Abstract
Background:
Long term exposure to particulate matter <2.5μm in diameter (PM2.5) and ozone has been associated with the development and progression of cardiovascular disease and, in the case of PM2.5, higher cardiovascular mortality. Whether exposure to PM2.5 and ozone is associated with patients’ health status and quality of life, is unknown. We used data from two prospective myocardial infarction (MI) registries to assess the relationship between long-term PM2.5 and ozone exposure with health status outcomes one year after a MI.
Methods and Results:
TRIUMPH and PREMIER enrolled patients presenting with MI at 31 U.S. hospitals between 2003 and 2008. One year later, patients were assessed with the disease-specific Seattle Angina Questionnaire (SAQ) and 5-year mortality was assessed with the Centers for Disease Control’s National Death Index. Individual patients’ exposures to PM2.5 and ozone over the year after their MI were estimated from the EPA’s Fused Air Quality Surface Using Downscaling tool that integrates monitoring station data and atmospheric models to predict daily air pollution exposure at the census tract level. We assessed the association of exposure to ozone and PM2.5 with 1-year health status and mortality over 5-years using regression models adjusting for age, sex, race, socioeconomic status, date of enrollment and comorbidities. In completely adjusted models, higher PM2.5 and ozone exposure were independently associated with poorer SAQ summary scores at 1-year (β estimate per +1 SD increase = −0.8 (95% CI −1.4, −0.3 p=0.002) for PM2.5 and −0.9 (95% CI-1.3, −0.4 p<0.001) for ozone). Moreover, higher PM2.5 exposure, but not ozone, was independently associated with greater mortality risk (HR = 1.13 per +1 SD (95% CI = 1.07-1.20, p<0.001).
Conclusions
In our study, greater exposure to PM2.5 and ozone was associated with poorer 1-year health status following an MI, and PM2.5 was associated with increased risk of 5-year death.
Background
Exposure to air pollutants is an important risk factor for premature morbidity and mortality.1 Fine particulate matter <2.5 μm in diameter (PM2.5) and ozone are the most studied air pollutants and have been associated with a wide range of diseases.1 Long-term exposure to PM2.5 has been implicated in the development and progression of cardiovascular disease.2-5 Complex and interlinked mechanisms have been proposed to explain this association with PM2.5 exposure, including higher rates of atherosclerosis6, 7 and the development of cardio-metabolic conditions, such as diabetes mellitus (DM), hypertension (HTN) and dyslipidemia.8 In contrast to PM2.5, there is some uncertainty between ozone exposure and cardiovascular outcomes. In a large study of over 400,000 participants, investigators did not find an association between ozone levels and cardiovascular mortality independent of PM2.5.9 Other studies have found an association between ozone exposure and mortality due to embolism10, ischemic heart disease11, heart failure12 and stroke13, but not all of these studies evaluated this association with ozone independent of PM2.5.
Beyond mortality, patients are equally or more concerned about their health status: their symptoms, function and quality of life (QoL). To date, there have been no studies examining the association between exposure to PM2.5 and ozone with patients’ health status among patients with coronary artery disease (CAD). A deeper understanding of the potential impact of air pollution may further guide public policy regarding air quality standards, as the health benefits of stricter standards are better understood. Accordingly, we used data from the two prospective myocardial infarction (MI) registries to assess the relationship between long term exposure to these important air pollutants with health status one year after MI.
Methods
The investigators are willing to work with others who are interested in validating or extending the analyses. Air quality data for the US is publically available. The analytical codes can be made available but patient specific data will not be publically available.
Study Population
For this study, we included data on patients with an acute MI from two prospective registries with detailed information on patients’ disease-specific health status. The TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction) and PREMIER (Prospective Registry Evaluating Myocardial Infarction: events and Recovery) studies enrolled patients form 31 US hospitals between 2003 and 2008. Detailed design and methods for these registries have previously been described.14, 15 Briefly, patients were required to have biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of MI, including prolonged ischemic signs/symptoms or electrocardiographic criteria of ST segment changes. Baseline data were obtained through chart abstraction and structured interviews by trained research coordinators. Health status data were obtained at baseline and during follow-up, including 1 year after the patients’ MI, using standardized interview conducted by trained study personnel. Each participating site obtained Institutional Research Board approval and all patients provided written informed consent for the interview.
Assessment of Air Pollution Exposures
Each patient’s exposure to ambient air PM2.5 and ozone was estimated using publicly available data from Community Multi-Scale Air Quality Model (CMAQ) and point measurements provided by United States Environment Protection Agency (EPA).16 For this study, exposure to PM2.5 was based on average daily concentrations, expressed in microgram per cubic meter (μg/m3). Exposure to ozone was based on the average of daily 8-hour maximum ozone levels, expressed in parts per million (ppm) moist air molecules in a fixed air volume. These metrics to assess long-term exposure are used commonly throughout the world and the EPA reports PM2.5 and ozone exposure using these metrics.16, 17
Daily average PM2.5 and 8-hour maximum ozone levels were derived from a Bayesian space-time downscaling fusion (DS) model by estimating concentrations at the census tract centroid of the patient’s residence on the basis of National Air Monitoring Stations/State and Local Air Monitoring Stations and CMAQ model data in 12 × 12 km grids. Downscaler PM2.5 and ozone estimates consider all monitors, as opposed to the most prevalent monitor, in areas where there are multiple monitors per site.18 The DS model performance using the predictive mean absolute error showed that the model outperformed ordinary kriging or CMAQ models.19
The main focus of our paper was to examine an association of exposure to air pollutants with health status. However we also wanted to assess if previously described association of greater exposure to air pollutants with mortality was similar in our study cohort. Different approaches for the measurement of air pollutant exposure were used for the health status and mortality comparisons. Because the health status comparison was 1 year after the MI, we used average pollutant exposure over the year after a patient’s MI. In contrast, as the mortality analyses examined survival after discharge, the air pollution exposure over the year prior to the MI was used.
