Abstract
A series of phosphinate-containing rhodol and fluorescein dyes are disclosed. These new fluorophores increase the color palette of phosphinate-based xanthenes in the far-red spectral region. The new chemical functionality of these scaffolds is leveraged to produce a sensitive, no-wash imaging probe for cellular esterase activity. The reported phosphinate-containing dyes provide platforms for the further development of imaging probes and self-reporting delivery vehicles.
Small molecule-based fluorescent probes represent attractive alternatives to fluorescent proteins for microscopy studies.1–3 Nonetheless, there is a need for the further development of red-shifted, photostable probes in order to enable the further development of robust bioprobes for imaging experiments. Towards this goal, recent efforts have focused on the introduction of heteroatoms into the bridging position of xanthene scaffolds.4–18 Our lab’s approach to this problem involves replacement of the bridging oxygen atom in the rhodamine scaffold with a phosphinate functionality (Fig. 1).19 The resulting Nebraska Red (NR) fluorophores retain the brightness and photostability of the rhodamine scaffold, while displaying >110 nm shifts in excitation and emission. Furthermore, the unique chemistry of the phosphinate functional group allows for the construction of new enzymatic probes,20 self-reporting small molecule delivery platforms,19 and provides inspiration for the development of new approaches for obtaining ratiometric probes.21 Building upon this, we sought to further increase the chemical functionality of NR dyes, through the synthesis and evaluation of rhodol- and fluorescein-based NR derivatives (Fig. 1). Importantly the phenol group present in these rhodol and fluorescein dyes provides a new chemical handle that can be leveraged to construct sensitive, red-shifted enzyme-activated probes for cellular imaging studies.22–25
Fig. 1.
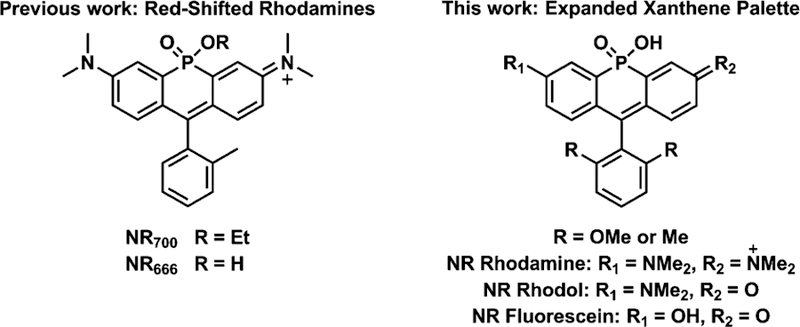
Structures of xanthene-based Nebraska Red family members.
Towards this goal, we first investigated synthetic routes capable of efficiently yielding rhodol and fluorescein NR derivatives. When compared to the synthesis of classical rhodamine dyes, insertion of phosphorous at the bridging position decreases the yield of the resulting fluorophore.9,19,26 Additional synthetic challenges arise in the case of fluorescein (due to the increased electron withdrawing capacity of the phenol group)10 and rhodol (due to the need to construct an asymmetric structure).27,28 During our investigation of different synthetic routes, the Yamaguchi group reported the direct conversion of phosphine oxide-containing rhodamines to rhodol and fluorescein via a hydrolytic deamination reaction under basic conditions (pH > 10).29 As an initial examination of this route in the context of NR dyes, we incubated NR700, a phosphinate ester-based tetramethylrhodamine derivative, with 0.1 M NaOH. After 3 h the products of the reaction were purified by HPLC, yielding the hydrolysis product (NR666) and the corresponding rhodol product (NR630, Fig. S1 and Scheme S1, ESI†). Upon incubation of NR700 with a 1 M solution of NaOH, instead of conversion to fluorescein we observed a rapid decrease in the color of the solution. This is likely due to attack at the C-9 position of the fluorophore as observed previously (Scheme S1, ESI†).29 In support of this hypothesis, we observed a restoration of the solution’s color upon addition of 2 M HCl. Nonetheless, we were very encouraged by the potential of this route to afford rhodol and fluorescein NR derivatives.
