Abstract
To describe the international landscape of clinical trials in carbon ion radiotherapy (CIRT), we reviewed the current status of 63 ongoing clinical trials (median: 47 subjects) involving CIRT identified from the clinicaltrials.gov trial registry and World Health Organization International Clinical Trials Platform Registry. We evaluated the potential for these trials to define the role of this modality in the treatment of specific cancer types, and to identify major challenges and opportunities to advance this technology. A significant body of literature suggests the potential for advantageous dose distributions and in preclinical biologic studies the enhanced effectiveness for CIRT compared to photons and protons. Additionally, clinical evidence, though limited, from phase I/II trials indicates the potential for CIRT to improve cancer outcomes. However, at present, high level phase III randomized clinical trial evidence does not exist. While there has been an increase in the number of trials investigating CIRT since 2010, and the number of countries and sites offering CIRT is slowly growing, this progress has excluded other countries. We propose several recommendations to study this modality in order to accelerate progress in the field, including to (1) increase the number of multi-national randomized clinical trials (2) leveraging existing CIRT facilities to launch larger multi-national trials directed at common cancers combined with high level quality assurance; and (3) developing more compact and less expensive “next generation” treatment systems integrated with radiobiological research and pre-clinical testing.
Keywords: Clinical Trials, Carbon, lons, Heavy Ion Radiotherapy, Particle therapy
Precis for use in the Table of Contents: Based on a reduced lateral penumbra and higher relative biological effectiveness (RBE), carbon ion radiotherapy (CIRT) should allow improved cancer control rates compared to x-ray or proton based external beam radiotherapy. We propose leveraging existing CIRT facilities to launch larger multi-national trials directed at common cancers combined with high level quality assurance, radiobiological research and major investments in developing more compact and less expensive “next generation” treatment systems.
INTRODUCTION
Nearly half of newly diagnosed cancer patients will undergo radiotherapy (RT), a form of treatment available for more than 100 years.1 Most of these patients are being treated with a form of external beam RT (EBRT) using photons (aka x-rays). Over the past 10 years, there has been increased interest in the development of more sophisticated forms of EBRT, the most popular being proton beam radiation therapy (PBRT).
A comprehensive review of the evidence for PBRT has recently been published.2 The authors concluded that although the proton therapy clinical trial portfolio was “expanding rapidly”, with “the majority of patients . accrued to observational studies”, they also acknowledged the need for these ongoing trials to “be evaluated in terms of comparative effectiveness . with conventional radiation modalities”. Most importantly, despite the comprehensiveness of their review, noticeably absent was the high-level evidence that proton-based treatment resulted in improved clinically significant results for any specific cancer type, site or age group, compared to other forms of radiotherapy. There are now ongoing randomized phase II and phase III studies comparing protons to photons for low grade brain tumors, cancers of the oropharynx, esophagus, lung, breast, prostate, and liver.
A growing number of facilities around the world are treating cancer patients with tumors that are considered resistant to photons with a highly targeted form of radiation withpotential physical and biological advantages over PBRT, namely carbon ion radiotherapy (CIRT)3,4. The purpose of this review is to describe the current status of this potentially more advanced form of radiotherapy, to list the major logistical and technical challenges hampering the wide spread adoption of this technology, to summarize the clinical work completed to date, and to provide recommendations for how these challenges might best be overcome.
Carbon Ion Radiotherapy (CIRT)
Based on the theoretical and early experimental biological advantages of CIRT compared to protons, several research centers around the world have chosen to pursue this treatment modality since the late 1970s3,5–8, even though the initial investment costs are much higher.9,10 Approximately 20,000 patients currently world-wide have been treated with CIRT according to the Particle Therapy Cooperative Group (PTCOG) database (https://ptcog.ch/index.php/clinical-protocols). The technology for radiotherapy with ions heavier than protons was originally developed in the United States of America (USA) at the Lawrence Berkeley National Laboratory (LBNL).11 The first Phase I/II trials with helium and neon ions were conducted in collaboration with LBNL at the University of California San Francisco (UCSF), more than 40 years ago.5,12–15
Unfortunately, funded follow-up of phase I/II clinical trial results of the UCSF/LBNL cohort ended shortly after enrollment due to a budget-forced closure of the facility.
