Abstract
A bench-top stable, bifunctional reagent for the redox-neutral cyclopropanation of olefins has been developed. Triethylammonium bis(catecholato)iodomethylsilicate can be readily prepared on multigram scale. Using this reagent in combination with an organic photocatalyst and visible light, cyclopropanation of an array of olefins, including trifluoromethyl- and pinacolatoboryl-substituted alkenes, can be accomplished in a matter of hours. The reaction is highly tolerant of traditionally reactive functional groups (carboxylic acids, basic heterocycles, alkyl halides, etc.) and permits the chemoselective cyclopropanation of poly-olefinated compounds. Mechanistic interrogation revealed that the reaction proceeds via a rapid anionic 3-exo-tet ring closure, a pathway consistent with experimental and computational data.
Graphical Abstract

INTRODUCTION
The cyclopropyl group is a common motif found in many pharmaceutical products1 and secondary metabolites.2 It is employed to increase metabolic stability, enhance potency, and decrease plasma clearance.1a Consequently, cyclopropane rings appear in 10 of the top 200 highest grossing pharmaceutical products from 20161b and in 124 approved or investigational drugs (Figure 1).1c Furthermore, the substitution pattern of these rings can impart bioisosteric properties. Trifluoromethyl-substituted cyclopropanes (TFCps) are salient examples of the unique bioisosterism possible with cyclopropanes; TFCps are tert-butyl isosteres that improve in vitro and in vivo stability while retaining biological activity.3–5 A TFCp was utilized in the development of a selective, brain-penetrating T-type calcium channel blocker (ACT-709478) with a safety profile suitable for advancement into Phase I clinical trials (Figure 1).3d
Figure 1.
Cyclopropane-containing pharmacons and trifluoromethyl cyclopropanes.
Among the more popular strategies for cyclopropane assembly are [2+1]-type reactions with olefins. Some examples include the use of carbenoids generated from diethylzinc and halomethanes (Simmons-Smith),6 or via diazo compounds.7 In some cases, a two-step process (Michael-type addition followed by intramolecular nucleophilic displacement) can be employed for the direct cyclopropanation of olefins (e.g., sulfur ylides with enones in the Corey-Chaykovsky reaction).7b,8 Although these routes are well-established in the literature, they are often lacking in broad functional group tolerance or operational simplicity, and rarely employ mild conditions. Specifically, state-of-the-art methods for TFCp synthesis by cyclopropanation rely on multi-step sequences involving treatment of the corresponding trifluoromethylalkene with diazomethane followed by a retro-[3+2]-cycloaddition in refluxing xylenes (Figure 2A).3a,4 Alternate strategies that do not rely on cyclopropanation of CF3-substituted olefins do exist (e.g., cationic ring closure,5a,b Minisci-type alkylation5c), but their scope is constrained by inherent mechanistic limitations.
Figure 2.
Synthesis of trifluoromethyl-substituted cyclopropanes.
Single-electron approaches for the cyclopropanations of alkenes are much less well-developed. Leading methods rely on the use of diazo compounds,9 pre-functionalized substrates,10 or methods that require large excesses of reagents and multiple additives.11 Thus, these methods are not easily employed in late-stage functionalization of complex molecules. Photoredox catalysis has enabled the operationally simple generation of radicals while maintaining the broad functional group tolerance and orthogonality to acidic or basic residues that is associated with processes proceeding through open shell intermediates.12 However, no photooxidizable C1 reagent for radical cyclopropanation is described in the literature. We thus directed our efforts toward designing such a species to fill this gap. We envisioned that a reagent furnishing a halomethyl radical could operate as a cyclopropanating reagent where, following radical addition to an olefin, a 3-exo-tet cyclization would forge the second C–C bond required for cyclopropanation (Figure 2B). In addition to serving as an effective cyclopropanating reagent, successful design of a reagent furnishing a halomethyl radical would allow the properties of this reactive odd-electron intermediate to be probed.13
DISCUSSION
Reagent Design and Development.
In devising this reagent, several parameters were considered: (i) an ability to be bifunctional in nature (i.e., able to engage in two distinct C–C bond forming events), (ii) bench stability, (iii) ease of photooxidation, (iv) a practical, inexpensive, direct preparation from commercial materials, and (v) potential to operate in a redox-neutral and metal-free reaction manifold. Such a reagent would not only leverage the wide functional group tolerance that is characteristic of photoredox catalyzed radical reactions, but would stand in contrast to the typical approaches to cyclopropanation (harsh bases, poor safety profiles, and/or toxic reagents). Although a variety of radical precursors were explored (e.g., potassium organotrifluoroborates, 4-alkyldihydropyridines, α-halocarboxylates, etc.), α-halomethyl bis(catecholato)silicates were ultimately identified as best fulfilling these criteria (see the Supporting Information for details on assessment of other halomethyl radical precursors). Alkylbis(catecholato)silicates can be prepared from commodity materials on multigram scale, have low, leveled oxidation potentials, and are typically free-flowing, bench-stable powders compatible with organic photocatalysts.14 We anticipated that single electron oxidation by the excited state of the photocatalyst would furnish the desired halomethyl radical intermediate. Once formed, the radical would readily add to an alkene 2, and an anionic (SN2) or radical 3-exo-tet (SH2) cyclization would furnish the desired cyclopropane 3 (Figure 2C).
To assess the feasibility of the envisioned process, three bifunctional silicates (α-chloro 1a, α-bromo 1b, and α-iodo 1c) were prepared from commercially available chloromethyltrimethoxysilane (~$0.26 per mmol) in either one or two simple chemical steps. These reagents could, in all cases, be prepared on multigram scale in good yield, without any chromatography, and were isolated as free-flowing powders.
Trifluoromethyl-substituted alkenes 2 were initially selected for exploring the proposed cyclopropanation given their known compatibility with photoredox catalysis and proficiency as radical acceptors.15,16 In addition, success here would allow the mild, one-step preparation of valuable TFCps 3 from trifluoromethyl-substituted alkenes. Given the ease with which these olefins can now easily be accessed from commercially available trifluoromethyl ketones,15b aryl halides,15c or organoboron reagents,15a success here would provide a regiospecific means to install the TFCp motif on virtually any scaffold (arene, hetereoarene, aliphatic, etc., Figure 3).
Figure 3.
Convergent strategies to TFCps from commercial materials.
In addition to their inherent synthetic value, the known ability for trifluoromethylalkenes to undergo a competitive anionic fluoride elimination would provide a handle through which insight could be gained as to whether ring closure would occur via an anionic or radical pathway (Figure 2C, see Mechanistic Studies for further details).16e–f,17
With these considerations in mind, we evaluated the selectivity for cyclization versus fluoride elimination (Scheme 1). Using 2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile (4CzIPN)18 as photocatalyst and dimethyl sulfoxide (DMSO) as solvent, we not only observed the desired reactivity, but found that the ratio of cyclopropane 3 to undesired gem-difluoroalkene 4 was strongly dependent on the halogen leaving group; a complete inversion in selectivity was observed when moving from the chloro- to bromo- to iodosilicate (Scheme 1).