In order to protect the C-9 position from nucleophilic attack, we adopted previous strategies for introduction of bulky groups into xanthenes,29,30 incorporating dimethyl or dimethoxy groups at the C-2′ and C-6′ of our NR dyes (Fig. 1). Our previously reported route to NR rhodamine analogues, relied on the condensation of 3-bromo-N,N-dimethylaniline with benzaldehydes19,21 or the use of a xanthone precursor.10,12,19,31 However, as mentioned above, these routes result in relatively low yields of the target fluorophores. Building upon a previously described route for phosphine oxide fluorophores, we found that yields of NR rhodamines could be significantly increased by first treating 3-bromo-N,N-dimethylaniline with sec-butyllithium and exposing the resulting lithiated reagent to ethyl dichlorophosphite.26 Subsequent oxidation with a 50% H2O2 solution yielded the phosphinate-bridged intermediate (2, Scheme 1a). A subsequent Vilsmeier–Haack reaction was used to generate the key intermediate 3. Reaction of 3 with the appropriate lithiated 2,6-disubstituted phenyl reagents (dimethyl or dimethoxy) generated the corresponding secondary alcohol, which was directly treated with 2 M HCl under reflux overnight. Finally, p-chloranil was used to generate the NR rhodamine analogues containing bulky substituents proximal to the C-9 position. Compared to our previously reported routes,19 the newly developed intermediate 3 is accessible in relatively higher yields, leading to a ≥4-fold increase in the yield of NR rhodamines.
Scheme 1.
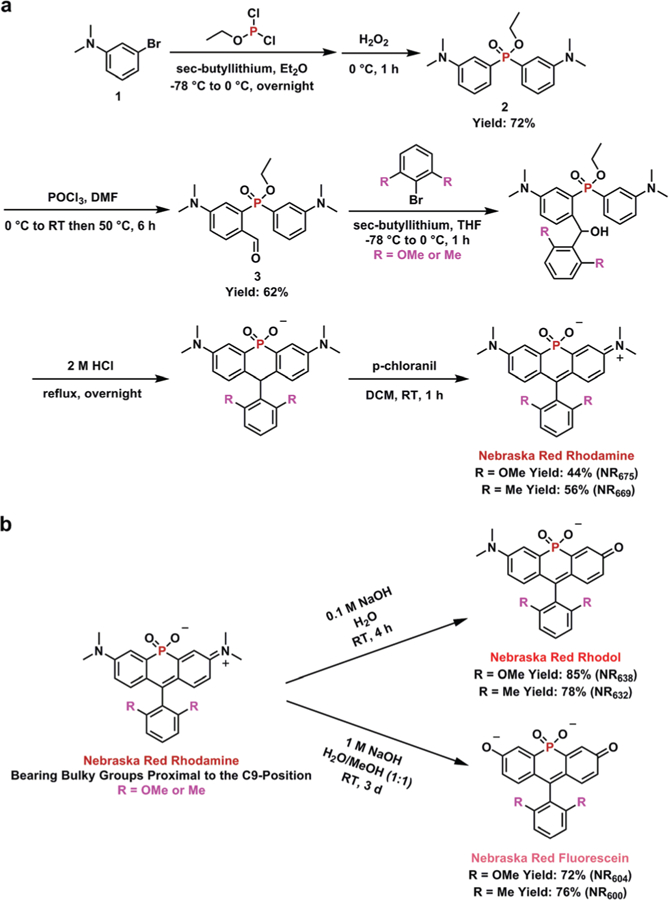
(a) Synthesis of phosphinate-based NR rhodamine analogues. (b) Direct transformation of NR rhodamine to NR rhodol or NR fluorescein.
