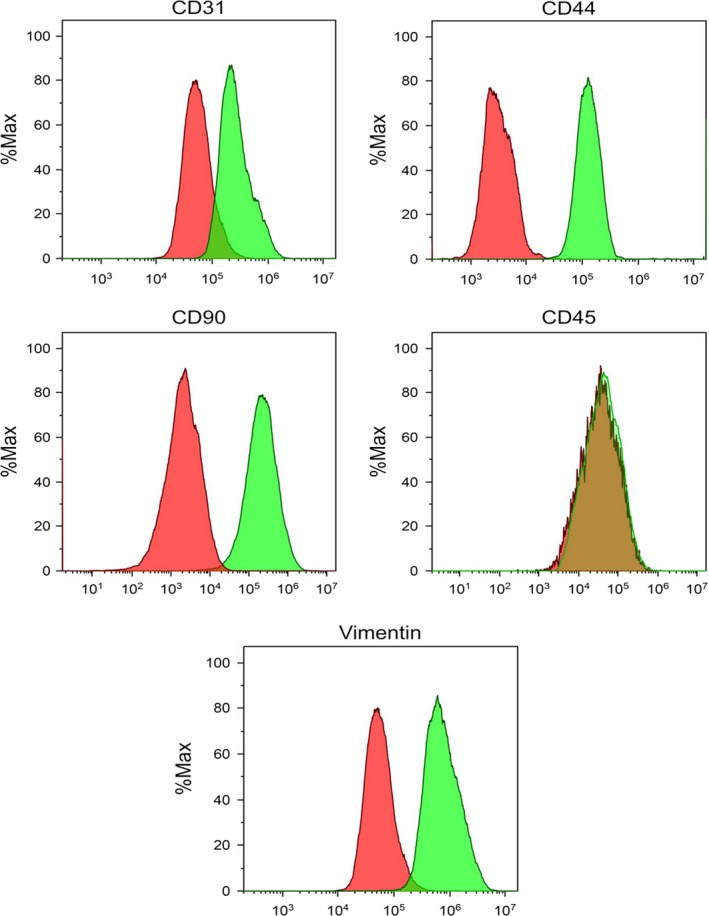
The immunophenotype of rat mesenchymal stem cells (MSC). MSCs used in experiments were screened for the expression of the indicated markers by flow cytometry. Histograms show expression of the indicated markers within a representative MSC population. Red lines indicate isotype controls, and green lines indicate labelled MSC [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Characteristics of alginate capsules and encapsulated MSC
Alginate capsules were designed to support MSC viability and secretory phenotype during the first 5‐10 days after intramyocardial transplantation (Figure 3A). Encapsulated viable cells were mechanically isolated from host cells, preventing the migration of MSCs, their integration into the recipient tissue and possible differentiation. Mesenchymal stem cells were encapsulated in spherical alginate microcapsules with a mean diameter of 225 ± 25 μm (Figure 3B). Confocal fluorescent microscopy showed that each microcapsule contained 35 ± 2 MSC. Stability analysis showed that microcapsules in phosphate‐buffered saline became slightly swollen on day 10, partially degraded on day 15 and completely degraded on day 21 (Figure 3B). In vitro viability testing using the LIVE/DEAD Cell Imaging Kit revealed that 76 ± 6% and 68 ± 9% of MSCs were viable 30 minutes and 5 days postencapsulation respectively (Figure 3C,D). Only 3 ± 2% of MSC remained viable on day 21 after encapsulation (Figure 3C,D).
Figure 3.
