Abstract
Background:
The high socioeconomic impact of osteoporosis and osteoporotic fracture is due to their high mortality, morbidity, and disease-related costs. Nowadays, bone mineral density (BMD) is a comparatively expensive way to diagnose and follow up patients with osteoporosis. Transforming growth factor-β3 (TGF-β3) is a protein categorized into cytokines. Some previous in vitro studies showed TGF-β3 effects on osteocytes and bone formation. Therefore, we conducted this study to find if there is any significant relationship between TGF-β3 and BMD results.
Materials and Methods:
This was an analytical cross-sectional study conducted in 2017. We included individuals who had been referred from their physicians to undergo BMD dual-energy X-ray absorptiometry. Blood samples were taken from 150 participants for measuring TGF-β3 with ELISA method.
Results:
The mean ± standard deviation of TGF-β3 serum level was 79 ± 30.8 pg/ml (minimum 41 pg/ml and maximum 210 pg/ml). There was a statistically significant and direct proportional relationship between TGF-β3 and T-score as a marker for the diagnosis and follow-up of osteoporosis and osteoporotic fracture (P = 0.001) (Pearson's correlation = +0.95).
Conclusion:
There was a significant relationship between TGF-β3 serum level and BMD. TGF-β3 serum level may be used as a marker for the diagnosis and follow-up of osteoporosis and osteoporotic fracture.
Keywords: Osteoporosis, osteoporotic fractures, transforming growth factor-β3
INTRODUCTION
Osteoporosis is a systemic skeletal disease caused by the deterioration of bone density. Osteoporosis is characterized by fragility of the bones that causes bone fracture. The disease develops slowly over several years and is often diagnosed when a minor trauma or falling causes bone fracture. The most common sites for fractures are hip, vertebrae, and forearm.[1] The worst osteoporotic fracture is hip fracture with mortality rate of about 20% in elderly patients.[2]
The reported lifetime osteoporotic fracture risk in the United States was 40%–50% in women and 13%–22% in men, with annual economic burden of osteoporotic fractures being approximately 14 billion dollars.[3]
Bone mineral content is determined by the amount of X-ray that is absorbed by calcium of the bone, and hence the bone mineral density is estimated by bone mineral content. There are some methods for the evaluation of bone mineral density such as dual-energy X-ray absorptiometry (DXA), single-energy X-ray absorptiometry, quantitative computed tomography, and quantitative ultrasound.[4]
Transforming growth factor-β3 (TGF-β3) was mentioned as a marker for osteoporosis severity in some studies. For the first time, TGF-β3 was isolated from porcine and human cDNA in 1988.[5]
There is about 80% similarity in the structure of TGF-β3 and TGF-β1.[5] Decline in TGF-β3 gene expression could decrease the osteoblastic activity. However, the investigation of osteoarthritic bone showed decreased TGF-β3 protein levels, but increased TGF-β3 mRNA in the bone.[6]
ten Dijke et al. compared the effects of recombinant TGF-β1 with TGF-β3 in osteoblast-enriched bone cell cultures, suggesting that both have mitogen-activated protein kinase and inhibitory effect of alkaline phosphatase activity.[7]
There is no sex-specific distribution for mRNA of TGF-β3. However, TGF-β3 serum protein level and mRNA decreased in the elderly population. Decline in TGF-β3 serum level can decrease the activity of bone osteoblasts, so that TGF-β3 may be used as an antosteoporotic marker.[8]
TGF-β3 serum level may be related with some conditions such as atherosclerosis[8] and some malignancies and tissue fibrosis.[9]
According to these findings and high burden of osteoporosis and high economic costs of bone mineral density (BMD) for the diagnosis and follow-up of patients, our study aimed to evaluate the correlation between TGF-β3 serum level and BMD results.
MATERIALS AND METHODS
We performed a cross-sectional distributive study on 150 patients who referred for BMD in one of the hospitals of Tehran, Iran, from November 2017 to July 2018. This research was approved by the Ethics Committee of AJA University of Medical Sciences, Tehran, Iran, with research project number 91000183 and ethical code IR.AJAUMS.REC.1396.058.