With the NR rhodamine analogues in-hand, we reassessed the ability to directly generate rhodol and fluorescein derivatives upon treatment with NaOH. Upon exposure to 0.1 M NaOH, the color of the solution quickly changed from green to purple, indicating the generation of NR rhodol (Scheme 1b). Monitoring by HPLC indicated that the reaction was complete after 4 h. As expected, the generation of NR fluorescein required a longer reaction time (3 days) and a higher concentration of base (1 M).29 After purification by HPLC, the structures of all six NR derivatives were confirmed by NMR and mass spectrometry (see ESI†).
The photophysical properties of the NR rhodamine, rhodol, and fluorescein derivatives were assessed in PBS (10 mM, pH = 7.4) (Fig. 2a–c). The NR rhodamine derivatives showed the longest absorption and emission wavelengths, similar to our previously reported analogues.19 However, the NR rhodol and NR fluorescein derivatives displayed 35 and 70 nm blue-shifts, respectively relative to NR rhodamine. Comparison to the spectral properties of Tokyo Green,32 containing oxygen at the bridging position, indicated that introduction of the phosphinate in NR fluorescein resulted in a ≥109 nm red-shift. Among the NR family members, the dimethoxy analogues were slightly red-shifted compared to their dimethyl counterparts, while the dimethyl NR analogues displayed relatively higher brightness (ε × ϕ) with the fluorescein dimethyl derivative (NR600) displaying the overall the highest brightness. These NR dyes displayed blue-shifted excitation and emission maxima as well as increased brightness compared to previously reported phosphine oxide-based fluorophores (Table S1, ESI†).10,29
Fig. 2.
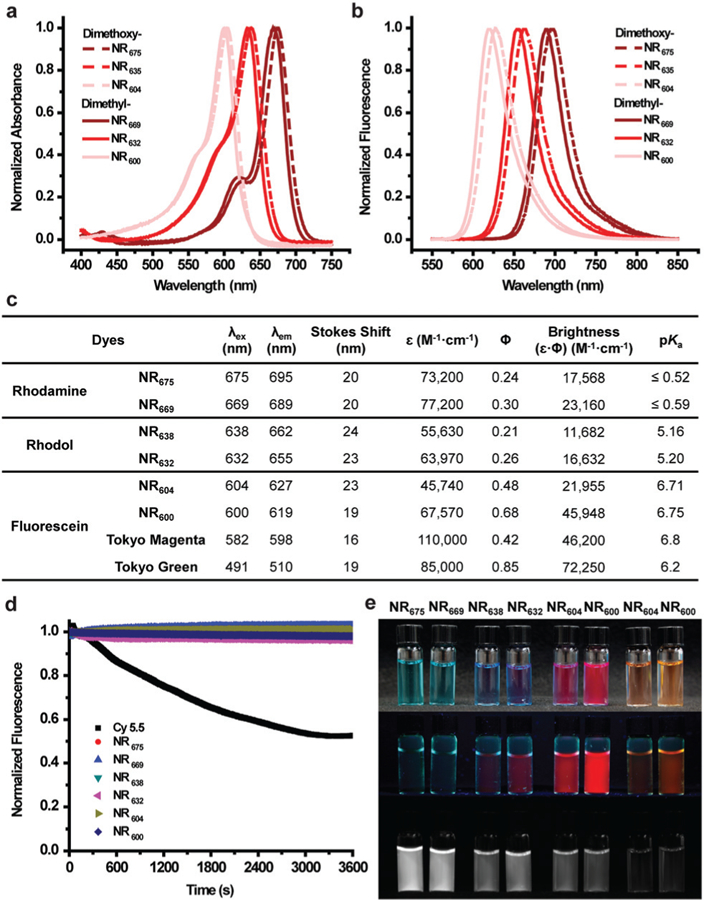
Photophysical properties and pKas of NR family members. All experiments were conducted in PBS buffer (10 mM, pH = 7.4 containing 1% DMSO). (a) Normalized absorption spectra. (b) Normalized emission spectra. (c) Summary of optical parameters including previously reported values for Tokyo Magenta5 and Tokyo Green.32 (d) Photostability in comparison to Cy 5.5. (e) Images corresponding to each dye solution. From top to bottom: white-light image, fluorescent image under a UV lamp (254 nm), and a NIR image with a 680 nm filter. From left to right: NR675, NR669, NR638, NR632, NR604, and NR600 in PBS buffer as well as NR604 and NR600 in buffer at pH = 4.0.