Scheme 1.
Synthesis and Optimization of the Bifunctional Silicate Reagent
aReaction conditions: halomethylsilicate (1.5 equiv, 0.15 mmol), alkene (1.0 equiv, 0.1 mmol), 4CzIPN (5 mol %, 0.005 mmol), DMSO (0.1 M), 3 h, irradiating with blue LEDs (30 W). See the Supporting Information for details. bRatio determined via 19F NMR.
Given its innate selectivity for cyclopropanation and inexpensive nature (~$1.10 per mmol), further optimization of this annulation process utilized iodomethyl-based silicate reagent 1c, providing reaction conditions that do not require the use of additives (see Supporting Information for details on optimization). Use of 30 W blue LEDs lamps as the light source allowed the shortest reaction times (0.5–24 h, depending on scale and substrate). The reaction proceeded equally well in the presence of other, more cost-effective, light sources (e.g., 21 W CFLs) with only the reaction time being impacted (See Supporting Information for detail on light source studies). Control reactions confirmed this indeed was a photocatalytic process; in the absence of light or photocatalyst, no reaction occurred (see Supporting Information). An additional control reaction where reaction progress was evaluated over alternating periods of irradiation and darkness (the so called “light/dark” experiment) was performed. More rigorous evaluation of the photochemical aspect of this process (e.g., Stern-Volmer emission quenching, quantum yield determination) was performed as well (see Mechanistic Studies for further details), ultimately ruling out an unassisted, self-sustaining chain process.
With these conditions in hand, the scope of the transformation was explored. A variety of structurally diverse α-trifluoromethyl alkenes were amenable to cyclopropanation, generally providing products in good yield and with excellent functional group compatibility (Table 1). Bromo-substituted arenes (3a-3d) reacted efficiently to provide the desired products. Steric effects did have some influence on the reaction. ortho-Substitution itself was not deleterious, but larger substituents did seem to have a more pronounced effect (e.g., 3c required longer reaction times and a slightly higher loading of 1c). The reaction tolerated a potentially reactive alkyne (3e) functional group. Additionally, a wide range of nitrogen-containing substrates, including aryl nitrile 3g, Boc-protected and N-methyl-anilines 3h-3j, a Boc-protected benzylic amine 3k, and a secondary amide 3l, all cyclopropanated successfully. The reaction even proceeds smoothly in the presence of an acidic ammonium chloride salt (3m). Furthermore, aldehyde 3n, phenol 3o, benzyl alcohol 3p, benzoic acid 3q, and methyl ester 3r were all compatible, illustrating both the broad functional group tolerance and the compatibility of protic groups. Other valuable synthetic lynchpins such as an aryl Bpin (3s), primary alkyl iodide (3t), and a secondary alkyl chloride (3u) were also tolerated. The chemoselectivity of the transformation was evaluated using the geraniol analog 3v, where cyclopropanation was observed exclusively at the electron-deficient trifluoromethyl-substituted olefin. The reaction was not limited to α-styryl trifluoromethyl-substituted alkenes. A representative trifluoromethyl-substituted aliphatic alkene (3w) also efficiently underwent cyclopropanation, albeit at an extended reaction time and with higher silicate loading. In addition, a variety of heteroaryl trifluoromethyl alkenes including indole 3x, indazole 3y, pyrido[2,3-b]pyrazine 3z, imidazole-pyrimidine 3aa, pyridine 3ab,19 a caffeine derivative 3ac, and thiophene 3ad were readily cyclopropanated. Finally, the reaction could be conducted on a 5 mmol scale with virtually no effect on the yield (3a). It should also be noted that these sorts of CF3-subsituted olefins fail to react under previously reported radical cyclopropanation methods (see Supporting information for comparison studies).
Table 1.

|
All values indicate the yield of the isolated product. pin = 2,3-dimethylbutane-2,3-diol.
General reaction conditions: iodosilicate (1.5 equiv, 0.75 mmol), alkene (1.0 equiv, 0.50 mmol), 4CzIPN (5 mol %, 0.025 mmol), DMSO (0.1 M), 3 h, irradiating with blue LEDs (30 W). See the Supporting Information for details.
Isolated yield on 5 mmol scale.
Conducted using 2.0 equiv of silicate.
Conducted using 1.75 equiv of silicate.
Reaction run for 24 h.
Isolated as a 95:5 mixture of product and 2-methylated product.
77:23 ratio of cyclopropane to fluoride elimination via 19F NMR of the crude reaction mixture; olefin removed via Ag-doped SiO2
92:8 ratio of cyclopropane to fluoride elimination, via 19F NMR of the crude reaction mixture; olefin removed via Ag-doped SiO2.
Alteration of the perfluoroalkyl chain was also explored. Both pentafluoroethyl and 1,1-difluoroethyl-substituted alkenes reacted to give the corresponding substituted cyclopropanes (3ae and 3af, respectively). Interestingly, the more electron-deficient and sterically encumbered pentafluoroethyl 2ae gave a 77:23 mixture of cyclopropane 3ae and fluoride elimination product, as determined by19F NMR of the crude reaction mixture, suggesting that the side chain can perturb the relative rates of cyclization versus elimination.
Lastly, to demonstrate the user-friendly nature of this reaction, the cyclopropanation of trifluoromethyl alkene 2d was conducted in the presence of an air atmosphere and using wet, non-degassed DMSO (Scheme 2). Although the reaction did proceed to full conversion, the product was isolated in a slightly lower yield. However, the result is still a testament to the robustness of the transformation.
Scheme 2.
Zero Precaution Reaction
We next explored the generality of this reagent for cyclopropanation, and thus other olefins were examined. Overall, various electronically distinct olefins were amenable to cyclopropanation, and the functional group tolerance matched that observed with trifluoromethyl-substituted alkenes. α,β-Unsaturated esters, amides, and ketones (6a–6e) readily underwent the cyclopropanation to give the corresponding trans products (Table 2). A key intermediate (6c) used in the synthesis of platelet aggregation inhibitor Ticagrelor (AstraZeneca, Figure 1)20 was prepared using this reagent and isolated as an approximately 1:1 mixture of trans-cyclopropane diastereomers. Several alkenes bearing 1,1-disubstitution were readily cyclopropanated (6f-6k). The reaction was further extended to 1,1-diarylethylenes to furnish 1,1-diaryl cyclopropanes (6i, 6j). Even a trisubstituted alkene was converted to its corresponding cyclopropane without issue (6k). Additionally, trans-anethole reacted to give only the corresponding trans-cyclopropane 6l, and terminally unsubstituted styrenes (which are typically incompatible with radical cyclopropanation)11a gave the corresponding mono-substituted cyclopropanes (6m and 6n). Furthermore, many examples (e.g., 6a, 6d, 6e, 6i, 6l, and 6m) displayed more successful reactivity when using 1c as compared to previously reported radical cyclopropanation methods.11
Table 2.

|
All values indicate the yield of the isolated product.