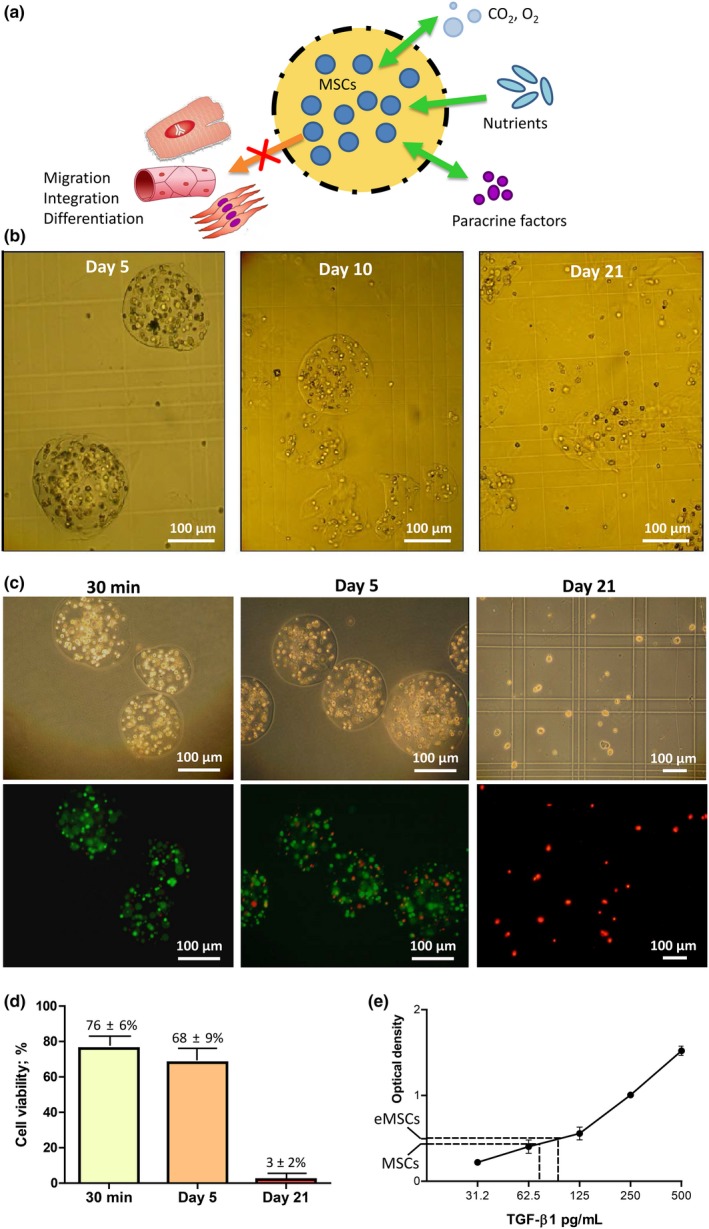
Experimental design and characteristics of alginate microcapsules and encapsulated mesenchymal stem cells. A, Schematic representation of encapsulation strategy. Rat bone marrow‐derived mesenchymal stem cells were encapsulated in alginate microcapsules, freely permeable to gases, nutrients, growth factors and cytokines. Encapsulation prevents viable cell release from the matrix, excluding putative protective effects related to cell migration, integration in the host tissue and transdifferentiation in myocardial cells. B, Light microscopy images of encapsulated cells incubated in phosphate‐buffered saline for 5, 10 and 21 days. C, Representative images of LIVE/DEAD cell staining 30 minutes and 5 and 21 days after encapsulation. Upper and lower panels show light and confocal microscopy images, respectively. D, Quantification of viable cell percentage in the corresponding time points. E, Enzyme‐linked immunosorbent assay (ELISA) analysis for transforming growth factor‐β1 in the media obtained from bare and encapsulated mesenchymal stem cells on day 5 postencapsulation [Colour figure can be viewed at wileyonlinelibrary.com]
The levels of TGF‐β1 in the media collected from encapsulated and intact MSCs on day 5 were 93 ± 7 pg/mL and 74 ± 9 pg/mL, respectively, indicating that encapsulation procedure had no negative impact on the secretory ability of MSC (Figure 3E).