BMD was determined using DXA method, and T-score ≤−2 indicated osteoporosis. Participants aged 30 years and above and those who referred for BMD examination were included in the study and participants with a history of hypothyroidism and glucocorticoid consumption and unwilling to participate in the study were excluded from the study.
A history of glucocorticoid consumption and bone fracture (including low-energy fracture) was taken from all the patients.[10] In all the patients, 5 ml of blood was taken, the serum was separated, and TGF-β3 was measured using an ELISA kit (Hangzhou Eastbiopharm Company, China).
The minimum sample size was calculated by the formula, 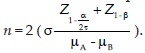 The study was done on 150 patients in three equal groups with 50 cases each. The patients of the first group (Group 1) did not have osteoporosis or bone fractures, the patients of the second group (Group 2) had osteoporosis without any history of osteoporotic fractures, and the patients of the third group (Group 3) had osteoporosis and a history of osteoporotic fractures in the last 2 years.
The study was done on 150 patients in three equal groups with 50 cases each. The patients of the first group (Group 1) did not have osteoporosis or bone fractures, the patients of the second group (Group 2) had osteoporosis without any history of osteoporotic fractures, and the patients of the third group (Group 3) had osteoporosis and a history of osteoporotic fractures in the last 2 years.
Initially, 165 cases were enrolled in the study, from which 15 cases were excluded due to unwillingness of the participants, and finally, the study was continued on 150 patients.
The data were entered into SPSS 23 (SPSS Inc., Chicago, IL, USA) software; Kolmogorov–Smirnov test and Mann–Whitney U-test were used to assess the normality of distribution of different variables and nonparametric variables, respectively. P < 0.05 was considered statistically significant.
RESULTS
In all 150 patients, 53 (35%) were male and 97 (65%) were female. The mean age of the patients was 65 ± 11.8 years (range, 30–87 years) and the mean of TGF-β3 serum level was 79 ± 30.8 pg/ml (range, 41–210 pg/ml). The mean of T-score was −2.4 ± 0.9 (range, −3.7–0.2).
T-scores of the male and female patients were −2.25 and −2.52, respectively [Figure 1]. There was no statistically significant difference between the BMD T-score of males and females (P > 0.05).
Figure 1.
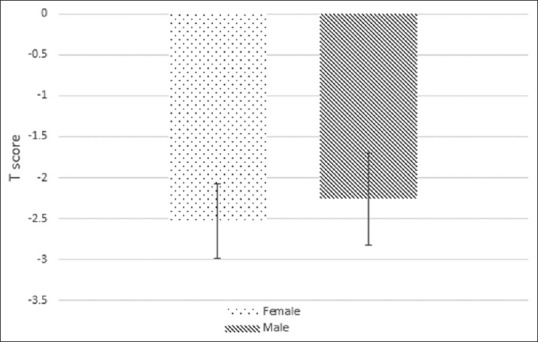
The mean of T-score by sex
TGF-β3 serum level had a significant direct correlation with T-score (P = 0.001) (Pearson's correlation = +0.952). It means that the patients with lower T-score had lower TGF-β3 serum level or the patients with severe osteoporosis had low TGF-β3 serum level.
Figure 2 shows the frequency of TGF-β3 with a history of fracture. The mean values of TGF-β3 serum level in patients with a history of osteoporotic fractures and patients without a history of fractures were 56 pg/ml and 91 pg/ml, respectively (P = 0.01).
Figure 2.
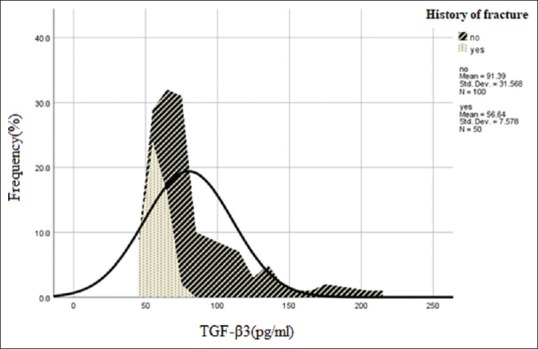
Frequency of transforming growth factor-β3 with a history of fracture
Figure 3 shows that, with increasing age, TGF-β3 serum level decreased, showing an inverse correlation between TGF-β3 serum level with age (P = 0.01). However, TGF-β3 serum level did not have any correlation with sex of the patients (P > 0.05).