General reaction conditions: iodosilicate (1.5 equiv, 0.75 mmol), alkene (1.0 equiv, 0.50 mmol), 4CzIPN (5 mol %, 0.025 mmol), DMSO (0.1 M), 3 h, irradiating with blue LEDs (30 W). See the Supporting Information for details.
Conducted using 2.0 equiv of silicate.
Reaction run for 18 h.
Conducted using 1.75 equiv of silicate.
Product isolated as a 94:6 mixture of product and starting material.
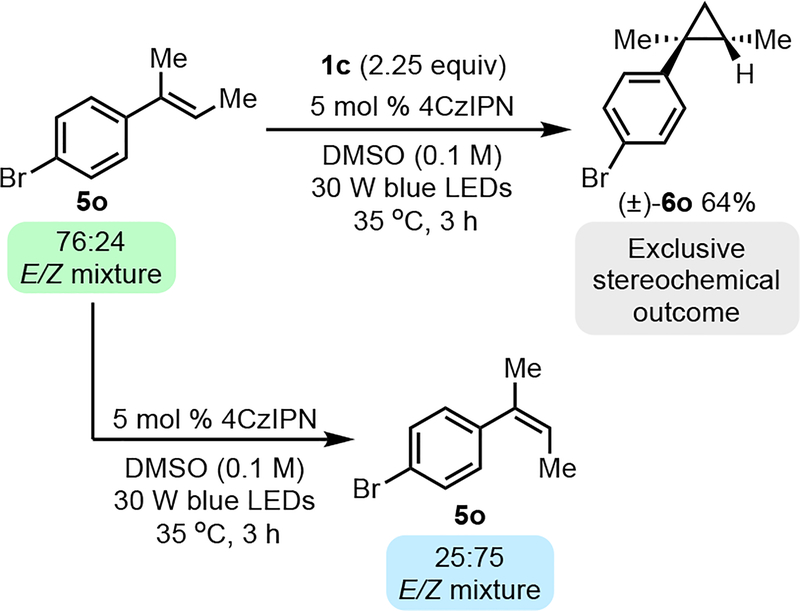
The stereochemical outcome when using trans-anethole prompted us to interrogate whether stereoconvergence occurred using the method outlined here. Cyclopropanation of an E/Z mixture of 5l as well as a trisubstituted alkene 5o provided trans-cyclopropane as the exclusive stereoisomer. In addition, use of pure cis- or pure trans β-methylstyrene resulted in the same stereochemical result. Exclusive formation of the trans-cyclopropane was observed, although these isomeric olefins appear to react at different rates. Stereoconvergence here is likely achieved via diastereoselective ring closure following radical addition to either isomer (made possible by the rapid interconversion of a radical intermediate). However, given that photochemical isomerization is a background reaction for some olefins (e.g., Scheme 3), stereoconvergence via preferential reaction with one alkene isomer cannot be conclusively ruled out for some systems (see Supporting Information for further details).
Scheme 3.
Stereoconvergent Cyclopropanation
Mechanistic Studies.
Having explored the synthetic scope and utility of this reagent, we next turned our attention to the reaction mechanism, specifically whether the proposed 3-exo-tet cyclization proceeded via an anionic or radical (SH2) pathway. Although the identity of the halogen of the halomethyl radical does dramatically impact the reaction pathway, that alone cannot distinguish between anionic or radical ring closure (i.e., iodide is both more prone to nucleophilic displacement and is more homolytically labile than bromine or chlorine). Experiments to explore the influence of electronics on product distributions were initially conducted. However, no strong electronic correlation between the ratio of cyclopropane to gem-difluoroalkene was observed (Scheme 4).
Scheme 4.
Insensitivity of Cyclopropanation to Electronicsa
aReaction conditions: iodosilicate (1.5 equiv, 0.75 mmol), alkene (1.0 equiv, 0.50 mmol), 4CzIPN (5 mol %, 0.025 mmol), DMSO (0.1 M), 3 h, irradiating with blue LEDs (30 W). See the Supporting Information for details.
Of note, the reaction could also be conducted in the presence of 3 equiv of trifluoroacetic acid (TFA) without any change in ratio or reaction conversion. Although intriguing, these results were not sufficient to preclude one of the two cyclization pathways. We next turned to quantum mechanical calculations to evaluate these mechanistic postulates more conclusively. Specifically, we sought to determine which of the two mechanisms more accurately described the observed results (i.e., the effect on product selectivity by the structure of α-halomethyl radical, etc.). Calculations were initially performed at the UM06–2X/DGDXVP level of theory in implicit solvent.21 Further, to assess the influence of dynamic correlation, open-shell, domain-based local pair, and natural orbital Coupled-Cluster calculations using single and double excitations with perturbative triple excitations (DLPNO-CCSD(T)) using def2-TZVPP basis using ORCA were also performed.22 This latter method provides accurate energies (within 3 kJ mol−1) with the computational cost comparable to DFT calculations.23 Single point energy calculations in implicit solvent using (D3(BJ)-B3LYP)/def2-TZVPP and UM06-2X/ def2-TZVPP were performed in parallel for comparison, which revealed identical trends and very similar energetic profiles as the DLPNO-CCSD(T) method (see Supporting Information).24 For simplicity, only DLPNO-CCSD(T) free energies are presented and discussed explicitly in the text. All 3D structures were generated using CYL-view.25
We initially sought the energetics of the radical 3-exo-tet cyclization. In this pathway (Figure 2C), radical intermediate 2′ presumably arises from Giese-type addition26 of the halomethyl radical 1′ to the alkene 2; which can undergo ring-closure via SH2–type mechanism to deliver the desired TFCp 3. Thus, we populated the potential energy surface of this pathway (Scheme 5). Note that energetics of the initial radical addition to the alkene show regioselective formation of α-radical INT-I, implying that α-radical formation is both kinetically and thermodynamically favored over β-radical formation (see the Supporting Information for additional details). With the exception of the iodo system (22.0 kcal mol−1 barrier of cyclization from INT-I), the barriers for the radical cyclization (SH2-type pathway, TS-II and TS-III, respectively) are not feasible under experimental conditions (barriers are 27.6 kcal mol−1 and 34.5 kcal mol−1). All other methods predicted similar barriers (See Supporting Information). This is surprising given that formation of cyclopropane was observed with all halomethyl silicates. Even more surprising was the fact that, in all cases, the reactions are net endergonic. Notably, the barriers and (endergonic) reaction energies are consistent with the strength of the C–X bond (C–X bond dissociation energies for C–I, C–Br, and C–Cl are ~57, ~71, and ~85 kcal mol−1, respectively).27 The computed barriers for a possible radical- mediated fluoride elimination to give the gem-difluoroalkene (not shown) were too high to be feasible and were universally highly endergonic (~63 kcal mol−1 between all adducts of halomethyl addition).
Scheme 5.
Influence of Halogen Substituent on the Barriers for the SH2-type 3-exo-tet Cyclization Pathway.a
aFree energies [DLPNO-CCSD(T)/def2-TZVPPTHF(SMD)//UM06–2X/DGDXVP-THF(SMD)] are in kcal mol−1, and selected distances are in Ångstroms.