Starting in 1994, CIRT technology was further developed for numerous tumor sites at the Heavy Ion Medical Accelerator (HIMAC) facility operated by the National Institute of Radiological Sciences (NIRS) in Chiba, Japan. Although the first carbon ion cancer treatments were initiated in Berkeley, California, the first Phase I/II trials with carbon ions were completed at the HIMAC16 and the Japanese program has accrued the most extensive experience with CIRT world-wide.3 During the “ramp-up phase” of the HIMAC
CIRT program between June 1994 and August 2003, more than 1,600 patients with tumors in various sites were enrolled in phase I/II dose-escalation studies and clinical phase II studies. These trials and the clinical experience gathered with them were summarized in a previous review.16 A second clinical facility for CIRT, the German Heidelberg Ion Therapy Center (HIT), started patient treatment in November 2009. This center followed the pioneering work at the experimental CIRT facility of the Gesellshaft für Schwerionen Forschung (GSI) in Darmstadt, Germany that advanced the use of 3D scanned beam delivery between 1998 and 2009 and was the first facility in the world to build a CIRT gantry. Additional clinical CIRT facilities have opened in Japan (5 facilities total), Germany (2 facilities total), Italy (1 facility), China (2 facilities) and Austria (1 facility as of 2017). Currently there are 11 CIRT facilities world-wide.
Figure 1 provides schematic examples allowing the readers to get an impression of the size of the CIRT facility, located in Gunma, Japan (http://heavy-ion.showa.gunma-u.ac.jp/en/page.php?id=5). Figure 1A displays the perimeter of this facility, 45 meters (m) by 65 m, about half the size of a professional soccer field at 45m by 120m. A linear accelerator provides acceleration to the carbon ions before they are injected into the synchrotron and they are accelerated up to 70% of the speed of light. Keeping the heavy ions on a circular track requires very large dipole magnets, making conventional (non-superconducting) synchrotrons for CIRT very large (~60 m circumference). The patients are irradiated with the accelerated carbon ions in the treatment room. Figure 1B shows a person standing next to the pioneering carbon ion gantry constructed at the HIT Facility in Heidelberg, Germany, again displaying the relative stature of this very large steel construction (25 m long, 13 m in diameter and 670 tons), The Japanese have developed a CIRT gantry that has half the weight of the HIT gantry and produces a 20 cm × 20 cm field.
Figure 1A.
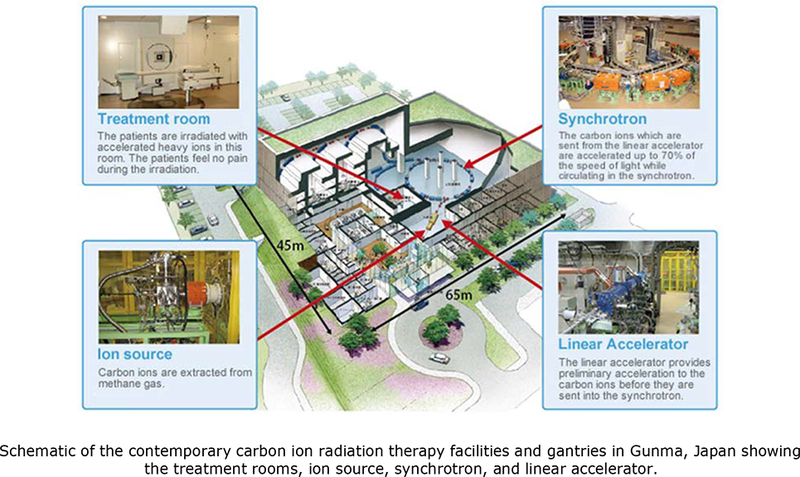

Schematic of the contemporary carbon ion radiation therapy facilities and gantries in Gunma, Japan showing the treatment rooms, ion source, synchrotron, and linear accelerator. Adapted from Noda et al., New Accelerator Facility for Carbon-Ion Cancer-Therapy. Journal of Radiation Research. 2007. 48: Suppl., A43-A54, by permission of Oxford University Press. 1B. Photo of the first carbon gantry built in Heidelberg, Germany shows the size of the gantry relative to a person. Reproduced with permission from Heidelberg University Hospital and the Heidelberg Ion-Beam Therapy Center.