Study Outcomes
Our main study outcome was health status at 1-year after MI. Disease-specific health status was assessed using the Seattle Angina Questionnaire (SAQ). The SAQ is a valid 19-item instrument with a 4-week recall period. It measures 5 domains of health in patients with coronary artery disease (CAD), which are angina frequency (SAQ AF), angina stability, QoL (SAQ QOL), physical limitation (SAQ PL) and treatment satisfaction.20 Domain scores range from 0 to 100, with higher scores indicating fewer symptoms and better QoL. The primary outcome for this study was the overall health status, which is summarized using the SAQ summary score (SAQ SS) and reflects the average of the SAQ PL, AF and QOL domains.21
In addition, we assessed the effect of air pollutants on other health status measures. These included physical health status, as measured by the Physical Component Summary of the Medical Outcomes Study 12-item Short Form (SF-12 PCS).22 The SF-12 is a reliable and valid measure of generic health status and provides summary component scales for overall physical and mental health.23 As dyspnea is a common angina equivalent in patients with coronary artery disease (CAD)24and might also be associated with higher air pollution levels, we also assessed the effect of air pollutants on dyspnea using a 4-level dyspnea item based on the Rose Dyspnea Scale (RDS). The RDS is a 4-item questionnaire with a 1-month recall period that assesses the patient’s level of dyspnea with common activities.25 Each activity associated with dyspnea is assigned 1 point, with scores of 0 indicating no dyspnea and increasing scores indicating greater limitation from dyspnea. The RDS has been used to assess symptoms in patients with coronary artery disease and has shown to be associated with QoL, rehospitalization, procedure success and long-term outcomes.26 Lastly, mortality status over 5-years was determined through a query of the Centers for Disease Control’s National Death Index.
Statistical Analysis
Baseline demographic and clinical characteristics of patients were compared across quartiles of average PM2.5 exposure. Differences in patient characteristics across quartiles of exposure were compared using analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. Categorical variables are presented as mean ± standard deviation (SD) or median with interquartile range. Categorical variables are presented as number (n) with percentage.
We evaluate the association between exposures to average daily ambient air PM2.5 and ozone with health status using generalized additive models with Gaussian errors for SAQ and SF-12 PCS scores and using a proportional odds logistic regression model for dyspnea scores. Smoothing or restricted cubic splines were used to allow for non-linear associations. Several models were defined a priori. In Model 1, we assessed unadjusted associations with PM2.5 and ozone without adjusting for any covariates, except for ozone levels when assessing PM2.5 and adjusting for PM2.5 when assessing the impact of ozone. In Model 2, we additionally adjusted for covariates known to be associated with health status in patients with CAD.27 These were demographics (age, sex and race), smoking, date of enrollment, and socioeconomic status (SES). SES has been shown to be associated with worse outcomes after MI28 and was quantified using patients’ education, insurance status, history of avoiding care due to costs, and end-of-the-month financial resources. We also adjusted for date of enrollment to account for temporal or seasonal effects. Since PM2.5 has been previously shown to be associated with the development of HTN, DM, chronic kidney disease (CKD) (defined as eGFR <60) and heart failure8, we constructed Model 3 to assess whether there remained an independent association between exposure to PM2.5 and ozone and health status after accounting for these factors; which could potentially have either confounding or mediating effects, or both. Next, to evaluate whether air pollutants were also associated with change in health status following MI we constructed an additional model using change in health status from baseline to 1 year as the outcome with adjustment for the covariates included in the second model, as well as baseline health status. Finally as higher concentrations of ozone can amplify the adverse cardiovascular effects of PM2.529 we augmented Model 2 to include an interaction between PM2.5 and ozone levels, and estimated the effect of PM2.5 at different ozone concentrations (10th and 90th percentiles and median of the distribution in our study).
While the main objective of our paper was to determine the association of air pollutant exposure with the health status of patients with stable ischemic heart disease who recently had a MI, we also wanted to see if the association of PM2.5 with mortality was similar in our study cohort as has been described in previous studies. Hence we examined the association of 12-month average air quality parameters prior to patient’s MI with all-cause mortality through 5 years after patients’ index MI. We calculated crude survival rates by quartiles of PM2.5 and ozone exposure using Kaplan-Meier methods, and we estimated hazard ratios using Cox regression models adjusted for patients’ demographics (age, sex and race), SES, smoking status and comorbidities, including HTN, DM, CKD, heart failure, left ventricular function, prior to MI and Global Registry of Acute Coronary Events (GRACE) mortality risk score. We additionally adjusted for ozone levels when assessing the associations between PM2.5 and outcomes and PM2.5 levels when assessing association with ozone. The proportional hazards assumptions were assessed using Schoenfeld residuals.