3.3. Arrhythmia incidence after intramyocardial injection of encapsulated or free MSCs in non‐infarcted animals
Life‐threatening arrhythmias such as ventricular tachycardia or ventricular fibrillation were not registered throughout the experiments. In all groups, the total number of arrhythmias was considerably higher on day 1 post‐transplantation compared to those on days 7 and 21 (Figure 4). There were no inter‐group differences in arrhythmia incidence.
Figure 4.

Arrhythmia incidence in non‐infarcted animals treated with free or encapsulated mesenchymal stem cells. Total number of arrhythmia episodes per 1 hour registered on days 1, 7 and 21 postoperatively. Arrhythmia incidence was significantly higher on day 1 after intramyocardial injection in all groups vs those on days 7 and 21 [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Post‐MI LV function in free MSC‐treated and encapsulated MSC‐treated animals
Left coronary artery ligation resulted in significant (P < 0.001) FS decrease (Figure 5B) in all groups compared to that in sham‐operated animals. In addition, there were no differences in FS between the groups subjected to MI. Two weeks after MI, FS was significantly higher in the MSCs and eMCS groups (24.8 ± 7.95% and 19.4 ± 4.12% respectively) than that in the Control MI and Capsule groups (14.1 ± 4.67% and 15.7 ± 3.46%, respectively, P < 0.05, Figure 5C). On day 28 post‐MI, FS remained significantly higher in the MSCs and eMCS groups (24.4 ± 4.78% and 20.8 ± 5.08% respectively) than in the Control MI and Capsule groups (13.4 ± 3.06% and 13.2 ± 3.28%, respectively, P < 0.05, Figure 5D). The overall dynamics of FS in the experimental groups over the period of 1‐28 days after MI are shown in Figure 5E. It was evident that transplantation of both free and encapsulated MSC resulted in significant amelioration of LV function compared to that in the control animals and the animals treated with empty capsule. FS values were not different between the MSCs and eMSC groups.
Figure 5.
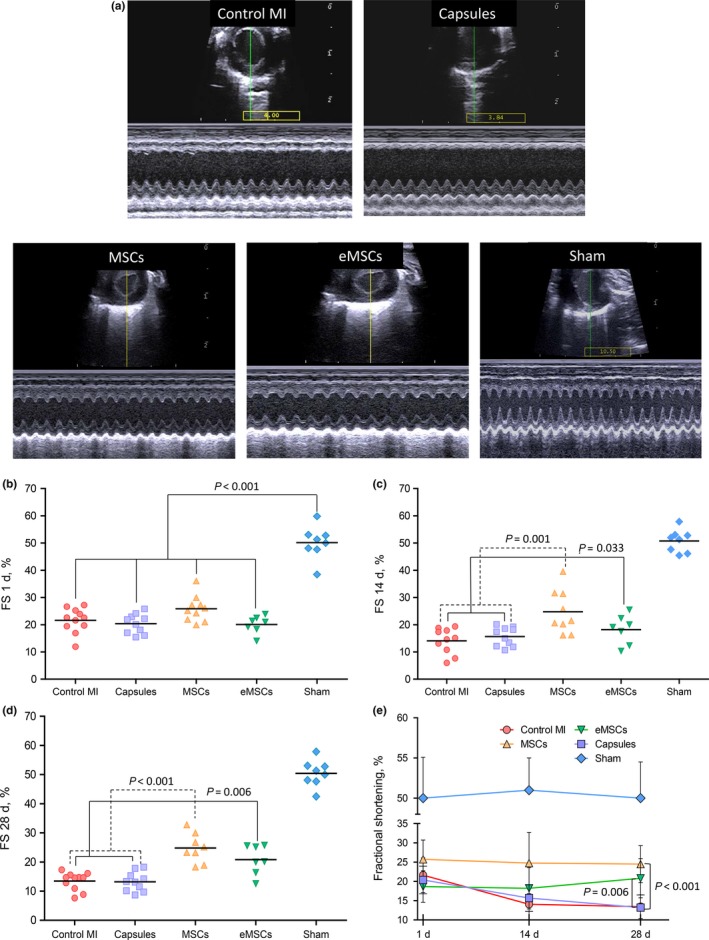