Due to the physical dose distributions of the carbon ion Bragg ionization curve (i.e., greater energy disposition within the Bragg peak and enhanced biological properties of the stopping ions with high linear energy transfer (LET)), carbon ion beams can potentially target tumor cells with greater precision while minimizing damage to surrounding tissues. Carbon ions can be used to reduce doses to surrounding normal tissue volumes due to their sharper lateral penumbra. With the use of heavier ions, such as carbon, high-LET radiation effects translate into an increased relative biological effectiveness (RBE) value by at least a factor of two-fold or three-fold relative to photons depending on the treatment volume17. Protons have RBE values that are, for the most part, only slightly higher than unity relative to photons18. While RBE is an important factor when selecting a radiation dose, there are additional advantages associated with high-LET radiation that can contribute to survival benefits8,19. For example, there are animal and human data suggesting that there is an increased immune stimulatory effect with CIRT compared to photons20.
The current clinical evidence from Phase I/II trials suggests a role for CIRT for a variety of relatively rare tumors. For example, Schulz-Ertner et al. reviewed their experience with CIRT in 2009 for a number of tumor sites including sarcomas of the base of skull (chordomas and chondrosarcomas).4 The patient cohorts were small, but the evidence suggested that there is likely to be an advantage for CIRT over PBRT, particularly for avoiding late normal tissue effects. A recent publication, however, suggests equivalent control rates and outcomes for 101 patients with skull-based chondrosarcomas treated with either protons or carbon ions using intensity-modulated active raster scanning21. Furthermore, the published evidence for long-term late effects from these and other therapies is sparse. There is the potential for considerable variability in the RBE of CIRT used clinically, and to a lesser degree of protons, depending on the biological models, treatment planning protocols, and treatment volumes currently in use22,23.
North American Interest in CIRT
Considering potential physical, biological, and cost-effective advantages have led us to pursue this field of research through the North American Particle Alliance (NAPTA), which was formed in 2013 to advance the development of therapy with carbon and other ions in the USA and world-wide.24 Our work was predicated on the assumption that there was a sufficient body of evidence to justify the re-establishment of a CIRT Center in the USA. However, the cost of a US facility with existing CIRT technology has been estimated to be $200 to $300 million US dollars. Consensus was reached among NAPTA investigators that in order to justify the cost associated with current CIRT technology, definitive studies, i.e., high level, phase III randomized clinical trials would be needed to prove the efficacy of CIRT over protons or other forms of advanced but less expensive x-ray-based therapy, such as intensity-modulated radiation (IMRT) or stereotactic body radiation therapy (SBRT).
This manuscript was prepared to catalog the status of recently completed and ongoing CIRT clinical trials, and to review the progress made to date in obtaining clinical evidence. We also set out to determine whether it is likely that the current clinical trials will provide compelling evidence to justify the routine use of CIRT in the treatment of cancer patients despite the higher cost associated with these treatments. More specifically, we sought to: (1) summarize the current status of recent and ongoing clinical trials; (2) assess whether these trials will provide the necessary high level evidence to establish superiority of the efficacy of CIRT over current forms of radiotherapy in different tumor sites; (3) summarize technical, radiobiological, and logistical challenges to further advance CIRT in the USA; (4) propose improvements in trial design (if justified); (5) propose strategies for insuring that the suggested trials are initiated as fiscally responsible as possible; (6) and finally, to the degree possible, laythe ground work for insuring that this technology becomes widely available when it is justified by the evidence.