All models included smoothing or restricted cubic splines for estimating effects of continuous variables to accommodate nonlinear relationships. In cases where no significant evidence of nonlinearity was found, associations were re-estimated using linear effects to simplify interpretation. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.5.2.30 P-values were evaluated at a 2-sided significance level of 0.05.
Results
Study Population
The PREMIER and TRIUMPH studies enrolled a total of 2,498 (between 1/1/2003-6/28/2004) and 4,340 patients (between 4/11/2005-12/31/2008), respectively. Some patients (n=1,198) could not be linked to EPA air quality data and the final study cohort for assessing 5-year mortality was 5,650. After excluding patients who did not have complete health status assessments due to death or loss to follow-up (n=1,727), our final study cohort for assessing 1-year health status was 3,913 patients (Supplemental Figure 1). Supplementary tables 1 and 2 compare the differences in patient characteristics between patients who were excluded and those who were not. There were significant differences in age, race, socioeconomic status and burden of comorbidities. Patients who were excluded were younger, had worse SES across all 4 SES variables that were assessed and a higher proportion had HTN, DM, CKD and heart failure. We adjusted for all these factors in our outcome analysis. Additionally patients who were excluded had on average lower peak troponin levels, lower GRACE mortality scores and a lower proportion presented with ST elevation myocardial infarction.
The mean age of the final analytical cohort was 60.6 ± 12.2, 33% were females and 25% were non-Caucasian. Comorbidities were common, with 64% of the patients having hypertension, 28% having diabetes and 40% having systolic dysfunction (EF < 50%). Fifteen percent of the patients were uninsured and 16% reported not having enough money at the end of the month to make ends meet. The mean 12-month average PM2.5 and ozone exposure per patient were 11.96 ± 2.11 μg/m3 (range = 4.3-20.5) and 0.0383 ± 0.0035 ppm (range= 0.0267-0.0534). There was no significant correlation between average PM2.5 exposure and average ozone exposure (Spearman r = −0.02, p=0.15).
Table 1 describes the baseline demographic and clinical characteristics, stratified according to quartiles of PM2.5 concentration. Patients in higher quartiles of PM2.5 concentration had lower Global Registry of Acute Coronary Events (GRACE) scores, lower peak troponin level, were more likely to avoid care due to costs, and were more likely to have HTN, prior MI, DM, heart failure, CKD, and chronic lung disease.
Table 1.
Baseline demographic characteristics of patients compared according to exposure to PM2.5 over 1 year after myocardial infarction.
| PM2.5 Concentration (μg/m3) | |||||
|---|---|---|---|---|---|
| Quartile 1 (4.3 to 10.6) n = 978 |
Quartile 2 (10.7 to 12.0) n = 978 |
Quartile 3 (12.1 to 13.2) n = 978 |
Quartile 4 (13.3 to 20.5) n = 979 |
p-value | |
| Demographics | |||||
| Age (Mean ± SD) | 60.4 ± 11.7 | 60.8 ± 12.1 | 60.4 ± 12.4 | 60.5 ± 12.7 | 0.860 |
| Female | 322 (32.9%) | 271 (27.7%) | 315 (32.2%) | 372 (38.0%) | < 0.001 |
| Caucasian Race | 826 (84.7%) | 841 (86.3%) | 740 (75.9%) | 518 (53.1%) | < 0.001 |
| Ozone exposure (ppm) (Mean ± SD) | 0.0384 ± 0.0036 | 0.0385 ± 0.0027 | 0.0382 ± 0.0036 | 0.0382 ± 0.0039 | 0.140 |
| Socioeconomic Status | |||||
| High school education | 843 (86.4%) | 818 (84.0%) | 798 (82.4%) | 715 (74.4%) | < 0.001 |
| Uninsured | 98 (10.4%) | 159 (16.5%) | 135 (14.3%) | 199 (21.2%) | < 0.001 |
| Avoiding care due to cost | 198 (20.4%) | 198 (20.5%) | 180 (18.8%) | 205 (21.7%) | 0.470 |
| Not enough money left at end of the month | 129 (13.4%) | 34 (14.1%) | 145 (15.3%) | 188 (19.8%) | < 0.001 |
| Comorbidities | |||||