Next, we investigated the equilibrium between protonated and deprotonated forms of the rhodol and fluorescein derivatives. Absorption of the NR rhodol and NR fluorescein dyes at varying pHs was recorded (Fig. S2, ESI†). From this data, the NR rhodol derivatives exhibited a pKa of ~5.2 while the pKas for the NR fluorescein derivatives was ~6.7. Last, the photostability of NR family members was compared to Cy 5.5 using continuous irradiation for 1 h (Fig. 2d). Gratifyingly, all six NR dyes showed virtually no evidence of photobleaching, compared to a 50% decrease in Cy 5.5 fluorescence under the same conditions. Thus, these new NR derivatives provide a robust, tuneable palette of fluorescent reagents with emissions ranging from the far-red to near-infrared (NIR) region (Fig. 2e).
To highlight to utility of these new NR family members, we sought to develop a sensitive bioprobe for imaging enzymatic activity. For this application, we chose the dimethyl phosphinate-based fluorescein NR600, due to its relative brightness. Importantly, previous work has clearly demonstrated the utility of acetoxymethyl (AM) ether groups for masking phenol functionalities within fluorophores.23–25,33 The resulting AM-protected probes display low background fluorescence, high chemical stability, and are readily cleaved by cellular esterases producing a turn-on fluorescent probe for esterase activity. Based on our previous results, we also anticipated that the negatively charged phosphinate of NR600 would prevent the fluorophore from entering cells.19 Thus, we envisioned a diAM-protected version of NR600, termed diAM-NR600 (Fig. 3a). To examine the chemical stability of diAM-NR600, an in vitro assay was conducted by monitoring the fluorescence generated by diAM-NR600 over a period of 1 h (Fig. 3b). In the presence of pig liver esterase (PLE) we observed a clear, 13.8-fold increase in fluorescence that stabilized after 10 min. However, in the presence of PBS alone virtually no increase in fluorescence was observed. Moreover, exposure to BSA or cell culture media containing 10% FBS produced relatively low background fluorescent signal (2.2- and 2.9-fold respectively). These data clearly indicate that diAM-NR600 can act as an esterase substrate, forming NR600 as a fluorescent product.
Fig. 3.
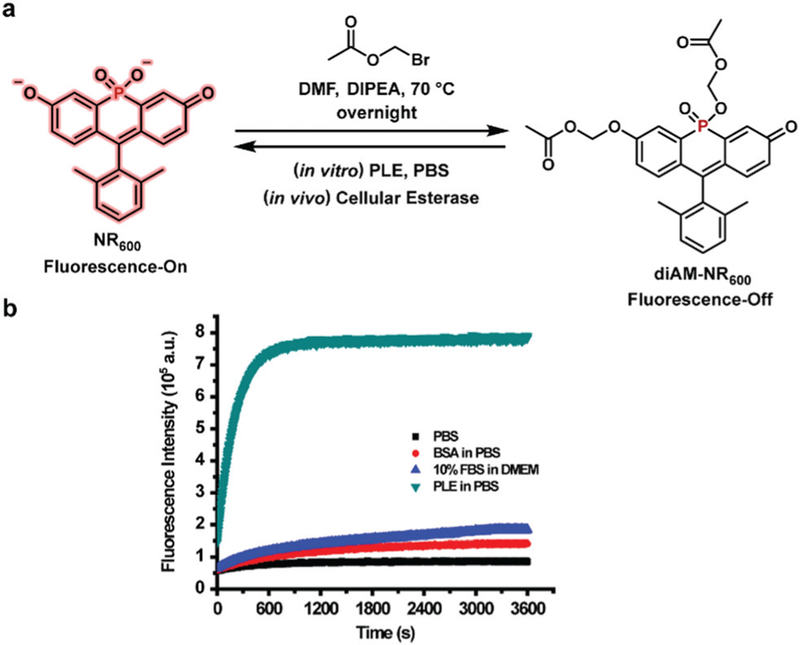
(a) Synthesis of the esterase sensor diAM-NR600. (b) In vitro stability of diAM-NR600 (5 μM) in PBS (10 mM, pH = 7.4) alone or in the presence of BSA (0.2 units) or PLE (0.2 unit). The fluorescence of diAM-NR600 in cell culture media (DMEM with 10% FBS) is also shown. Fluorescence emission at 619 nm was monitored using excitation at 600 nm.