Echocardiographic characteristics of left ventricular function. A, Representative two‐dimensional echocardiography‐guided M‐mode images in parasternal short‐axis (PSAX) position obtained at day 28 postoperatively. B, Fractional shortening (FS, %) as registered immediately after intramyocardial injections. A significant decrease in FS was registered in all groups compared to sham‐operated animals (Sham). C, FS at day 14 after surgery was significantly higher in the mesenchymal stem cells (MSCs) and encapsulated MSC (eMSC) groups compared to those in the Control MI and Capsule groups. D, FS on day 28 after surgery remained significantly higher in the MSCs and eMSC groups compared to those in the Control MI and Capsule groups. E, Dynamic changes in FS over the period of 28 days after surgery [Colour figure can be viewed at wileyonlinelibrary.com]
3.5. Effects of bare and encapsulated MSCs on scar size and LV remodelling
Histological parameters describing postinfarct scar and LV remodelling are presented in Table 1. Intramyocardial transplantation of MSCs and encapsulated MSC resulted in significant reduction in both scar size and scar area compared to those in the Control MI (Table 1, Figure 6). Scar size and scar area were not different between the MSCs and eMSC groups. The administration of empty alginate microcapsules had no effect on myocardial histological parameters. Parameters such as total LV wall area, LV hypertrophy index and index of dilatation increased in all groups compared to those in the sham‐operated group. No other inter‐group differences were found. On day 28, histopathological examination has not revealed any signs of chronic inflammation in the myocardium adjacent to the sites of microcapsule or MSC injection.
Table 1.
Histological parameters of postinfarct scar and cardiac remodelling
| Parameter | Sham | Control MI | Capsule | MSC | eMSC |
|---|---|---|---|---|---|
| Scar size (%) | ‐ | 21.7 ± 6.88 | 21,9 ± 6,12 | 12.1 ± 6.83a | 14.7 ± 4.26a |
| Scar area (mm2) | ‐ | 20.2 ± 6.39 | 21.5 ± 5.87 | 12.2 ± 6.63a | 15.3 ± 5.42a |
| Total LV wall area (mm2) | 79.1 ± 5.36 | 93.7 ± 10.71b | 91.9 ± 9.66b | 103.9 ± 11.97b | 102.6 ± 12.23b |
| Scar thickness/LV anterior wall thickness (mm) | 1.7 ± 0.12 | 1.2 ± 0.29b | 1.2 ± 0.23b | 1.4 ± 0.37b | 1.2 ± 0.36b |
| Interventricular septum thickness (mm) | 1.8 ± 0.04 | 1.7 ± 0.37 | 1.8 ± 0.29 | 1.9 ± 0.24 | 1.9 ± 0.39 |
| LV hypertrophy index | 1.0 ± 0.05 | 1.5 ± 0.44b | 1.5 ± 0.47b | 1.5 ± 0.42b | 1.8 ± 0.93b |
| Index of dilatation (%) | 15.6 ± 1.34 | 31.5 ± 11.09b | 29.1 ± 7.82b | 25.1 ± 4.58b | 24.0 ± 2.04b |
MI, myocardial infarction; LV, left ventricular; MSC, mesenchymal stem cells; eMSC, encapsulated mesenchymal stem cells.
P < 0.05 vs Control MI.
P < 0.05 vs Sham.
Figure 6.