| Current Smoker | 168 (17.6%) | 176 (18.3%) | 192 (20.5%) | 202 (21.8%) | 0.08 |
| Hypertension | 594 (60.7%) | 601 (61.5%) | 613 (62.7%) | 711 (72.6%) | < 0.001 |
| Prior MI | 173 (17.7%) | 173 (17.7%) | 197 (20.1%) | 210 (21.5%) | 0.085 |
| Prior PCI | 137 (14.0%) | 182 (18.6%) | 215 (22.0%) | 173 (17.7%) | < 0.001 |
| Prior CABG | 93 (9.5%) | 115 (11.8%) | 126 (12.9%) | 132 (13.5%) | 0.035 |
| Diabetes | 238 (24.3%) | 251 (25.7%) | 291 (29.8%) | 326 (33.3%) | < 0.001 |
| Heart failure | 51 (5.2%) | 60 (6.1%) | 55 (5.6%) | 125 (12.8%) | < 0.001 |
| Lung disease | 63 (6.4%) | 76 (7.8%) | 91 (9.3%) | 102 (10.4%) | 0.009 |
| CKD | 46 (4.7%) | 51 (5.2%) | 59 (6.0%) | 112 (11.4%) | < 0.001 |
| MI presentation | |||||
| STEMI | 462 (47.2%) | 496 (50.7%) | 470 (48.1%) | 346 (35.3%) | < 0.001 |
| Normal LV function | 622 (63.6%) | 556 (57.0%) | 580 (59.4%) | 585 (59.9%) | < 0.001 |
| Peak troponin (Median (IQR)) | 7.6 (1.6, 31.6) | 9.7 (2.3, 50.1) | 8.7 (2.1, 37.7) | 4.1 (0.9, 16.1) | < 0.001 |
| GRACE score (Median (IQR)) | 7.6 (1.6, 31.6) | 9.7 (2.3, 50.1) | 8.7 (2.1, 37.7) | 4.1 (0.9, 16.1) | < 0.001 |
CABG= Coronary Artery Bypass Graft surgery, CKD= Chronic Kidney Disease, MI= Myocardial Infarction, PCI= Percutaneous Coronary Intervention
Supplementary table 3 describes the baseline demographics and clinical characteristics of patients stratified according to quartiles of ozone exposure. There were significant differences in the prevalence of diabetes, heart failure and chronic kidney disease in groups stratified by ozone exposure.
Association between PM2.5 and Ozone with Health Status
The mean SAQ SS at 1 year was 89.2 ± 15.7. The mean dyspnea score was 0.80 ± 1.08 and the mean SF-12 PCS score was 44.1 ±11.8 respectively. Table 2 compares health status scores at one year in patients according to quartiles of PM2.5 exposure. Patients with higher exposure to PM2.5 had worse health status scores.
Table 2.
Health status of patients at baseline and one year after MI compared according to exposure to PM2.5 over 1 year after myocardial infarction.
| PM2.5 Concentration (μg/m3) | |||||
|---|---|---|---|---|---|
| Quartile 1 (4.3 to 10.6) n = 978 |
Quartile 2 (10.7 to 12.0) n = 978 |
Quartile 3 (12.1 to 13.2) n = 978 |
Quartile 4 (13.3 to 20.5) n = 979 |
p-value | |
| Seattle Angina Questionnaire Summary Score | |||||
| Baseline | 79.3 ± 17.2 | 78.6 ± 16.0 | 79.0 ± 17.3 | 75.4 ± 19.0 | < 0.001 |
| 1-year | 90.3 ± 14.5 | 90.2 ± 14.3 | 89.2 ± 15.4 | 87.1 ± 18.2 | < 0.001 |
| Dyspnea Score | |||||
| Baseline | 1.02 ± 1.08 | 0.94 ± 1.07 | 0.93 ± 1.09 | 1.21 ± 1.23 | < 0.001 |
| 1-year | 0.71 ± 1.03 | 0.75 ± 1.07 | 0.79 ± 1.06 | 0.97 ± 1.15 | < 0.001 |
| SF-12 PCS | |||||
| Baseline | 43.9 ± 12.1 | 44.0 ± 12.0 | 43.8 ± 12.0 | 41.9 ± 12.6 | < 0.001 |
| 1-year | 45.1 ± 11.7 | 44.6 ± 11.5 | 44.3 ± 11.8 | 42.2 ± 12.1 | < 0.001 |
SF-12 PCS=Physical Component Summary of the Medical Outcomes Study 12-item Short Form
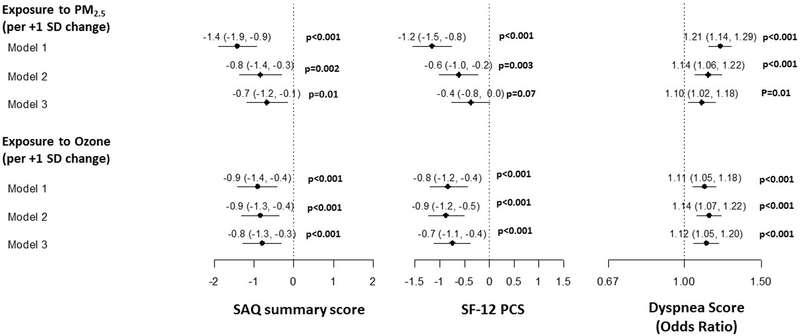
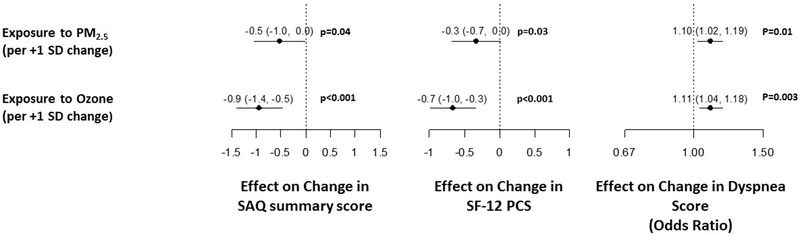
In unadjusted analysis (Model 1), there was a significant association of higher PM2.5 concentration and ozone level with worse generic and disease-specific health status (Figure 1). Adjusting for demographics, smoking, SES and date of enrollment (Model 2) did not attenuate these associations. Even in Model 3, which additionally adjusted for comorbidities associated with air pollution, there remained a significant association with worse SAQ SS, dyspnea and SF-12 PCS scores. No significant nonlinearity was found for the effects of either PM2.5 or ozone on any of the health status outcomes (p>0.1 for all), so all associations are summarized as linear effects. Furthermore, higher PM2.5 and ozone concentration was significantly associated with worse recovery (i.e., change) in health status 1-year after an MI (Figure 2).