The energetic inconsistency of the radical cyclization pathway with our experimental observations led us next to investigate whether anionic pathways might be operative for both cyclization and gem-difluoroalkene formation (Scheme 6). In excellent agreement with experiment, the computed barriers for the anionic pathway: 1) are feasible under experimental conditions (~4–9 kcal mol−1) and exergonic (~ −4 to −43 kcal mol−1), and 2) qualitatively and quantitatively replicate the experimental trends (Scheme 1C). Further, although the barriers for the SN2-type cyclization increase with the leaving group ability (I > Br > Cl), the barriers for the elimination (E1cB pathway) remained relatively unchanged for all halo systems (~7 kcal mol−1). Therefore, for the iodo system, cyclopropane formation P-IV (via SN2-type transition state) is kinetically favored by 3.8 kcal mol−1 over the alkene product (P-VII). However, for the bromo system, the energy difference between these two competing pathways decreases (ΔΔG‡ is 1.1 kcal mol−1 for TS-V versus TS-VIII) in favor of gem-difluoroalkene formation. Finally, in the chloro-system, the elimination transition state (TS-IX) is favored by 2 kcal mol−1 over the substitution pathway (via TS-IV), thus further favoring the formation of the alkene.
Scheme 6.
Energetics of Anionic 3-exo-tet Cyclization Versus E1cb-type Fluoride Eliminationa
aFree energies [DLPNO-CCSD(T)/def2-TZVPP-THF(SMD)//UM06–2X/DGDXVP-THF(SMD)] are in kcal mol−1, and selected distances are in Ångstroms.
Not only do the barriers for cyclization explain the observed selectivity (and, because of their less energy-demanding nature, rule out the SH2-type pathway), but they agree with our experimentally observed results. Both pathways rule against intermolecular (bimolecular) protonation as a viable reaction outcome because the reaction is likely proceeding at catalytic concentrations of anionic and radical intermediates, and the barriers for intramolecular cyclization and elimination are low (<9 kcal mol−1). This rationalizes not only how the reaction proceeds in the presence of an ammonium but also how it can operate even when a highly acidic species such as TFA is added. This same insensitivity toward acid likely rules out the formation of an iodomethyl anion (and thus the formation of carbene). Moreover, it explains how gem-difluoroalkene formation is the predominant pathway when using non-bifunctional radicals, rather than traditional Giese-type hydroalkylation.16d,f Finally, the very low barrier for cyclization from the iodomethyl-derived anion (Scheme 5, TS-IV) explains the relative insensitivity to varying electronics of the arene.
Next, we wondered whether the mechanistic findings would translate to the non-trifluoromethylated systems successfully cyclopropanated using 1c. More specifically, the possibility of a mechanistic spectrum where the electronics of an α-substituent would control whether the cyclization proceeds through a radical or anionic pathway. Whereas photoredox Giese-type processes are established for α-carbonyl28 and α-boryl29 olefins, similar reactivity in more electron-rich olefins is not established. Thus, we evaluated the energetics of anionic and radical ring closure for a representative system, 1,1-diphenylethylene (Scheme 7). The result of this set of calculations rules out a mechanistic spectrum; the energetic trends are similar to the α-trifluoromethyl systems. That is, the barrier for radical cyclization is ~20 kcal mol−1 (via TS-XIII) from the corresponding 1,1-diphenyl-3-iodopropyl radical, while the barrier for the cyclization from the corresponding 1,1-diphenyl-3-iodopropyl anion is virtually barrierless (~3 kcal mol−1; via TS-X). Importantly, the anionic mechanism is contingent on a rather fast (and favorable) SET reduction of the intermediate radical. Other halomethyl radical additions show similar trends.
Scheme 7.
Energetics of Anionic Versus Radical 3-exo-tet Cyclization in a Non-α-Trifluoromethyl Systema
aFree energies [DLPNO-CCSD(T)/def2-TZVPP-THF(SMD)//UM06–2X/DGDXVP-THF(SMD)] are in kcal mol−1 and selected distances are in Ångstroms.
The transition states for the reaction of olefin 5i with various halomethyl radicals is suggestive that, if an anionic pathway is indeed operative, cyclization should be feasible under experimental conditions with even the less reactive chloromethyl radical. In the absence of a fluoride elimination, only intermolecular side reactions are possible in systems such as 5i. To probe this prediction, the cyclopropanation of 5i using chloromethylsilicate 1a was attempted under the conditions optimized for 1c (Table 3). Indeed, cyclopropanation of 5i gave a comparable yield when using chloromethylsilicate 1a in place of iodomethylsilicate 1c. Further, examination of the cyclopropanation of other non-CF3-bearing alkenes using 1a resulted in varying levels of success. Overall, the isolated yields were lower when using 1a, reflective of the higher barrier to cyclization onto an alkyl chloride versus alkyl iodide. Seemingly less stabilized anions were more likely to engage in appreciable cyclization. Substrates that proceed through stabilized enolate anions (5a′, 5d, 5f) provide no cyclized product. Additionally, some substrates preferentially underwent protonation to give the uncyclized, alkyl chloride Giese-type addition product (5g, 5n). On the whole, the observations here are consistent with the proposed anionic cyclization pathway.
Table 3.

|
All values indicate the yield of the isolated product.
General reaction conditions: iodosilicate (1.5 equiv, 0.75 mmol), alkene (1.0 equiv, 0.50 mmol), 4CzIPN (5 mol %, 0.025 mmol), DMSO (0.1 M), 3 h, irradiating with blue LEDs (30 W). See the Supporting Information for details.
Conducted using 2.0 equiv of silicate.
Conducted using 1.75 equiv of silicate.
Isolated 66% yield of noncyclized Giese-type addition product.
Reaction run for 18 h.
Isolated product contained 2% yield of non-cyclized Giese-type addition product.
Isolated 60% yield of non-cyclized Giese-type addition product. See Supporting Information for details.
To bracket the rate of radical reduction experimentally, 5p was prepared and subjected to cyclopropanation. Surprisingly, the major product was bis-cyclopropane 6p, albeit in low isolated yield. Giese-type adduct 7 was not observed (Scheme 8). This implies that SET reduction exceeds the known rate constant of ring opening for the related α-cyclopropyl benzyl radical of 6.1 × 104 s−1, which corresponds to a barrier of ~11.1 kcal mol−1 at 298 K.30 The experimental results are again borne out in the computational model (Scheme 9). The calculated thermodynamics of the ring opening process for this system shows that the barrier for SET reduction must be lower than the calculated barrier for ring-opening (13.3 kcal mol−1). This is in agreement with the rate data. In addition, the low barrier for cyclization from INT-VI (2.7 kcal mol−1) explains the facile formation of bis-cyclopropane 6p.
Scheme 8.
Bracketing Experiment.
Scheme 9.
Energetics of Ring Opening Versus Anionic Cyclizationa
aFree energies [DLPNO-CCSD(T)/def2-TZVPP-THF(SMD)//UM06–2X/DGDXVP-THF(SMD)] are in kcal mol−1, and selected distances are in Ångstroms.