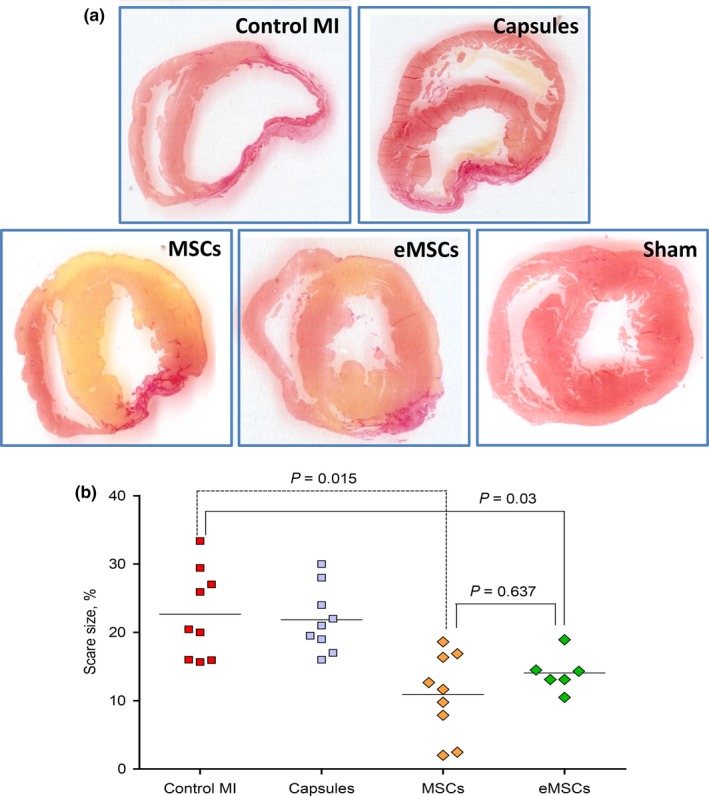
Scar size in different experimental groups. A, Representative Picro‐Sirius red‐stained cross‐sections of the heart from different groups. B, Quantitative values of scar size defined as a percentage of scar area to the total left ventricular wall area. The data are expressed as dot plots with median values. Scar size was significantly smaller in the mesenchymal stem cells (MSCs) and encapsulated MSC (eMSC) groups compared to those in the Control MI and Capsule groups [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The major finding of this study is that intramyocardial administration of free MSCs in the rat model of MI had a cardioprotective effect similar to that of dose‐matched MSC encapsulated in alginate microcapsules, preventing viable cell release while supporting their secretory phenotype during the acute stage of MI. These data provided strong evidence that MSC‐derived growth factors and cytokines are crucial for cardiac protection elicited by MSC transplantation in the peri‐infarct area.
Notably, a greater cardioprotective effect of encapsulated stem cells compared to that of free cells was documented in several studies using different types and sources of stem cells, animal species and experimental endpoints.19, 20, 26 In particular, Levit et al embedded either free or alginate‐encapsulated human bone marrow‐derived MSCs in a hydrogel patch, which was attached to the rat heart surface after permanent coronary ligation.26 Increased LV function and reduced scar size were noted only in the animals treated with encapsulated MSCs, which were associated with better retention and survival of transplanted cells. Blocki et al showed that intramyocardial transplantation of MSC encapsulated in agarose supplemented with collagen type I, fibrin and dextran sulphate resulted in increased myocardial cell retention compared to bare MSCs.18 In another study, human adipose tissue MSCs were encapsulated in genipin‐cross‐linked alginate‐chitosan microcapsules and transplanted to the infarcted rats.27 Administration of microencapsulated cells resulted in improved cell retention, decreased scar size and augmented LV function compared to free cells. In all of the above‐mentioned studies, stem cells have been encapsulated in semi‐permeable material in order to protect them from various injurious factors present in the recipient heart during the early postimplantation period. The characteristics of hydrogels and cross‐linking agents have been selected to ensure gradual release of viable cells from the eroding capsule, followed by their migration and integration in the host tissue. In contrast with this approach, the present study used encapsulation resulting in death of practically all embedded cells prior to capsule degradation. Using this protocol, we intended to estimate the relative contribution of two major mechanisms: (a) paracrine signalling through secreted growth factors/cytokines and (b) integration of stem cells in the host myocardium with transdifferentiation into cardiac cells for MSC‐mediated cardiac protection. Assuming that MSC differentiation contributes to the overall cardioprotective effect of MSCs, the encapsulated cells would be less efficacious in terms of scar reduction and functional improvement. However, our results showed no difference in efficacy between non‐encapsulated and encapsulated cells, suggesting that differentiation of MSCs in cardiac cells had minimal, if any, contribution to MSC‐mediated anti‐remodelling effect. This finding is in line with the results of Meier et al's study on the mechanisms of anti‐fibrotic effect of MSCs in murine models of liver fibrosis.28 To address the problem, MSC encapsulation into alginate‐polyethylene glycol microspheres was used to avoid direct contact between transplanted and recipient cells, allowing the analysis of solely the effects of soluble factors released by MSCs. With this approach, it was concluded that secreted factors play a key role in both anti‐fibrotic and anti‐inflammatory effects of MSCs.