Figure 1.
Association of PM2.5 and Ozone exposure with health status one year after MI.
MI= Myocardial Infarction, PM2.5= Particulate Matter < 2.5μm
Figure 2.
Association of PM2.5 and Ozone exposure with change in health status one year after MI
MI= Myocardial Infarction, PM2.5= Particulate Matter < 2.5μm
To assess whether ozone levels moderated the association of PM2.5 and health status, we modified Model 2 to include an interaction between PM2.5 and ozone. Although there was no significant interaction for disease specific health status (SAQ SS) and SF-12 PCS scores, higher ozone levels were associated with worse dyspnea for the same PM2.5 concentration (Supplemental Figure 2).
Association between PM2.5 and Ozone with 5-year All-cause Mortality
There was no loss to follow-up in the ascertainment of all-cause mortality at 5 years outcome. Higher quartiles (Q) of PM2.5 exposure in the year leading up to the MI were associated with lower crude 5-year survival (Q1: 85.2%, Q2: 82.1%, Q3: 76.8%, Q4: 73.1%; p<0.001). The association with ozone levels, while nominally significant in unadjusted analyses, was weaker (Q1: 79.7%, Q2: 80.7%, Q3: 79.6%, Q4: 77.1%; p=0.03) (Supplemental Figure 3). In Cox regression analysis adjusting for patient factors, higher PM2.5 exposure was associated with greater mortality risk (HR = 1.13 per +1 SD, 95% CI = 1.07-1.20, p<0.001), while ozone level was not (HR = 1.01 per +1 SD, 95% CI = 0.96-1.06, p=0.67). The proportional hazards assumption was satisfied for both parameters (p>0.3), and no evidence of nonlinearity was found (p=0.59).
Discussion
US policy on air pollution standards is in flux. Recent changes to the regulation of air pollution seeks to promote fossil fuels and abandon prior efforts to curb greenhouse gas emissions. This has raised concerns regarding the deleterious health effects of air pollutants such as PM2.5 and ozone.31 In this first analysis of the association of long-term exposure to PM2.5 and ozone with health status and all-cause mortality after MI, we found small but statistically significant linear associations of higher long-term exposure to ambient air PM2.5 and ozone with worse disease-specific and generic health status in patients with MI, as well as increased risks of 5-year mortality with higher exposures to PM2.5 particles.
Previous studies have shown an association of increased long-term exposure to PM2.5 with higher all-cause mortality11 and increased cardiovascular events32. In patients with MI, long term exposure to PM2.5 was associated with higher cardiovascular mortality.33 Although some prior studies did not show an association of ozone with cardiovascular outcomes independent of PM2.5 exposure9, studies using more precise estimates of ozone exposure, independent of PM2.5, show ozone to also be associated with cardiovascular mortality.13 Our study confirms findings from previous studies regarding association of higher PM2.5 exposure with mortality and adds to the evaluation of long term PM2.5 and ozone exposure with cardiovascular outcomes by demonstrating that both PM2.5 and ozone are independently associated with poorer 1-year health status after MI. Moreover, finding similar associations with PM2.5 and post-MI mortality with previously reported studies (and the increased prevalence of comorbidities associated with air pollution in our study) provide external validity of our novel results regarding worse health status with higher air pollution levels.
Health status outcomes directly assess the impact of disease on patient’s symptoms, function and QoL. In patients with cardiovascular disease, optimizing health status and disease-specific QoL have been recognized as important goals of treatment.34 In addition to defining treatment factors that influence health status, it is important to consider other risk factors that may negatively affect patients’ health status. Our analysis identifies PM2.5 and ozone exposure as two such factors that negatively affect long-term health status of patients with cardiovascular disease. The mechanisms underlying the association of ozone and PM2.5 with poorer health status are likely to be complex. There is abundant pathophysiologic evidence supporting the development and progression of coronary disease with air pollution. Long term exposure of PM2.5 has been shown to be associated with oxidative stress35 endothelial dysfunction36 and increased propensity to coagulation.37 These processes are thought to drive the association of exposure to PM2.5 and development/progression of cardiovascular disease. Additionally, exposure to PM2.5 is strongly associated with several risk factors for cardiovascular disease including HTN38, DM39 dyslipidemia40 , CKD41, obesity42 and breathing disorders43. Ozone has also been shown to enhance the toxic effects of PM2.529, 44 and could have independent effects contributing to adverse cardiovascular outcomes.45, 46 This complex interplay and biochemical relationships could underlie the observed associations between these air pollutants with health status and mortality after MI.