STATUS OF CIRT TRIALS
A total of 63 clinical trials involving CIRT met the inclusion criteria outlined in the Supporting Information. The vast majority of the 63 trials were non-randomized (84%) compared to 10 randomized trials (16%). The median intended enrollment was 47 subjects (minimum of 6 to maximum of 689 subjects). As expected, the trials with non-random allocation (n=53) had a lower median enrollment goal of 40 subjects (minimum of 6 to maximum of 689 subjects) compared to the larger median enrollment of 152 (minimum of 50 to maximum of 436 subjects) for those with random allocation. Nearly all of the clinical trials recruited adults only (54 trials; 86%) or adults and pediatric patients (8 trials; 13%), with one trial (2%) exclusively studying children only. Of the 63 clinical trials, most were conducted in Japan (38 trials; 60%) followed by Germany (16 trials; 25%), China (7 trials. 11%), Italy (1 trial; 2%). One trial (2%) of radio-resistant head and neck tumors was developed in France and it will be conducted at the HIT Center in Germany. Two non-randomized and a single randomized trial were considered completed, while nearly half of the trials (49%) were still recruiting subjects. Nine trials (14%) were not yet recruiting, seven trials (11%) were no longer recruiting, nine trials (14%) had unknown recruitment status, and three trials (5%) were terminated before completion.
Clinical endpoints and study phase of CIRT trials
The primary endpoint for the majority (32 trials/63 trials; 51%) of clinical trials was adverse events (13 trials) or toxicity and/or dose response (19 trials) followed by local control in 15 trials (24%), progression-free survival in 9 trials (14%), and overall survival in 7 trials (11%). Of the 10 randomized trials, one (10%) phase II trial included overall survival as the primary endpoint, 2 (20%) used progression-free survival endpoint, 4 (40%) used an adverse event or toxicity and/or dose response endpoint, and 3 (30%) focused on local control. Of note, of the 10 randomized clinical trials, 30% were classified as phase III, and those phase III trials represent 5% (n=3/63) of all trials involving CIRT.
Figure 2 presents the year of initial enrollment (or intended enrollment) of the CIRT trials according to their study phase. Before 2010 there were very few trials activated with the largest increase in activation of trials in 2010 and 2016. Nearly half of the 11 CIRT facilities (45%) opened by 2010 in Japan, China and Germany. The additional six facilities opened after 2010 with the most recent addition in Austria (2017). The number of phase II trials increased in 2010, but not phase III. In fact, very few phase III trials were initiated in the last 5 years.
Figure 2:

Summary of the expected (or intended) first year of enrollment of study trials extracted from the clinicaltrials.gov registry and WHO. Gold color indicates the total number of phase III clinical trials, dark blue color indicates the total number of phase II clinical trials, and light blue indicates the total number of phase I or phase I/II clinical trials.
Figure 3 displays the study disease site of CIRT trials according to study phase. Most sites studied were phase I and phase II trials, or combined phase I/II trials. Phase III trials were limited to the base of skull (n=2) and head and neck (H&N) (n=1). Most trials with random allocation were activated in Germany, i.e., 7 trials (70%), 2 trials (20%) in Japan, and 1 trial (10%) was conceived in France, the latter will be conducted at HIT in Germany. All of these trials randomized adults comparing carbon therapy to either PBRT (7 trials; 70%), photon therapy (2 trials; 20%), or neoadjuvant therapy (1 trial; 10%) by using alpha-galactosylcerimide pulsed antigen followed by carbon ion radiotherapy).