The results here obtained may have important clinical implications. The use of living cells for treatment of MI is a complicated and expensive procedure, which is also associated with certain risks of the patients. The consistency of our data with the results of other experimental studies on the major role of MSC secretome components in cardiac protection further supports the hypothesis that the use of live, though encapsulated, cells for MI therapy may be replaced with heart‐targeted delivery of certain growth factors and cytokines. To date, MSC secretome analysis has identified >200 unique proteins.29 Therapeutic use of some growth factors from MSCs such as vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF‐2) was promising in preclinical models of MI 30, 31 but was not associated with sustained benefits in randomized clinical trials.32, 33 Several reasons accounted for the negative results of clinical trials. First, the instability of systemically administered protein in the circulation results in a short‐term effect. Second, angiogenic factors may cause the formation of capillary‐like structures, which lack maturity because of the transient nature of the stimulus. Third, VEGF and FGF‐2 may have serious side effects when used at the doses required for infarct size limitation. At least in part, these limitations could be circumvented by the use of locally released growth factors.34 Hydrogels such as alginate and gelatine are successfully used for sustained delivery of several growth factors with different release kinetics.35, 36, 37 Hao et al studied the effects of VEGF‐ and platelet‐derived growth factor (PDGF)‐loaded alginate hydrogel in rats with MI.36 They showed that intramyocardial injection of hydrogel incorporating both growth factors resulted in greater angiogenic effect and better LV function than the administration of hydrogels selectively loaded with VEGF or PDGF. Ruvinov et al tested alginate hydrogel sequentially releasing insulin‐like growth factor‐1 (IGF‐1) and hepatocyte growth factor (HGF).37 Animal experiments demonstrated increased scar thickness, decreased apoptosis and enhanced angiogenesis in the animals treated with growth factor‐loaded hydrogel compared to those in animals treated with growth factors in saline. Similar results were obtained with albumin‐alginate microcapsules that sequentially released FGF‐2 and HGF.35 Finally, subcutaneous implantation of alginate scaffold incorporating three growth factors (VEGF, PDGF and TGF‐β1) resulted in the maximal vascularization in the rat model.38 Despite the positive effects observed, further improvement of this approach by optimization of parameters such as growth factor immobilization technique and its release kinetics is required before clinical trials can be contemplated.
An additional goal of this study was to evaluate the arrhythmia incidence in the animals treated with intramyocardial injections of free or encapsulated MSCs. It is generally known that myocardial transplantation of certain types of stem cells (eg skeletal myoblasts) increases the incidence of arrhythmias, resulting in increased risk/benefit ratio.39 The data on the putative proarrhythmic effects of MSC transplantation are contradictory. Some studies demonstrated that MSCs could elicit arrhythmias in vitro 40 and in vivo, 41 while others documented decreased severity of postinfarct ventricular arrhythmias after MSC administration.42 In this study, intramyocardial transplantation of either free or encapsulated MSCs did not increase the number of cardiac arrhythmias in non‐infarcted rats compared to sham operation, although the surgery itself caused increased arrhythmia incidence during the first postoperative day.