The observed effect sizes of greater air pollutant exposure to health status are small, although statistically significant. However, it is important to consider the results of our study in the context of the range of PM2.5 and ozone exposure throughout the US and the world. While in our study the range for PM2.5 was 4.3-20.5μg/m3, in the US the range of average exposure to PM2.5 is estimated to range from 5-50μg/m3.47 Worldwide, the range is even higher, with levels greater than 100μg/m3 in some developing countries.8 As different metrics for ozone concentration are used worldwide, a global comparison is difficult to make. However it is generally thought that ozone levels measured by daily average of 8-hour maximum vary considerably worldwide.17 Given that we found a linear relationship with both health status and mortality (for PM2.5), the true impact of both ozone and PM2.5, could be even greater in patients exposed to higher concentrations of these air pollutants. The Clean Air Act48 requires the EPA to set National Ambient Air Quality Standards, and the primary standard for average (per year) concentration of PM2.5 has been set at 12μg/m3 and 0.070ppm for ozone.8 We observed a significant impact on mortality and health status at concentrations below these standards.
Our findings should be interpreted in the context of the following potential limitations. This study focused on the impact of ambient air PM2.5 and ozone exposure on health status and mortality in patients after an MI and the primary exposure metric only focused on daily outdoor concentrations. Patients spend their time in various microenvironments, for example in their homes, offices, shopping malls, and could also be exposed to second-hand smoke. As there is likely to be a lot of variability in air pollutant concentrations in these different environments, it is possible that the total exposure of each patient to could have been misclassified, which would have biased our findings to the null. However, outdoor PM2.5 and ozone levels have been shown to be strong proxies for total exposure for an individual patient.49, 50 A second concern is that although we adjusted extensively for comorbid conditions, there is the possibility of residual confounding in this observational study. For example, we did not adjust for recruitment site, as there was a strong correlation between both PM2.5 and ozone exposure and recruitment site (intraclass correlation coefficient (ICC) 79% for both), which is not unexpected. Nevertheless, there is the possibility that site-specific factors could have influenced the results, although the intra-class correlations between health status and site were much smaller (range 3-6%) than for air pollutant exposure (ICC = 79%). Importantly, even after adjusting for comorbidities that may be along the causal pathway between air pollution and cardiovascular outcomes, a significant association between PM2.5 and ozone remained. Finally a number of patients were excluded as they had missing 1-year health status data or could not be linked to EPA air quality data, making it possible that our results could have been slightly different if we had complete data for all patients enrolled. However, it seems unlikely that patients with missing data would exhibit a different association between exposure and health status than those with complete data, particularly after adjustment for other factors.
Conclusions
We found a strong association of high long-term exposure to air pollutants with patients’ 1-year health status and 5-year all-cause mortality after MI. These findings suggest that greater air pollution not only increases patients’ risks for dying, but also worsens their symptoms, function and QoL. While further studies are needed to determine the mechanism of this increased risk, ongoing debates about the regulation of air pollution should consider the impact of higher exposure to PM2.5 and ozone on patients’ health status.
Supplementary Material
WHAT IS KNOWN?
Long-term exposure to ambient air particulate matter <2.5μm (PM2.5) and ozone has been associated with development and progression of coronary artery disease (CAD) and, in the case of PM2.5, higher long-term mortality risk after an acute myocardial infarction (MI).
WHAT THE STUDY ADDS.
In patients with CAD 1 year after an MI, we found higher exposure to both PM2.5 and ozone to be modestly but independently associated with poorer generic and disease-specific health status.
These associations were not attenuated after adjusting for demographics, socioeconomic status and prevalence of comorbidities.
The associations were also preserved after adjusting for baseline health status, indicating that poor air quality was also associated with recovery of health status following an MI.
Similar to previous studies, higher exposure to PM2.5 was associated with higher risk of mortality over 5 years after MI.
Acknowledgments
Sources of Funding:
The TRIUMPH study was funded by National Heart, Lung, and Blood Institute grant (P50 HL077113) and CV Outcomes, Kansas City, Missouri. The PREMIER study was funded by CV Therapeutics, Palo Alto, California. Dr. Malik, Dr. Peri-Okonny and Dr. Hejjaji are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures:
Dr. Spertus owns copyright for the Seattle Angina Questionnaire. He serves as a consultant to United Healthcare, Bayer and Novartis (modest). He has research grants from Abbott Vascular, Novarits and is the PI of an analytic center for the American College of Cardiology (significant). He has an equity interest in Health Outcomes Sciences (significant). The other authors report no disclosures.