Figure 3:
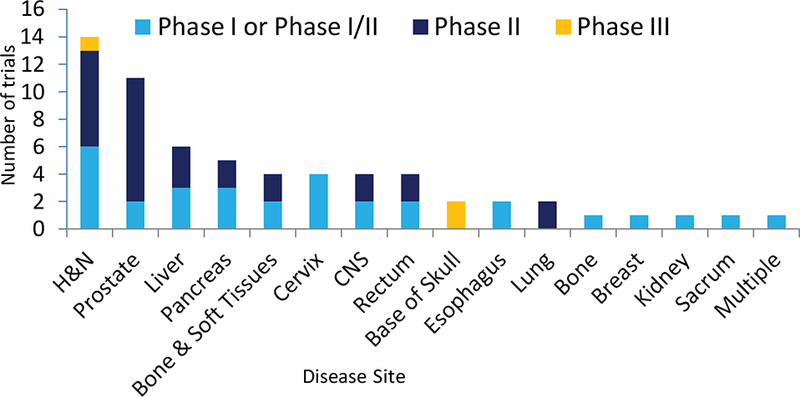
Summary of the disease site extracted from the clinicaltrials.gov registry and WHO. Gold color indicates the total number of phase III clinical trials, dark blue color indicates the total number of phase II clinical trials, and light blue indicates the total number of phase I or phase I/II clinical trials. H&N denotes head and neck site and multiple denotes two or more sites.
A variety of disease sites are or were under investigation including in order of frequency: 14 head and neck (H&N) trials (22%), 11 prostate trials (18%), and 6 liver trials (10%). Among the 10 randomized trials, 3 were central nervous system (CNS) trials (30%), 2 head and neck (H&N) trials (20%), 2 base of skull trials (20%), altogether accounting for 70% of the ongoing randomized trials, with one of the most common cancer diseases, prostate cancer, only tested in a single randomized trial (10%).
CHALLENGES AND FUTURE DIRECTIONS
This review assesses the CIRT trial landscape to evaluate where we stand in terms of clinical evidence and how rapidly progress is advancing. Mark Twain has been credited with saying “the best predictor of future behavior is past behavior”. Although this aphorism is directed at predicting human behavior, we believe it is also very likely to be predictive of the direction of scientific and clinical research in general and progress in CIRT specifically. This saying may also provide insights as to what clinical sites might be selected to study in the context of moving forward with phase III trials (see our discussion below). As described above, although there are a modest number of trials involving CIRT that have been completed in recent times or are currently being conducted, a minority are randomized, a smaller minority are phase III, and even fewer involve an overall survival (OS) primary endpoint.
Despite a recent international consensus for model-based approach to particle beam therapy that includes patient selection, non-randomized trials based on highly selected patients are inadequate for providing high level evidence for the efficacy of CIRT required to justify the construction of new facilities25. Only 5% of all of the CIRT trials reviewed here are phase III comparative randomized trials and none of these involves OS as endpoint. Only one randomized trial involves prostate, the most common cancer disease in the Western world, and very few randomized trials have been initiated in the past 3 years. Thus, the prospects are slim for the completion of a “registration type” trial to definitively establish the value of CIRT in the near future. If substantial progress in the distant future is to be made in clinical CIRT research, it appears most likely that it would involve Germany and/or Japan, the two countries with the largest current activity in CIRT, prostate trials and basic research26–31. However, the current trials being conducted have provided inspiration, direction, and potentially useful resources for the future of CIRT.
Furthermore, even when the same carbon ion radiation dose is prescribed in a trial from Japan or a European site, the tumors (and normal tissues) may be receiving different carbon doses of radiation due to facility-dependent protocols and assumptions in the biological models used for treatment planning of effective doses. Thus, going forward we would propose reaching a consensus where possible, but at a minimum capturing detailed dosimetric and clinical information so that clinical outcomes can be used to adjudicate these differences of opinion32,16,33.