The present study has several limitations. Cell retention in the myocardium was not measured, because we aimed to analyse the final effect of free and encapsulated cells administered at a dose previously shown to elicit maximal possible infarct‐limiting effect in a similar model.13 The maintenance of MSC secretory phenotype was confirmed only by the determination of the levels of TGF‐β1, which we considered sufficient to prove the fact that encapsulated MSC retained the ability to produce key secretome components.
5. CONCLUSION
In conclusion, we developed an encapsulation protocol preventing the integration of MSCs in the host myocardium. This approach was used as a tool to investigate the mechanisms of MSC‐mediated cardiac protection. It was demonstrated that the secretion of paracrine factors represented the only mechanism of cell‐based MI therapy with MSCs. These data may contribute to a more frequent use of growth factor combinations proved to be optimal for cardiac protection after irreversible ischaemic injury.
CONFLICT OF INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Supporting information
ACKNOWLEDGEMENTS
This study was supported by Government of Russian Federation (Grant 074‐U01) and Russian Foundation for Basic Research (Grant 18‐29‐02057 mk).
Karpov AA, Puzanov MV, Ivkin DY, et al. Non‐inferiority of microencapsulated mesenchymal stem cells to free cells in cardiac repair after myocardial infarction: A rationale for using paracrine factor(s) instead of cells. Int. J. Exp. Path. 2019;100:102–113. 10.1111/iep.12312
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nigro P, Bassetti B, Cavallotti L, et al. Cell therapy for heart disease after 15 years: unmet expectations. Pharmacol Res. 2018;127:77‐91. [DOI] [PubMed] [Google Scholar]
- 3. Nunez Garcia A, Sanz‐Ruiz R, Fernandez Santos ME, Fernandez‐Aviles F. “Second‐generation” stem cells for cardiac repair. World J Stem Cells. 2015;7:352‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karpov AA, Uspenskaya YK, Minasian SM, et al. The effect of bone marrow‐ and adipose tissue‐derived mesenchymal stem cell transplantation on myocardial remodelling in the rat model of ischaemic heart failure. Int J Exp Pathol. 2013;94:169‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9‐20. [DOI] [PubMed] [Google Scholar]
- 6. Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010‐2015). Stem Cell Res Ther. 2016;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afzal MR, Samanta A, Shah ZI, et al. Adult bone marrow cell therapy for ischemic heart disease: evidence and insights from randomized controlled trials. Circ Res. 2015;117:558‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Jong R, Houtgraaf JH, Samiei S, et al. Intracoronary stem cell infusion after acute myocardial infarction: a meta‐analysis and update on clinical trials. Circulation. 2014;7:156‐167. [DOI] [PubMed] [Google Scholar]
- 9. Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134‐2139. [DOI] [PubMed] [Google Scholar]
- 10. Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198‐2202. [DOI] [PubMed] [Google Scholar]
- 11. Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150‐I156. [DOI] [PubMed] [Google Scholar]
- 12. Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114‐1122. [DOI] [PubMed] [Google Scholar]
- 13. Paul A, Ge Y, Prakash S, Shum‐Tim D. Microencapsulated stem cells for tissue repairing: implications in cell‐based myocardial therapy. Regen Med. 2009;4:733‐745. [DOI] [PubMed] [Google Scholar]
- 14. Al Kindi AH, Asenjo JF, Ge Y, et al. Microencapsulation to reduce mechanical loss of microspheres: implications in myocardial cell therapy. Eur J Cardiothorac Surg. 2011;39:241‐247. [DOI] [PubMed] [Google Scholar]
- 15. Karpov AA, Udalova DV, Pliss MG, Galagudza MM. Can the outcomes of mesenchymal stem cell‐based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif. 2017;50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maureira P, Marie PY, Yu F, et al. Repairing chronic myocardial infarction with autologous mesenchymal stem cells engineered tissue in rat promotes angiogenesis and limits ventricular remodeling. J Biomed Sci. 2012;19:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei HJ, Chen CH, Lee WY, et al. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29:3547‐3556. [DOI] [PubMed] [Google Scholar]