References
- 1.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Balde AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potocnik J, Preker AS, Ramesh J, Rockstrom J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K and Zhong M. The Lancet Commission on pollution and health. Lancet (London, England). 2018;391:462–512. [DOI] [PubMed] [Google Scholar]
- 2.Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L and Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart (British Cardiac Society). 2014;100:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Perier MC, Marijon E, Vernerey D, Empana JP and Jouven X. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. Jama. 2012;307:713–21. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Kindzierski W and Kaul P. Air Pollution and Acute Myocardial Infarction Hospital Admission in Alberta, Canada: A Three-Step Procedure Case-Crossover Study. PloS one. 2015;10:e0132769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weichenthal S, Lavigne E, Evans G, Pollitt K and Burnett RT. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study. Environmental health : a global access science source. 2016;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akintoye E, Shi L, Obaitan I, Olusunmade M, Wang Y, Newman JD and Dodson JA. Association between fine particulate matter exposure and subclinical atherosclerosis: A meta-analysis. European journal of preventive cardiology. 2016;23:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Diez Roux AV, Gassett AJ, Jacobs DR Jr.,, Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA and Watson KE Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet (London, England). 2016;388:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajagopalan S, Al-Kindi SG and Brook RD. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2018;72:2054–2070. [DOI] [PubMed] [Google Scholar]
- 9.Jerrett M, Burnett RT, Pope CA 3rd, Ito K, Thurston G, Krewski D, Shi Y, Calle E and Thun M Long-term ozone exposure and mortality. The New England journal of medicine. 2009;360:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoek G, Brunekreef B, Fischer P and van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology (Cambridge, Mass). 2001;12:355–7. [DOI] [PubMed] [Google Scholar]
- 11.Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, Villeneuve PJ, Jerrett M, Goldberg MS, Pope CA 3rd, Brauer M, Brook RD, Robichaud A, Menard R and Burnett RT Ambient PM2.5, O(3), and NO(2) Exposures and Associations with Mortality over 16 Years of Follow-Up in the Canadian Census Health and Environment Cohort (CanCHEC). Environmental health perspectives. 2015;123:1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner MC, Jerrett M, Pope CA 3rd, Krewski D, Gapstur SM, Diver WR, Beckerman BS, Marshall JD, Su J, Crouse DL and Burnett RT Long-Term Ozone Exposure and Mortality in a Large Prospective Study. American journal of respiratory and critical care medicine. 2016;193:1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong YC, Lee JT, Kim H, Ha EH, Schwartz J and Christiani DC. Effects of air pollutants on acute stroke mortality. Environmental health perspectives. 2002;110:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM and Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circulation Cardiovascular quality and outcomes. 2011;4:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C and Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. American heart journal. 2006;151:589–97. [DOI] [PubMed] [Google Scholar]
- 16.United States EPA. Air Data: Air Quality Data Collected at Outdoor Monitors Across the US. 2018;2019. [Google Scholar]
- 17.Lefohn AS, Malley CS, Smith L, Wells B, Hazucha M, Simon H, Naik V, Mills G, Schultz MG, Paoletti E, De Marco A, Xu X, Zhang L, Wang T, Neufeld HS, Musselman RC, Tarasick D, Brauer M, Feng Z, Tang H, Kobayashi K, Sicard P, Solberg S and Gerosa G. Tropospheric ozone assessment report: Global ozone metrics for climate change, human health, and crop/ecosystem research. Elementa (Washington, DC). 2018;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States EPA. Downscaler Model for predicting daily air pollution. 2019. [Google Scholar]
- 19.Berrocal VJ, Gelfand AE and Holland DM. Space-time data fusion under error in computer model output: an application to modeling air quality. Biometrics. 2012;68:837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M and Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. Journal of the American College of Cardiology. 1995;25:333–41. [DOI] [PubMed] [Google Scholar]
- 21.Chan PS, Jones PG, Arnold SA and Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circulation Cardiovascular quality and outcomes. 2014;7:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware J Jr., Kosinski M and Keller SD A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Nordhorn J, Roll S and Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart (British Cardiac Society). 2004;90:523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qintar M, Grantham JA, Sapontis J, Gosch KL, Lombardi W, Karmpaliotis D, Moses J, Salisbury AC, Cohen DJ, Spertus JA and Arnold SV. Dyspnea Among Patients With Chronic Total Occlusions Undergoing Percutaneous Coronary Intervention: Prevalence and Predictors of Improvement. Circulation Cardiovascular quality and outcomes. 2017;10:e003665. doi: 10.1161/CIRCOUTCOMES.117.003665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose GA and Blackburn H. Cardiovascular survey methods. Monograph series World Health Organization. 1968;56:1–188. [PubMed] [Google Scholar]
- 26.Arnold SV, Spertus JA, Jones PG, Xiao L and Cohen DJ. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: results from the PREMIER registry. American heart journal. 2009;157:1042–9.e1. [DOI] [PubMed] [Google Scholar]