To interrogate the ability of diAM-NR600 to act as a no wash probe for cellular esterase activity, HeLa cells were incubated with the esterase probe and Cell Tracker Green CMFDA, in order to verify localization. Cells were then imaged, without washing, using confocal microscopy (Fig. 4a). Indeed, the formation of NR600 in the cytosol could clearly be observed within cells (Fig. S3a, ESI†), yielding a 10-fold increase in signal over background (Fig. 4b). Similar signal to noise was obtained in HeLa cells that were washed prior to imaging (Fig. S3b, ESI†). In addition, formation of NR600 was also observed in the cytosol of NIH-3T3 and RAW 264.7 cells, indicating the generality of diAM-NR600 (Fig. S4 and S5, ESI†). Decreased nuclear localization of NR600 was consistently observed relative to Cell Tracker Green CMFDA (a fluorescein derivative), indicating a potential difference in the nuclear accumulation of these dyes. Toxicity assays also clearly demonstrated that diAM-NR600 was not toxic at concentrations up to 10 mM (Fig. S6, ESI†). These experiments verify the ability of diAM-NR600 to act as a far-red emitting, no-wash probe for cellular esterase activity.
Fig. 4.
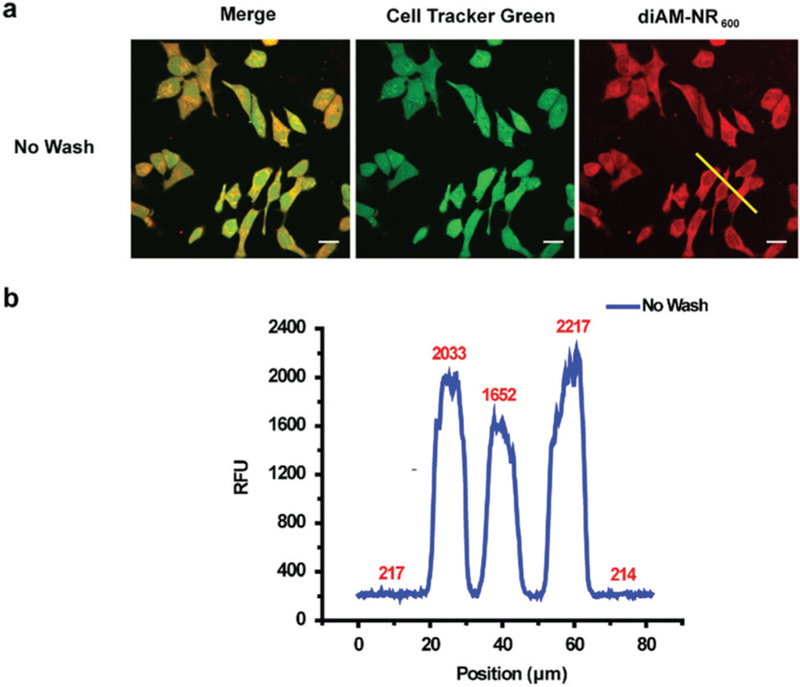
(a) Confocal fluorescence microscopy imaging of HeLa cells incubated with 0.6 mM Cell Tracker Green CMFDA for 20 min followed by 0.6 μM diAM-NR600 for 30 min (Scale bar: 20 mm). (b) Quantification of fluorescence intensity from panel a (yellow line in red channel).