We previously coined the mnemonic “RESIDUE” to call attention to 7 major challenges that need to be solved to advance this field and allow CIRT to be widely available.24
These 7 major “RESIDUE” challenges are:
Radiobiological knowledge to address uncertainty in optimal fraction sizes and doses and relative biological effectiveness for each tumor site (biological)
Exchange of technology, funding and infrastructure between academic centers, health care payers, industry and funding agencies (operational)
Size/weight of accelerators and gantries (engineering/physics and cost)
Integration of technology to advance key areas from beam acceleration and delivery, through treatment planning and image guidance (engineering/physics to create equipose with x-ray based treatment)
Define the patient population to be studied; that is, identify and select patients who will benefit the most from CIRT (clinical)
Uncertainties of effective dose and range at the end of the Bragg peak (physics and accuracy)
Evidence of clinical effectiveness and cost-effectiveness (societal)
The first issue will give direction and create new hypotheses, but will ultimately require validation in humans, as animal models may not be accurate enough to accurately predict the benefits of this technology in humans, although even this issue is highly debated.34,35. It may be useful to leverage the human cancer transcriptome based data to move this field forward.36 The open-access Human Pathology Atlas database (www.proteinatlas.org/pathology) allows for genome-wide exploration of the impact of individual proteins on clinical outcome in major human cancers. If existing patient biopsy materials become available from CIRT patients, specific tumor protein profiles could be correlated with clinical outcome and could be used to prognostically identify patients for whom CIRT is appropriate.
Issues 2 and 3 (above), represent true challenges to building new CIRT facilities in the USA, but are also an opportunity that could lead to improved cost-effectivness. Issues 4 and 6 (above) can be readily accomplished via ongoing research in existing facilitiess (such as work currently being performed by NAPTA members)24 and future research-oriented facilities. This leaves primarily issues 5 and 7 to be addressed. To this end, robust large trials with clinically meaningful endpoints (foremost OS, or time to metastatic disease (e.g. prostate cancer)) should be developed (issue 5), allowing us to eventually address issue 7 and justifying further investment into new technology development37. These trials must include not only rare and challenging diseases, but also common diseases (discussed in more detail below).
Cancer sites suitable for clinical trials
Japan and Germany have demonstrated that there are a number of selected sites for which CIRT might potentially be beneficial. In order for widespread acceptance, it is important that the utility of CIRT not be limited to rare radiation resistant tumors. The tumor site most frequently treated in Japan with CIRT has been prostate cancer. In order to establish CIRT as a standard treatment option, common cancers should be studied in prospective trials treated definitively with a very limited number of fractions. This argument is based on the observation that stereotactic body radiotherapy (SBRT) appears to be at least as effective as dose escalated conventionally fractionated photons for a number of common solid tumors (e.g. lung and prostate cancers) and based on the fact that physical dose targeting of the tumor by CIRT spares normal tissues and reduces the need to fractionate the dose38,39. There may also be additional biological mechanisms underlying improved treatment outcome of CIRT when it is administered in a few fractions.
The use of different treatment planning systems, techniques and assumptions concerning RBE and LET models could potentially complicate the interpretation of outcomes for patients treated in Japan, Europe, and elsewhere32. For example, Japan had relied on their prior extensive neutron experience (involving similar high-LET effects) to guide their initial clinical approach to CIRT carbon ions for treatment planning to have similar effects. The Japanese program has now matured to a modified MKM (microdosimetic kinetic model)-based approach with a plateau carbon ion17,40,41. For several European centers, on the other hand, a local effects model is used that calculates the probablity of a lethal event based upon nanometer features of track structure. We believe that it is critical that future trials of a given cancer site not be confined to one center with CIRT capability. We must first standardize certain elements of treatment and insure a very high level of quality assurance. To this end we have begun a program to utilize anthromorphic human as well as virtual phantoms to investigate issues like “range uncertainty” and to incorporate image guidance techniques into these trials42,43,44.