With all these data in hand, we suggest the following order of events as the operative mechanism (Figure 4) under the developed reaction conditions: 1) Visible light-mediated photoexcitation of 4CzIPN to its excited state. 2) Reductive quenching of 4CzIPN* by halomethyl silicate 1. This mode of quenching is supported by Stern-Volmer emission quenching experiments (see Supporting Information) and also by the generally low, favorable oxidation potentials of silicates (E1/2 = +0.4 – 0.7 vs SCE) and the high, unfavorable reduction potentials of primary iodides (e.g., E1/2 = −1.44 V vs SCE for CH2I2)11a Subsequent fragmentation of oxidized 1 furnishes halomethyl radical 1′. 3) After radical generation, Giese-type addition by the halomethyl radical generates adducts 2′ or 5′; 4) SET reduction of these adducts by the reduced state of 4CzIPN gives anions 2′′ or 5′′ and returns 4CzIPN to its original ground state; 6) Anionic cyclization of 2′′ or 5′′ furnishes cyclopropanes 3 or 6. Ultimately, this process is a closed cycle and does not appear to operate by a chain mechanism. The quantum yield of this process was determined to be 0.066, implying that an assisted chain process is not operative.
Figure 4.
Plausible Mechanism for Cyclopropanation.
In cases where the olefin is 1,2-disubstituted or trisubstituted, stereoconvergence is observed. Stereoconvergence likely arises from rapid equilibration of Giese-adduct 2/5 to the most stable conformation followed by stereoretentive reduction and rapid ring closure (see Supporting Information for calculations supporting this hypothesis). Alternatively, because photochemical isomerization of the starting olefin is possible, a dynamic kinetic resolution-type scenario may arise.
The results obtained and subsequent mechanistic postulate stands in stark contrast to literature reports employing the iodomethyl radical, which propose ring closure through an SH2-type 3-exo-tet cyclization.11 At the request of a reviewer, a direct comparison using a representative set of substrates was made between the method reported here and the method previously reported by Suero and coworkers (Table 4). For some substrates, the reactions gave comparable yields (6d, 6e, and 6l), while for others distinctly different reactivities were observed. Specifically, alkenes 5i and 5l gave exclusive non-cyclized iodoalkylation products under the conditions previously reported by Suero et al., whereas under the redox-neutral, radical/polar crossover conditions reported here, the same alkenes reacted to give solely cyclopropanated product.31Given that the putative active species is the same in both cases, the disparity in reactivity ultimately suggests that the redox environment in which the radical is generated (i.e., the order of SET events, presence of additives, etc.) dramatically influences the reaction outcome. More broadly, this implies the redox environment can open up, or close off, alternative mechanistic pathways. This latter conclusion may be useful for methods design in other reaction manifolds beyond cyclopropanation.
Table 4.

|
All values indicate the yield of the isolated product.
Reactions using condition B were performed exactly as described in reference 11a.
Yield reported in reference 11b.
Yield reported in reference 11a.
CONCLUSIONS
Herein, the successful development of a new reagent for the redox-neutral cyclopropanation of olefins under mild, photocatalytic conditions is disclosed. This reagent has resulted in the generation of not only a suite of TFCps, but it has enabled radical/polar crossover cyclopropanation of a diverse range of olefins to be accomplished with ease and excellent functional group compatibility. Combined theoretical and experimental Mechanistic Studies revealed that the reaction likely proceeds via a photooxidatively generated iodomethyl radical addition to an olefin followed by radical SET reduction, culminating in an anionic 3-exo-tet ring closure. Further applications for this reagent are under development and will be reported in due course. More generally, the findings disclosed here not only enable facile access to halomethyl radicals, but begin to shed light on the unique capabilities of these C1 synthons.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful for the financial support provided by NIGMS (R01 GM 113878 to G.M.) and NSF (CAREER, 1751568 to O.G.). J.P.P. is grateful for an NIH NRSA fellowship (F32 GM125241). C.B.K. is grateful for an NIH NRSA postdoctoral fellowship (F32 GM117634). O.G. is grateful to the University of Maryland College Park for start-up funds and computational resources from UMD Deepthought2 and MARCC/BlueCrab HPC clusters and XSEDE (CHE160082 and CHE160053). We thank Dr. David Primer (Celgene) and Rebecca Wiles (UPenn) for stimulating discussions. We thank Dr. Charles W. Ross, III (UPenn) for his assistance in obtaining HRMS data. We especially thank Dr. Mirna El Khatib (UPenn) for assistance with quantum yield determination. We thank Kessil Lighting for the generous donation of a prototype PR160 Rig.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details and spectral data (PDF)
REFERENCES
- 1.(a) Talele TT The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem 2016, 59, 8712–8756. [DOI] [PubMed] [Google Scholar]; (b) Njardson Lab. Top 200 Brand Name Drugs by Retail Sales in 2016. http://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/2016Top200PharmaceuticalRetailSalesPosterLowResV3_0.pdf (accessed Feb 23, 2018); (c) The Drugbank. Chemical Structure Search https://www.drugbank.ca/structures/search/small_molecule_drugs/structure#results (accessed Feb 23, 2018).