Figure 3.
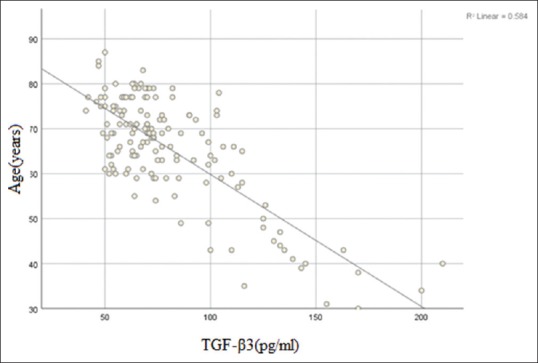
Distribution of transforming growth factor-β3 with age
Moreover, the mean values of T-score in patients with a history of osteoporotic fractures and those without a history of osteoporotic fractures were −2.99 and −2.15, respectively (P = 0.001). The mean values of T-score in female and male cases were −2.52 and −2.25, respectively (P > 0.05). However, there was a significant correlation between T-score and age of the patients (P < 0.001) [Figure 4].
Figure 4.
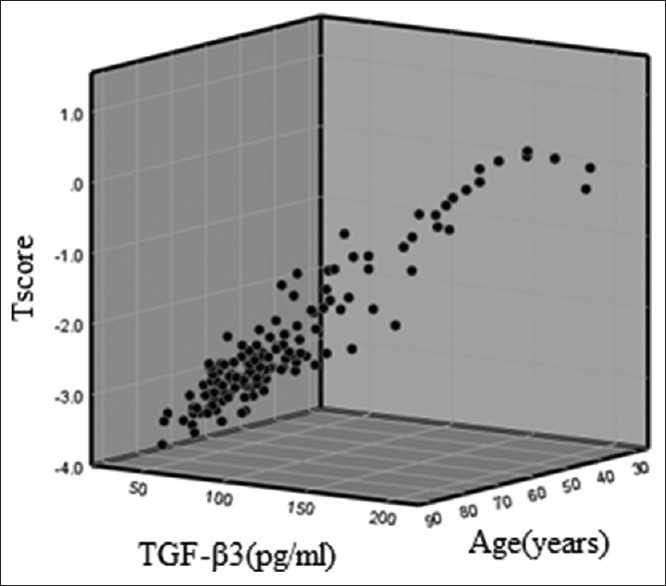
Three-dimensional graph of the relation of transforming growth factor-β3 with T-score and age
This study showed that, with an increase in age, the number of cases with a positive history of osteoporotic fractures increased significantly (P = 0.005). However, there was no significant correlation between the history of fracture and sex (P > 0.05).
DISCUSSION
The study showed that TGF-β3 serum level had a significant direct correlation with BMD T-score; on the other hand, TGF-β3 was a protective factor of bone density. In addition, there was an inverse association of TGF-β3 serum level with a history of osteoporotic fractures in the past 2 years.
Jahn found that in vitro adding of TGF-β3 protein to serum leads to better survival of osteocytes.[11] Furthermore, Hering et al.[7] found the correlation of TGF-β3 serum level with TGF-β3 mRNA expression in bone. Positive survival effect of different types of TGF-β on cultured osteoblast-lineage cells was approved by Dufour et al.,[12] Karsdal et al.[13] and Jilka et al.[14] The effect of TGF-β3 on osteocytes and osteoblasts may be more potent than TGF-β1 or TGF-β2.[15] The results of our study had some similarity with the abovementioned studies regarding the protective effect of TGF-β3 on BMD.
In our study, the mean of TGF-β3 serum level was 30 pg/ml that was less than the level obtained by Hering et al.[7] in their study. Controversy in this result may be due to the difference in the number of cases, so we evaluated the patients who referred for BMD due to bone pain or bone fracture; however, Hering et al. evaluated the normal population.[7]
There was an inverse correlation between TGF-β3 serum level and age of the patients mentioned earlier; however, TGF-β3 serum level had not any correlation with sex of the patients. These findings were similar to the results of Hering et al.[7]
We found an adverse correlation of T-score with age of the patients. Osteoporosis is an age-related disease,[11] and according to the findings, TGF-β3 was age related too.