As shown in Figure 3 based on available empirical clinical data, cancers of the H&N, prostate, liver, pancreas as well bone and soft tissues, appear to have been found by investigators to be most suitable for enrollment onto phase I or I/II trials involving CIRT. Thus, we would favor focusing on patients with locally advanced disease (e.g. T3–T4) in these sites. Two phase III trials involving pancreatic cancer have recently been launched (described below8) and those involving sarcomas and liver primaries are in the planning stages. Another phase III trial launched in France (and to be conducted at the HIT Center in Germany) plans to study radio-resistant head and neck tumors. This is an excellent start to multi-national trials. However, all of these phase III trials are being conducted at single centers, and we believe multi-center international trials are essential for optimal recruitment and generalizability. A detailed discussion of trials required for each of these cancer sites is beyond the scope of this paper but, in general, multiple stratification variables may be needed to be incorporated into the design to insure balanced recruitment, particularly for sites with large patient heterogeneity (e.g. H&N cancers).
One site under discussion by our own group (NAPTA), in collaboration with the leadership of a number of centers with CIRT capability involves the design of a large phase II/III clinical trial for unfavorable intermediate and high-risk prostate cancer. The rationale for choosing this cancer site is as follows: (1) prostate cancer is one of the most common cancers treated definitively with radiation in the world, and is the most common cancer treated with CIRT3,29; (2) increasingly SBRT is being used to treat both intermediate and high grade prostate cancer either as a monotherapy in 4 to 5 fractions or as a boost with 2 fractions after pelvic radiotherapy and recently a number of investigators have begun studying the feasibility of treating prostate cancer with a single fraction39 (ClinicalTrials.gov Identifier: NCT03294889); (3) there are theoretical reasons to believe that CIRT, due to the tighter penumbra, should allow increased sparing of neurovascular structures and the penile bulb resulting in improved erectile function and favorably impacting quality of life45–47; (4) the higher RBE and sharper lateral fields of CIRT, permitting biological and physical dose escalation, should improve local control more than might be expected with photon or proton based SBRT43,44; (5) hypoxia and other mechanisms of radiation resistance thought to confer resistance to photon based EBRT may be overcome with CIRT26,48–50; (6) intermediate endpoints such as PSA nadir and /or time to metastatic disease could be used to support the viability of CIRT37,39,51,52.
As noted above, prostate cancer patients (including patients from the USA), would initially be treated with 4 or 5 fractions of SBRT using photons (x-rays) vs. protons vs. CIRT (with photons considered the standard to which the other are compared). Subsequently we would evaluate the feasibility of managing this disease with a single fraction. This would allow us to answer questions related to dose distributions advantages (charged particles vs. photons) and RBE (photons and protons vs. carbon). One concern that might be voiced by some authorities is whether radiation limited to the prostate (excluding pelvic lymph nodes) would be appropriate in patients with intermediate or high-risk disease. Fortunately, it is anticipated that the results of RTOG 0924 (a phase III trial testing the value of whole pelvic radiotherapy in patients with unfavorable intermediate and high risk disease) should be available in the next few years (anticipated completed accrual n=2580, May 2019, personal communication, Mack Roach III, MD, Principal Investigator)53. This should allow investigators to complete the due diligence required to address the technical challenges associated with range uncertainty and image guidance. This should guarantee the required accuracy for designing and launching the appropriate clinical trial24,54.
Limitations of review
There are a number of limitations to this review. Although we provide an up-to-date summary, a small number of trials may have been missed due to oversight or because they were not included due to absence of sufficient available detail. Additional trials are being planned in Japan and may open soon.55 For example we have become aware of two trials that are being planned in the USA, to be conducted in foreign centers, but were not yet registered in clinicaltrials.gov or WHO at the time of this review. This includes the trial designed by investigators from the University of Texas Southwestern Medical Center (Dallas, Texas, USA) known as CIPHER, carbon ion versus photon therapy for pancreatic cancer, and another phase III trial funded by the USA National Cancer Institute (NCI) to study CIRT vs. IMRT for pancreatic cancer in patients at the Shanghai Proton and Heavy Ion Center (SPHIC; Shanghai, China). In the planned CIPHER trial, the CIRT will be administered to U.S. patients at the NIRS or the Italian National Center of Oncological Hadrontherapy (CNAO; Pavia, Italy). The CIPHER trial is designed to detect an increase in 2-year overall survival (OS) from 22% in IMRT to 48% in carbon ions8.