In summary, we have broadened the color palette of our NR dyes to include rhodol and fluorescein derivatives. The resulting NR rhodol and fluorescein dyes display significantly red-shifted fluorescence relative to their oxygen analogues and display increased photostability compared to commonly used labels such as Cy 5.5. The new chemical functionality afforded by these scaffolds enabled the development of a red-shifted, no-wash probe for cellular esterase activity. Current efforts in our lab are focused on lowering the pKa of the NR fluorescein series to further increase the brightness at physiological pH. These new NR dye scaffolds also provide the starting point for the development of additional probes for enzymatic activity,34 reagents for advanced imaging experiments,35–37 and self-reporting delivery reagents.19
We gratefully acknowledge the Morrison Microscopy Core Research Facility and Prof. Christian Elowsky for assistance with confocal fluorescence microscopy as well as Prof. Edward Harris for use of cell culture equipment. We also thank the Research Instrumentation/NMR facility and the Nebraska Center for Mass Spectrometry for assistance with characterization of new compounds. This work was funded by the NIH (R35GM119751), NSF (CHE-1757957), and the University of Nebraska-Lincoln. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: Experimental details, synthesis and characterization of new compounds, and supplementary figures. See DOI: 10.1039/c9cc02492a
Conflicts of interest
The authors declare no conflicts of interest.
Notes and references
- 1.Lavis LD and Raines RT, ACS Chem. Biol, 2008, 3, 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavis LD and Raines RT, ACS Chem. Biol, 2014, 9, 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavis LD, Biochemistry, 2017, 56, 5165–5170. [DOI] [PubMed] [Google Scholar]
- 4.Koide Y, Urano Y, Hanaoka K, Terai T and Nagano T, ACS Chem. Biol, 2011, 6, 600–608. [DOI] [PubMed] [Google Scholar]
- 5.Egawa T, Koide Y, Hanaoka K, Komatsu T, Terai T and Nagano T, Chem. Commun, 2011, 47, 4162–4164. [DOI] [PubMed] [Google Scholar]
- 6.Koide Y, Urano Y, Hanaoka K, Piao W, Kusakabe M, Saito N, Terai T, Okabe T and Nagano T, J. Am. Chem. Soc, 2012, 134, 5029–5031. [DOI] [PubMed] [Google Scholar]
- 7.Koide Y, Kawaguchi M, Urano Y, Hanaoka K, Komatsu T, Abo M, Terai T and Nagano T, Chem. Commun, 2012, 48, 3091–3093. [DOI] [PubMed] [Google Scholar]
- 8.Grimm JB, Sung AJ, Legant WR, Hulamm P, Matlosz SM, Betzig E and Lavis LD, ACS Chem. Biol, 2013, 8, 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai X, Cui X, Wang B, Yang F, Cai Y, Wu Q and Wang T, Chem. – Eur. J, 2015, 21, 16754–16758. [DOI] [PubMed] [Google Scholar]
- 10.Fukazawa A, Suda S, Taki M, Yamaguchi E, Grzybowski M, Sato Y, Higashiyama T and Yamaguchi S, Chem. Commun, 2016, 52, 1120–1123. [DOI] [PubMed] [Google Scholar]
- 11.Grimm JB, Gruber TD, Ortiz G, Brown TA and Lavis LD, Bioconjugate Chem, 2016, 27, 474–480. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Sun YQ, Zhang H, Shi H, Shi Y and Guo W, ACS Appl. Mater. Interfaces, 2016, 8, 22953–22962. [DOI] [PubMed] [Google Scholar]