The limitations of our study were the small sample size and mismatching of the patients of three groups based on age and sex, so with a view to exploring and achieving accurate and wider results, studies with larger sample size and randomization in selection of the patients should be conducted. Because TGF-β3 serum level may have been changed in the developmental age in the adolescent population,[16] we suggest doing the studies with correlation of TGF-β3 with age and BMD in patients aged <18 years also.
CONCLUSION
Based on the results of the study, TGF-β3 serum level can be used as a valuable and available marker for the evaluation and follow-up of osteoporosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Prevention WSGo, Osteoporosis Mo, World Health Organization. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group. World Health Organization; 2003. [Google Scholar]
- 2.Larijani B, Resch H, Bonjour J, Meybodi HA, Tehrani MM. Osteoporosis in Iran, overview and management. Iran J Public Health. 2007;1:1–13. [Google Scholar]
- 3.Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl 2):S3–7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: Scientific review. JAMA. 2002;288:1889–97. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 5.Derynck R, Lindquist PB, Lee A, Wen D, Tamm J, Graycar JL, et al. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988;7:3737–43. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centrella M, Casinghino S, Ignotz R, McCarthy TL. Multiple regulatory effects by transforming growth factor-beta on type I collagen levels in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1992;131:2863–72. doi: 10.1210/endo.131.6.1446624. [DOI] [PubMed] [Google Scholar]
- 7.Hering S, Isken F, Janott J, Jost C, Pommer A, Muhr G, et al. Analysis of TGFbeta3 gene expression and protein levels in human bone and serum. Exp Clin Endocrinol Diabetes. 2001;109:107–15. doi: 10.1055/s-2001-14830. [DOI] [PubMed] [Google Scholar]
- 8.Grainger DJ, Kemp PR, Metcalfe JC, Liu AC, Lawn RM, Williams NR, et al. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat Med. 1995;1:74–9. doi: 10.1038/nm0195-74. [DOI] [PubMed] [Google Scholar]
- 9.Anscher MS, Peters WP, Reisenbichler H, Petros WP, Jirtle RL. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N Engl J Med. 1993;328:1592–8. doi: 10.1056/NEJM199306033282203. [DOI] [PubMed] [Google Scholar]
- 10.Yoo JH, Kim SW, Kwak YH, Kim HK, Hwang JH, Kim JU, et al. Low energy fractures: what is the difference? specific features and clinical outcomes by minimally invasive locking plates. Biomedical Research. 2017;28(1) [Google Scholar]
- 11.Jahn K. Ex vivo and in vitro Culture Models of Human Osteoblast-Like Cells and the Effects of TGFbeta3 and Serum-Free Culture: Cardiff University. 2010 [Google Scholar]
- 12.Dufour C, Holy X, Marie PJ. Transforming growth factor-beta prevents osteoblast apoptosis induced by skeletal unloading via PI3K/Akt, bcl-2, and phospho-bad signaling. Am J Physiol Endocrinol Metab. 2008;294:E794–801. doi: 10.1152/ajpendo.00791.2007. [DOI] [PubMed] [Google Scholar]
- 13.Karsdal MA, Fjording MS, Foged NT, Delaissé JM, Lochter A. Transforming growth factor-beta-induced osteoblast elongation regulates osteoclastic bone resorption through a p38 mitogen-activated protein kinase- and matrix metalloproteinase-dependent pathway. J Biol Chem. 2001;276:39350–8. doi: 10.1074/jbc.M008738200. [DOI] [PubMed] [Google Scholar]
- 14.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 15.ten Dijke P, Iwata KK, Goddard C, Pieler C, Canalis E, McCarthy TL, et al. Recombinant transforming growth factor type beta 3: Biological activities and receptor-binding properties in isolated bone cells. Mol Cell Biol. 1990;10:4473–9. doi: 10.1128/mcb.10.9.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo . Development. 1991;111:131–43. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]