- 2.Chen DY-K; Pouwer RH; Richard J-A Recent advances in the total synthesis of cyclopropane-containing natural products. Chem. Soc. Rev 2012, 41, 4631–4642. [DOI] [PubMed] [Google Scholar]
- 3.(a) Barnes-Seeman D; Jain M; Bell L; Ferreira S; Cohen S; Chen X-H; Amin J; Snodgrass B; Hatsis P Metabolically Stable tert-Butyl Replacement. ACS Med. Chem. Lett 2013, 4, 514–516. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lazerwith SE; Lew W; Zhang J; Morganelli P; Liu Q; Canales E; Clarke MO; Doeffler E; Byun D; Mertzman M; Ye H; Chong L; Xu L; Appleby T; Chen X; Fenaux M; Hashash A; Leavitt SA; Mabery E; Matles M; Mwangi JW; Tian Y; Lee Y-J; Zhang J; Zhu C; Murry BP; Watkins WJ Discovery of GS-9669, a Thumb Site II Non-Nucleoside Inhibitor of NS5B for the Treatment of Genotype 1 Chronic Hepatitis C Infection. J. Med. Chem 2014, 57, 1893–1901. [DOI] [PubMed] [Google Scholar]; (c) Westphal MV; Wolfstädter BT; Plancher J-M; Gatfield J; Carreira EM Evaluation of tert-Butyl Isosteres: Case Studies of Physicochemical and Pharmacokinetic Properties, Efficacies, and Activities. Chem. Med. Chem 2015, 10, 461–469. [DOI] [PubMed] [Google Scholar]; (d) Bezencon Olivier; Heidmann Bibia; Siegrist R; Stamm S; Sylvia R; Pozzi D; Corminboeuf O; Roch C; Kessler M; Ertel EA; Reymond I; Pfeifer T; de Kanter R; Toeroek-Schafroth M; Moccia LG; Mawet J; Moon R; Rey M; Capeleto B; Fournier E Discovery of a Potent, Selective T-type Calcium Channel Blocker as a Drug Candidate for the Treatment of Generalized Epilepsies. J. Med. Chem 2017, 60, 9769–9789. [DOI] [PubMed] [Google Scholar]
- 4.Reviews on CF3 cyclopropanes:; (a) Bos M; Poisson T; Pannecoucke X; Charette AB; Jubault P Recent Progress Toward the Synthesis of Trifluoromethyl- and Difluoromethyl-Substituted Cyclopropanes, Chem –Eur. J 2017, 23, 4950–4961; [DOI] [PubMed] [Google Scholar]; (b) Grygorenko OO; Artamonov OS; Komarov IV; Mykhailiuk PK Trifluoromethyl-substituted cyclopropanes Tetrahedron 2011, 67, 803 [Google Scholar]
- 5.Some alternate strategies to access these type of CF3 cyclopropanes: Cationic ring closure:; (a) Mercadante MA; Kelly CB; Hamlin TA; Delle Chiaie KR; Drago MD; Duffy KK; Dumas MT; Fager DC; Glod BLC; Hansen KE; Hill CR; Leising RM; Lynes CL; MacInnis AE; McGohey MR; Murray SA; Piquette MC; Roy SL; Smith RM; Sullivan KR; Truong BH; Vailonis KM; Gorbatyuk V; Leadbeater NE; Tilley LJ 1,3-γ-Silyl-elimination in electron-deficient cationic systems Chem. Sci 2014, 5, 3983–3994.; [Google Scholar]; (b) Kelly CB; Mercadante MA; Carnaghan ER; Doherty MJ; Fager DC; Hauck JJ; MacInnis AE; Tilley LJ; Leadbeater NE Synthesis of perfluoroalkyl-substituted vinylcyclopropanes by way of enhanced neighboring group participation Eur. J. Org. Chem 2015, 4071–4076.; Via Minisci-type alkylation of heteroarenes: [Google Scholar]; (c) Gianatassio R; Kawamura S; Eprile CL; Foo K; Ge J; Burns AC; Collins MR; Baran PS Simple sulfinate synthesis enables C–H trifluoromethylcyclopropanation Angew. Chem., Int. Ed 2014, 53, 9851–9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Simmons HE; Smith RD A new synthesis of cyclopropanes from olefins. J. Am. Chem. Soc 1958, 80, 5323–5324. [Google Scholar]; (b) Simmons HE A New Synthesis of Cyclopropanes. J. Am. Chem. Soc 1959, 81, 4256–4264. [Google Scholar]; (c) Furukawa J; Kawabata N; Nishimura J A novel route to cyclopropanes from olefins. Tetrahedron Lett 1966, 7, 3353–3354. [Google Scholar]; (d) Denmark SE; Edwards JP A comparison of (chloromethyl)- and (iodomethyl)zinc cyclopropanation reagents. Org. Chem 1991, 56, 6974–6981. [Google Scholar]; (e) Charette AB; Francoeur S; Martel J Wilb N New Family of Cyclopropanating Reagents: Synthesis, Reactivity, and Stability Studies of Iodomethylzinc Phenoxides. Angew. Chem. Int., Ed 2000, 39, 4539–4542. [PubMed] [Google Scholar]; (f) Charette AB; Beauchemin A; Francoeur S Acyloxymethylzinc Reagents: Preparation, Reactivity, and Solid-State Structure of This Novel Class of Cyclopropanating Reagents. J. Am. Chem. Soc 2001, 123, 8139–8140. [DOI] [PubMed] [Google Scholar]; (g) Charette AB; Beauchemin A Simmons-Smith Cyclopropanation Reaction. Org. React 2001, 58, 1–395. [Google Scholar]
- 7.(a) Morandi B; Carreira EM Iron-Catalyzed Cyclopropanation in 6 M KOH with in Situ Generation of Diazomethane. Science 2012, 335, 1471–1474. [DOI] [PubMed] [Google Scholar]; (b) Ebner C; Carreira EM Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev 2017, 117, 11651–11679. [DOI] [PubMed] [Google Scholar]
- 8.(a) Johnson AW; LaCount RB The Chemistry of Ylids. VI. Dimethylsulfonium Fluorenylide—A Synthesis of Epoxides. J. Am. Chem. Soc 1961, 83, 417–423. [Google Scholar]; (b) Corey EJ Dimethyloxosulfonium Methylide ((CH3)2SOCH2) and Dimethylsulfonium Methylide ((CH3)2SCH2). Formation and Application to Organic Synthesis. J. Am. Chem. Soc 1965, 87, 1353–1364. [Google Scholar]
- 9.For select examples using diazo compounds, see:; (a) Huang L; Chen Y; Gao G-Y; Zhang XP Diastereoselective and Enantioselective Cyclopropanation of Alkenes Catalyzed by Cobalt Porphyrins. J. Org. Chem 2003, 68, 8179–8184. [DOI] [PubMed] [Google Scholar]; (b) Dzik WI; Xu X; Zhang XP; Reek JNH; de Bruin B ‘Carbene Radicals’ in CoII(por)-Catalyzed Olefin Cyclopropanation. J. Am. Chem. Soc 2010, 132, 10891–10902. [DOI] [PubMed] [Google Scholar]; (c) Wang Y; Wen X; Cui X; Wojtas L; Zhang XP Asymmetric Radical Cyclopropanation of Alkenes with In Situ-Generated Donor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis. J. Am. Chem. Soc 2017, 139, 1049–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu X; Wang Y; Cui X; Wojtas L; Zhang XP Metalloradical activation of α-formyldiazoacetates for the catalytic asymmetric radical cyclopropanation of alkenes. Chem. Sci 2017, 8, 4347–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sarabia FJ Radical Cation Cyclopropanations via Chromium Photooxidative Catalysis. Org. Lett 2017, 19, 2865–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Čeković Ž; Saičić R Radical cyclization reactions. Cyclopro-pane ring formation by 3-exo-cyclization of 5-phenylthio-3-pentenyl radicals. Tetrahedron Lett 1990, 31, 6085–6088. [Google Scholar]; (b) Ye L; Gu Q-S; Tian Y; Meng X; Chen G-C; Liu X-Y Radical asymmetric intramolecular α-cyclopropanation of aldehydes towards bicyclo[3.1.0]hexanes containing vicinal all-carbon quaternary stereocenters. Nat. Commun 2018, 9, Article 227. https://www.nature.com/articles/s41467-017-02231-7 (accessed Jan 22, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) del Hoyo AM; Herraiz AG; Suero MG A Stereoconvergent Cyclopropanation Reaction of Styrenes. Angew. Chem. Int., Ed 2017, 56, 1610–1613. [DOI] [PubMed] [Google Scholar]; (b) del Hoyo AM; Suero MG Photoredox-Catalyzed Cyclopropanation of Michael Acceptors. Eur. J. Org. Chem 2017, 2122–2125. [Google Scholar]