- 27.Arnold SV, Jang JS, Tang F, Graham G, Cohen DJ and Spertus JA. Prediction of residual angina after percutaneous coronary intervention. European heart journal Quality of care & clinical outcomes. 2015;1:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahimi AR, Spertus JA, Reid KJ, Bernheim SM and Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. Jama. 2007;297:1063–72. [DOI] [PubMed] [Google Scholar]
- 29.Weichenthal S, Pinault LL and Burnett RT. Impact of Oxidant Gases on the Relationship between Outdoor Fine Particulate Air Pollution and Nonaccidental, Cardiovascular, and Respiratory Mortality. Scientific reports. 2017;7:16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2019. [Google Scholar]
- 31.Samet JM, Burke TA and Goldstein BD. The Trump Administration and the Environment - Heed the Science. The New England journal of medicine. 2017;376:1182–1188. [DOI] [PubMed] [Google Scholar]
- 32.Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, de Faire U, Erbel R, Eriksen KT, Fratiglioni L, Galassi C, Hampel R, Heier M, Hennig F, Hilding A, Hoffmann B, Houthuijs D, Jockel KH, Korek M, Lanki T, Leander K, Magnusson PK, Migliore E, Ostenson CG, Overvad K, Pedersen NL, J JP, Penell J, Pershagen G, Pyko A, Raaschou-Nielsen O, Ranzi A, Ricceri F, Sacerdote C, Salomaa V, Swart W, Turunen AW, Vineis P, Weinmayr G, Wolf K, de Hoogh K, Hoek G, Brunekreef B and Peters A. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ (Clinical research ed). 2014;348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Burnett RT, Copes R, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, Kopp A and Tu JV. Ambient Fine Particulate Matter and Mortality among Survivors of Myocardial Infarction: Population-Based Cohort Study. Environmental health perspectives. 2016;124:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rumsfeld JS, Alexander KP, Goff DC Jr., Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D and Zerwic JJ Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–49. [DOI] [PubMed] [Google Scholar]
- 35.Cachon BF, Firmin S, Verdin A, Ayi-Fanou L, Billet S, Cazier F, Martin PJ, Aissi F, Courcot D, Sanni A and Shirali P. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM(2.5) and PM(>2.5)) collected from Cotonou, Benin. Environmental pollution (Barking, Essex : 1987). 2014;185:340–51. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Qimuge A, Wang H, Xing C, Gu Y, Liu S, Xu H, Hu M and Song L. IRE1alpha/XBP1s branch of UPR links HIF1alpha activation to mediate ANGII-dependent endothelial dysfunction under particulate matter (PM) 2.5 exposure. Scientific reports. 2017;7:13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Chan TC, Guo C, Chang LY, Lin C, Chuang YC, Jiang WK, Ho KF, Tam T, Woo KS, Lau AKH and Lao XQ. Long-term exposure to ambient particulate matter (PM2.5) is associated with platelet counts in adults. Environmental pollution (Barking, Essex : 1987). 2018;240:432–439. [DOI] [PubMed] [Google Scholar]
- 38.Chan SH, Van Hee VC, Bergen S, Szpiro AA, DeRoo LA, London SJ, Marshall JD, Kaufman JD and Sandler DP. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environmental health perspectives. 2015;123:951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiering E, Markevych I, Bruske I, Fuertes E, Kratzsch J, Sugiri D, Hoffmann B, von Berg A, Bauer CP, Koletzko S, Berdel D and Heinrich J. Associations of Residential Long-Term Air Pollution Exposures and Satellite-Derived Greenness with Insulin Resistance in German Adolescents. Environmental health perspectives. 2016;124:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklin BA, Brook R and Arden Pope C 3rd., Air pollution and cardiovascular disease. Current problems in cardiology. 2015;40:207–38. [DOI] [PubMed] [Google Scholar]
- 41.Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, Vokonas PS and Schwartz JD. Long-Term Exposure to Ambient Fine Particulate Matter and Renal Function in Older Men: The Veterans Administration Normative Aging Study. Environmental health perspectives. 2016;124:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Dorans KS, Wilker EH, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Fox CS and Mittleman MA. Residential proximity to major roadways, fine particulate matter, and adiposity: The framingham heart study. Obesity (Silver Spring, Md). 2016;24:2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing YF, Xu YH, Shi MH and Lian YX. The impact of PM2.5 on the human respiratory system. Journal of thoracic disease. 2016;8:E69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosson J, Pourazar J, Forsberg B, Adelroth E, Sandstrom T and Blomberg A. Ozone enhances the airway inflammation initiated by diesel exhaust. Respiratory medicine. 2007;101:1140–6. [DOI] [PubMed] [Google Scholar]
- 45.Miller DB, Ghio AJ, Karoly ED, Bell LN, Snow SJ, Madden MC, Soukup J, Cascio WE, Gilmour MI and Kodavanti UP. Ozone Exposure Increases Circulating Stress Hormones and Lipid Metabolites in Humans. American journal of respiratory and critical care medicine. 2016;193:1382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paffett ML, Zychowski KE, Sheppard L, Robertson S, Weaver JM, Lucas SN and Campen MJ. Ozone Inhalation Impairs Coronary Artery Dilation via Intracellular Oxidative Stress: Evidence for Serum-Borne Factors as Drivers of Systemic Toxicity. Toxicological sciences : an official journal of the Society of Toxicology. 2015;146:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L and Kaufman JD Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. [DOI] [PubMed] [Google Scholar]
- 48.United States EPA. Summary of the Clean Air Act. 2019. [Google Scholar]
- 49.Brown KW, Sarnat JA, Suh HH, Coull BA and Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. The Science of the total environment. 2009;407:3754–65. [DOI] [PubMed] [Google Scholar]
- 50.Liu LJ, Koutrakis P, Leech J and Broder I. Assessment of ozone exposures in the greater metropolitan Toronto area. Journal of the Air & Waste Management Association (1995). 1995;45:223–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.