- 13.Grimm JB, Brown TA, Tkachuk AN and Lavis LD, ACS Cent. Sci, 2017, 3, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piao W, Hanaoka K, Fujisawa T, Takeuchi S, Komatsu T, Ueno T, Terai T, Tahara T, Nagano T and Urano Y, J. Am. Chem. Soc, 2017, 139, 13713–13719. [DOI] [PubMed] [Google Scholar]
- 15.Lutkus LV, Irving HE, Davies KS, Hill JE, Lohman JE, Eskew MW, Detty MR and McCormick TM, Organometallics, 2017, 36, 2588–2596. [Google Scholar]
- 16.Hirayama T, Mukaimine A, Nishigaki K, Tsuboi H, Hirosawa S, Okuda K, Ebihara M and Nagasawa H, Dalton Trans, 2017, 46, 15991–15995. [DOI] [PubMed] [Google Scholar]
- 17.Fischer C and Sparr C, Angew. Chem., Int. Ed, 2018, 57, 2436–2440. [DOI] [PubMed] [Google Scholar]
- 18.Deng F and Xu D, Chin. Chem. Lett, DOI: 10.1016/j.cclet.2018.1012.1012. [DOI] [Google Scholar]
- 19.Zhou X, Lai R, Beck JR, Li H and Stains CI, Chem. Commun, 2016, 52, 12290–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai X, Xiao J, Li M, Wang C, An H, Li C, Li Y, Zhang D, Cui X and Wang T, Chem. – Eur. J, 2018, 24, 14506–14512. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Lesiak L, Lai R, Beck JR, Zhao J, Elowsky CG, Li H and Stains CI, Angew. Chem., Int. Ed, 2017, 56, 4197–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavis LD, Chao TY and Raines RT, ACS Chem. Biol, 2006, 1, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavis LD, Chao TY and Raines RT, Chem. Sci, 2011, 2, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian L, Yang Y, Wysocki LM, Arnold AC, Hu A, Ravichandran B, Sternson SM, Looger LL and Lavis LD, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, 4756–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chyan W and Raines RT, ACS Chem. Biol, 2018, 13, 1810–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukazawa A, Usuba J, Adler RA and Yamaguchi S, Chem. Commun, 2017, 53, 8565–8568. [DOI] [PubMed] [Google Scholar]
- 27.Sednev MV, Wurm CA, Belov VN and Hell SW, Bioconjugate Chem, 2013, 24, 690–700. [DOI] [PubMed] [Google Scholar]
- 28.Roth A, Li H, Anorma C and Chan J, J. Am. Chem. Soc, 2015, 137, 10890–10893. [DOI] [PubMed] [Google Scholar]
- 29.Grzybowski M, Taki M and Yamaguchi S, Chem. – Eur. J, 2017, 23, 13028–13032. [DOI] [PubMed] [Google Scholar]
- 30.Lei Z, Li X, Li Y, Luo X, Zhou M and Yang Y, J. Org. Chem, 2015, 80, 11538–11543. [DOI] [PubMed] [Google Scholar]
- 31.Best QA, Sattenapally N, Dyer DJ, Scott CN and McCarroll ME, J. Am. Chem. Soc, 2013, 135, 13365–13370. [DOI] [PubMed] [Google Scholar]
- 32.Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K and Nagano T, J. Am. Chem. Soc, 2005, 127, 4888–4894. [DOI] [PubMed] [Google Scholar]
- 33.Levine SR and Beatty KE, Chem. Commun, 2016, 52, 1835–1838. [DOI] [PubMed] [Google Scholar]
- 34.Casey GR and Stains CI, Chem. – Eur. J, 2018, 24, 7810–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martineau M, Somasundaram A, Grimm JB, Gruber TD, Choquet D, Taraska JW, Lavis LD and Perrais D, Nat. Commun, 2017, 8, 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Taki M, Sato Y, Fukazawa A, Higashiyama T and Yamaguchi S, J. Am. Chem. Soc, 2017, 139, 10374–10381. [DOI] [PubMed] [Google Scholar]
- 37.Grzybowski M, Taki M, Senda K, Sato Y, Ariyoshi T, Okada Y, Kawakami R, Imamura T and Yamaguchi S, Angew. Chem., Int. Ed, 2018, 57, 10137–10141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