- 12.For reviews on photoredox catalysis, see:; (a) Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tellis JC; Kelly CB; Primer DN; Jouffroy M; Patel NR; Molander GA Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3–sp2 Cross-Coupling. Acc. Chem. Res 2016, 49, 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev 2016, 116, 10075–10166. [DOI] [PubMed] [Google Scholar]; (d) Matsui JK; Lang SB; Heitz DR; Molander GA Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development. ACS Catal 2017, 7, 2563–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Twilton J; Le CC; Zhang P; Shaw MH; Evans RW; Macmillan DWC The merger of transition metal and photocatalysis. Nat. Rev. Chem 2017, 1, Article 52. https://www.nature.com/articles/s41570-017-0052 (accessed Jan 25, 2018). [Google Scholar]
- 13.For examples, see:; (a) Blomstrom DC; Herbig K; Simmons HE Photolysis of Methylene Iodide in the Presence of Olefins. J. Org. Chem 1965, 30, 959–964. [Google Scholar]; (b) Kropp PJ; Pienta NJ; Sawyer JA; Polniaszek RP Photochemistry of alkyl halides—VII : Cyclopropanation of alkenes. Tetrahedron 1981, 37, 3229–3236. [Google Scholar]; (c) Kropp PJ Photobehavior of alkyl halides in solution: radical, carbocation, and carbene intermediates. Acc. Chem. Res 1984, 17, 131–137. [Google Scholar]; (d) Phillips DL; Fang W-H; Zhang X Isodiiodomethane Is the Methylene Transfer Agent in Cyclopropanation Reactions with Olefins Using Ultraviolet Photolysis of Diiodomethane in Solutions: A Density Functional Theory Investigation of the Reactions of Isodiiodomethane, Iodomethyl Radical, and Iodomethyl Cation with Ethylene. J. Am. Chem. Soc 2001, 123, 4197–4203. [DOI] [PubMed] [Google Scholar]
- 14.For selected reactions using bis(catecholato)silicates, see:; (a) Corcé V; Chamoreau L-M; Derat E; Goddard J-P; Ollivier C; Fensterbank L Silicates as Latent Alkyl Radical Precursors: Visible-Light Photocatalytic Oxidation of Hypervalent Bis-Catecholato Silicon Compounds. Angew. Chem. Int., Ed 2015, 54, 11414–11418. [DOI] [PubMed] [Google Scholar]; (b) Jouffroy M; Primer DN; Molander GA Base-Free Photoredox/Nickel Dual-Catalytic Cross-Coupling of Ammonium Alkylsilicates. J. Am. Chem. Soc 2016, 138, 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Patel NR; Kelly CB; Siegenfeld AP; Molander GA Mild, Redox-Neutral Alkylation of Imines Enabled by an Organic Photocatalyst. ACS Catal 2017, 7, 1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lin K; Kelly CB; Jouffroy M; Molander GA Preparation of Diisopropylammonium Bis(catecholato)-cyclohexylsilicate. Org. Synth, 2017, 94, 16–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.For representative examples of CF3 alkene synthesis see:; (a) Pan R-Q; Liu X-X; Deng M-ZJ Fluorine Chem 1999, 95, 167–170. [Google Scholar]; (b) Hamlin TA; Kelly CB; Cywar RM; Leadbeater NE Methyle-nation of Perfluoroalkyl Ketones using a Peterson Olefination Approach. J. Org. Chem 2014, 79, 1145–1155. [DOI] [PubMed] [Google Scholar]; (c) Phelan JP; Wiles RW; Lang SB; Kelly CB; Molander GA Rapid Access to Diverse Trifluoromethyl-Substituted Alkenes Using Complementary Strategies. Chem. Sci 2018, 9, 3215–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.For radical additions to trifluoromethylalkenes, see:; (a) Bergstrom DE; Ng MW; Wong JJ Addition Reaction of 3,3,3-Trifluoropropene to Tetrahydrofuran. J. Chem. Soc., Perkin Trans 1 1983, 741–745. [Google Scholar]; (b) Narita T; Hagiwara T; Hamana H; Kitamura K; Inagaki Y; Yoshida Y Radical Addition Reaction of 2-Benzoyloxypentafluoropropene onto Cycloalkenes. J. Fluorine Chem 1999, 97, 263–265. [Google Scholar]; (c) Hosoya A; Umino Y; Narita T; Hamana H Carbon–carbon Bond Formation by Radical Addition of α-Trifluoromethylacrylate with Cyclic Ethers J. Fluorine Chem 2008, 129, 91–96. For examples using photoredox catalysis, see: [Google Scholar]; (d) Xiao T; Li L; Zhou L Synthesis of Functionalized gem-Difluoroalkenes via a Photocatalytci Decarboxylative/Defluorinative Reaction. J. Org. Chem 2016, 81, 7908–7916. [DOI] [PubMed] [Google Scholar]; (e) Chen H; Xiao T; Li L; Anand D; He Y; Zhou L Synthesis of Fluorinated Benzo[a]quinolizidines via Visible Light-induced Tandem Substitution of Two Fluorine Atoms in a CF3 Group. Adv. Synth. Catal 2017, 359, 3642–3647. [Google Scholar]; (f) Lang SB; Wiles RJ; Kelly CB; Molander GA Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics. Angew. Chem. Int., Ed 2017, 56, 15073–15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For E1cB-type fluoride elimination, see:; (a) Bégué J-P; Bonnet-Delpon D; Rock MH A concise synthesis of functionalised gemdifluoroalkenes, via the addition of organolithium reagents to α-trifluoromethylstyrene. Tetrahedron Lett 1995, 36, 5003–5006. [Google Scholar]; (b) Bégué J-P; Bonnet-Delpon D; Rock MH Addition of organolithium reagents to α-(trifluoromethyl)styrene: concise synthesis of functionalised gem-difluoroalkenes. J. Chem. Soc. Perkin Trans 1 1996, 1409–1413. For a theoretical study, see: [Google Scholar]; (c) Alunni S; de Angelis F; Ottavi L; Papavasileiou M; Tarantelli F Evidence of a Borderline Region between E1cb and E2 Elimination Reaction Mechanisms: A Combined Experimental and Theoretical Study of Systems Activated by the Pyridine Ring. J. Am. Chem. Soc 2005, 127, 15151–15160. [DOI] [PubMed] [Google Scholar]
- 18.Luo J; Zhang J Donor–Acceptor Fluorophores for Visible-Light-Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)–C(sp2) Cross-Coupling. ACS Catal 2016, 6, 873–877. [Google Scholar]