- 18. Blocki A, Beyer S, Dewavrin JY, et al. Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long‐term retention of MSCs in infarcted myocardium. Biomaterials. 2015;53:12‐24. [DOI] [PubMed] [Google Scholar]
- 19. Ban K, Park HJ, Kim S, et al. Cell therapy with embryonic stem cell‐derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair. ACS Nano. 2014;8:10815‐10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mayfield AE, Tilokee EL, Latham N, et al. The effect of encapsulation of cardiac stem cells within matrix‐enriched hydrogel capsules on cell survival, post‐ischemic cell retention and cardiac function. Biomaterials. 2014;35:133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150‐156. [DOI] [PubMed] [Google Scholar]
- 23. Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93‐98. [DOI] [PubMed] [Google Scholar]
- 24. Yang W, Zheng H, Wang Y, et al. Nesprin‐1 has key roles in the process of mesenchymal stem cell differentiation into cardiomyocyte‐like cells in vivo and in vitro. Mol Med Rep. 2015;11:133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curtis MJ, Hancox JC, Farkas A, et al. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther. 2013;139:213‐248. [DOI] [PubMed] [Google Scholar]
- 26. Levit RD, Landazuri N, Phelps EA, et al. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. 2013;2:e000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paul A, Chen G, Khan A, et al. Genipin‐cross‐linked microencapsulated human adipose stem cells augment transplant retention resulting in attenuation of chronically infarcted rat heart fibrosis and cardiac dysfunction. Cell Transplant. 2012;21:2735‐2751. [DOI] [PubMed] [Google Scholar]
- 28. Meier RP, Mahou R, Morel P, et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol. 2015;62:634‐641. [DOI] [PubMed] [Google Scholar]
- 29. Ge L, Jiang M, Duan D, et al. Secretome of olfactory mucosa mesenchymal stem cell, a multiple potential stem cell. Stem Cells Int. 2016;2016:1243659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopez JJ, Laham RJ, Stamler A, et al. VEGF administration in chronic myocardial ischemia in pigs. Cardiovasc Res. 1998;40:272‐281. [DOI] [PubMed] [Google Scholar]
- 31. Virag JA, Rolle ML, Reece J, et al. Fibroblast growth factor‐2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol. 2007;171:1431‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359‐1365. [DOI] [PubMed] [Google Scholar]
- 33. Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor‐2: double‐blind, randomized, controlled clinical trial. Circulation. 2002;105:788‐793. [DOI] [PubMed] [Google Scholar]
- 34. Ruvinov E, Cohen S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv Drug Deliv Rev. 2016;96:54‐76. [DOI] [PubMed] [Google Scholar]
- 35. Banquet S, Gomez E, Nicol L, et al. Arteriogenic therapy by intramyocardial sustained delivery of a novel growth factor combination prevents chronic heart failure. Circulation. 2011;124:1059‐1069. [DOI] [PubMed] [Google Scholar]
- 36. Hao X, Silva EA, Mansson‐Broberg A, et al. Angiogenic effects of sequential release of VEGF‐A165 and PDGF‐BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178‐185. [DOI] [PubMed] [Google Scholar]
- 37. Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF‐1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32:565‐578. [DOI] [PubMed] [Google Scholar]
- 38. Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity‐binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122‐2131. [DOI] [PubMed] [Google Scholar]
- 39. Fernandes S, Amirault JC, Lande G, et al. Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc Res. 2006;69:348‐358. [DOI] [PubMed] [Google Scholar]
- 40. Chang MG, Tung L, Sekar RB, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113:1832‐1841. [DOI] [PubMed] [Google Scholar]
- 41. Fukushima S, Varela‐Carver A, Coppen SR, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254‐2261. [DOI] [PubMed] [Google Scholar]
- 42. Wang D, Zhang F, Shen W, et al. Mesenchymal stem cell injection ameliorates the inducibility of ventricular arrhythmias after myocardial infarction in rats. Int J Cardiol. 2011;152:314‐320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