- 19.The product was isolated as a 95:5 mixture of product and 2-methylated product, presumably due to a Minisci type radical substitution. For select example on Minisci reactions using photoredox catalysis, see:; (a) MacMillan DWC Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 2011, 480, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DiRocco DA; Dykstra K; Krska S; Vachal P; Conway DV; Tudge M Late-Stage Functionalization of Biologically Active Heterocycles Through Photoredox Catalysis. Angew. Chem. Int., Ed 2014, 53, 4802–4806. [DOI] [PubMed] [Google Scholar]; (c) Matsui JK; Primer DN; Mo-lander GA Metal-free C–H alkylation of heteroarenes with alkyltrifluoroborates: a general protocol for 1°, 2° and 3° alkylation. Chem. Sci 2017, 8, 3512–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Garza-Sanchez RA; Tlahuext-Aca A; Tavakoli G; Glorius F Visible Light-Mediated Direct Decarboxylative C–H Functionalization of Heteroarenes. ACS Catal 2017, 7, 4057–4061. [Google Scholar]; (e) Nuhant P; Oderinde MS; Genovion J; Juneau A; Gagné Y; Allais C; Chinigo GM; Choi C; Sach NW; Bernier L; Fobian YM; Bundesmann MW; Khunte B; Frenette M; Fadeyi OO Visible-Light-Initiated Manganese Catalysis for C–H Alkylation of Heteroarenes: Applications and Mechanistic Studies. Angew. Chem. Int., Ed 2017, 56, 15309–15313. [DOI] [PubMed] [Google Scholar]
- 20.For biological activity of Ticagrelor, see:; (a) Wijeyeratne YD; Joshi R; Heptinstall S Ticagrelor: a P2Y12 antagonist for use in acute coronary syndromes. Expert Rev. Clin. Pharmacol 2012, 5, 257–269. For preparation of Ticagrelor, see: [DOI] [PubMed] [Google Scholar]; (b) Larsson U; Magnusson M; Musil T; Palmgren A Novel Triazolo Pyrimidine Compounds. WO 0192263 December 6, 2001. [Google Scholar]; (c) Clark A; Jones E; Larsson U; Minidis A Process for the Preparation of Cyclopropyl Carboxylic Acid Esters and Derivatives. U.S. Patent 7,122,695B2, October 17, 2006. [Google Scholar]; (d) Wang J; Sánche-Roselló Aceña, J. L.; del Pozo C; Sorchinsky AE; Fustero S; Soloshonok VA; Liu H Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev 2014, 114, 2432–2506. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y; Truhlar D The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc 2008, 120, 215–241. [Google Scholar]
- 22.(a) Neese F The ORCA program system Wiley Interdiscip. Rev.: Comput. Mol. Sci 2012, 2, 73–78. [Google Scholar]; (b) Riplinger C; Sandhoefer B; Hansen A; Neese F Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys 2013, 139, 134101–134113. [DOI] [PubMed] [Google Scholar]
- 23.(a) Liakos DG; Sparta M; Kesharwani MK; Martin JML; Neese F Exploring the Accuracy Limits of Local Pair Natural Orbital Coupled-Cluster Theory. J. Chem. Theory Comput, 2015, 11, 1525–1539. [DOI] [PubMed] [Google Scholar]; (b) Paulechka E; Kazakov A Efficient DLPNO- CCSD(T)-Based Estimation of Formation Enthalpies for C-, H-, O-, and N-Containing Closed-Shell Compounds Validated Against Critically Evaluated Experimental Data. J. Phys. Chem. A, 2017, 121, 4379–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.For a recent study disclosing similar observations, see:; Li X; Liao T; Chung LW Computational Prediction of Excited-State Carbon Tunneling in the Two Steps of Triplet Zimmerman Di-π-Methane Rearrangement. J. Am. Chem. Soc 2017, 139, 16438–16441. [DOI] [PubMed] [Google Scholar]
- 25.Legault CY CYLview, 1.0b; Université de Sherbrooke: Sherbrooke, Canada, 2009, (http://www.cylview.org). [Google Scholar]
- 26.For selected reviews, see:; (a) Renaud R; Gerster M Use of Lewis Acids in Free Radical Reactions. Angew. Chem., Int. Ed 1998, 37, 2562. [DOI] [PubMed] [Google Scholar]; (b) Sibi MP; Porter NA Enantioselective Free Radical Reactions Acc. Chem. Res 1999, 32, 163. [Google Scholar]; (c) Zhang W Intramolecular free radical conjugate additions. Tetrahedron 2001, 57, 7237–7262. [Google Scholar]; (d) Sibi MP; Manyem S; Zimmerman J Enantioselective Radical Processes Chem. Rev 2003, 103, 3263. [DOI] [PubMed] [Google Scholar]; (e) Srikanth GSC; Castle SL Advances in radical conjugate additions. Tetrahedron 2005, 61, 10377–10441. [Google Scholar]
- 27.Blanksby SJ; Ellison GB Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res 2003, 36, 255–263. [DOI] [PubMed] [Google Scholar]
- 28.For selected reports, see:; (a) Chu L; Ohta C; Zuo Z; MacMillan DWC Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-Pregabalin. J. Am. Chem. Soc 2014, 136, 10886–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Millet A; Lefebvre Q; Rueping M Visible-Light Photoredox-Catalyzed Giese Reaction: Decarboxylative Addition of Amino Acid Derived a-Amino Radicals to Electron-Deficient Olefins. Chem. Eur. J 2016, 22, 13464–13468. [DOI] [PubMed] [Google Scholar]; (c) Ramirez NP; Gonzalez-Gomez JC Decarboxylative Giese-Type Reaction of Carboxylic Acids Promoted by Visible Light: A Sustainable and Photoredox-Neutral Protocol. Eur. J. Org. Chem 2017, 2154–2163. [Google Scholar]
- 29.For selected recent reports, see:; (a) Noble A; Mega RS; Pflästerer D; Myers EL; Aggarwal VK Visible-Light-Mediated Decarboxylative Radical Additions to Vinyl Boronic Esters: Rapid Access to γ-Amino Boronic Esters Angew. Chem., Int. Ed 2018, 57, 2155–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lovinger GJ; Morken JP Ni-Catalyzed Enantioselective Conjunctive Coupling with C(sp3) Electrophiles: A Radical-Ionic Mechanistic Dichotomy. J. Am. Chem. Soc 2017, 139, 17293–17296. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Silvi M; Sandford C; Aggarwal VK Merging Photoredox with 1,2-Metallate Rearrangements: The Photochemical Alkylation of Vinyl Boronate Complexes. J. Am. Chem. Soc 2017, 139, 5736–5739. [DOI] [PubMed] [Google Scholar]; (d) Kischkewitz M; Okamoto K; Mück-Lichtenfeld C; Studer A Radical-polar crossover reactions of vinylboronate complexes. Science 2017, 355, 936–938. [DOI] [PubMed] [Google Scholar]; (e) Fernandez Reina D; Ruffoni A; Al-Faiyz YSS; Douglas JJ; Sheikh NS; Leonori D Visible-Light-Mediated Reactions of Electrophilic Radicals with Vinyl and Allyl Trifluoroborates. ACS Catal 2017, 7, 4126–4130. [Google Scholar]; (f) Quiclet-Sire B; Zard SZ Radical Instability in Aid of Efficiency: A Powerful Route to Highly Functional MIDA Boronates J. Am. Chem. Soc 2015, 137, 6762–6765. [DOI] [PubMed] [Google Scholar]
- 30.Halgren TA; Roberts JD; Horner JH; Martinez FN; Tronche C; Newcomb M Kinetics and Equilibrium Constants for Reactions of α-Phenyl-Substituted Cyclopropylcarbinyl Radicals. J. Am. Chem. Soc 2000, 122, 2988–2994. [Google Scholar]
- 31.For a more in-depth discussion and computation analysis of the process reported in Ref 11a, see Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.