CONCLUSIONS
Our international colleagues should be congratulated for moving the investigations involving CIRT much further along than we or any other investigators in the USA.
Through their hard work and dedication, at least 11 centers have been built to date. We must now join and support their efforts by facilitating patient accrual to phase III randomized treatment trials. The available data makes it clear that we are far from making this potentially very powerful treatment modality available for the everyday care of cancer patients. However, we are very optimistic that by leveraging the existing expertise and existing facilities and planning the work described above that critical trials could be launched in the immediate future.
We propose designing “proof-of-priniciple” international trials optimizing treatment of the most common cancer sites (RESIDUE issues 5 and 7) via collaboration between a number of national and international investigators. We favor launching a series of trials leveraging existing technology to promote and extend investigations seeking to improve CIRT survival and local control of selected cancers ranging from rare to common cancers. We also support the call for a registry by the Particle Therapy Co-Operative Group (PTCOG): https://ptcog.ch/index.php/clinical-protocols to facilitate definitive comparisons. However, we believe a more aggressive but cost-conscious strategy to include deliberate approvals for international medical regulations and standards is greatly needed at this time. This is already underway in that American cancer patients are seeking CIRT in Japan, Germany, Shanghai and Italy (some covered by their private health insurance).
For patients from the USA, we would require insurance coverage (including Medicare, which would literally require an Act of Congress) to participate in a very cost-effective set of trials. Such trials should involve patients from the USA to be sent to other countries, such as Germany or Japan, with existing CIRT facilities. Exceptions could be made to existing federal laws to allow Medicare and other insurance companies to share an appropriate portion of the cost with differences made up by Federal research support. This would allow potentially billions of dollars to be saved in expensive investments. Concurrent with these international trials, we would recommend to support basic research and accelerator technology advancements to be carried out in National Labs or other national centers for developing more compact, more accurate and less expensive CIRT technology (“better machines”).
Before building additional new CIRT facilities using existing technology (which could become obsolete as ongoing technologies in this field continue to advance), we recommend that CIRT must be documented with unbiased high level 1 evidence of increased survival and quality of life benefits. If these trials, conducted at existing CIRT facilities, demonstrate within the next 10 years that there are benefits, then compact and more cost-effective technologies, also to be developed over the next 5–10 years, could then be rapidly implemented in new or existing CIRT centers. These “improved accelerators” could be ready for wide-spread production at lower cost and more refined accuracy. In addition, new radiobiological rationales or hypotheses developed in ion-therapy research centers could lead to new clinical trials at that time.
In order to determine what doses and dose constraints should be used for key trials providing level I evidence, we urgently need to address the uncertainty of RBE. The existing phase I/II trials may allow us to answer issue 1 above for all critical biological system responses (including metabolic, immune, stem cell, and tissue homeostatic). Fractionation schemes based on existing RBE and assumptions will be validated using robust studies of patients previously irradiated with CIRT. In addition, following the example of our pioneering Japanese colleagues, validation studies should be conducted using a variety of fraction sizes and dose constraints, allowing esimates of safety and efficacy to be confirmed.
We therefore call to standardize CIRT treatment planning across CIRT centers. That way when patient outcomes from CIRT trials are compared, the carbon ion dose and fraction size will mean the same for patients treated across CIRT centers and multi-center trials across CIRT centers can be designed. Thus, participation of multiple carbon ion centers in a multi-center Phase III randomized clinical trial would accelerate accrual, allow cross validation of differing carbon ion treatment beam models and generalize the applicability of the trial results.
Supplementary Material
Acknowledgments
FUNDING SUPPORT: This work was supported by the US NIH P20 Planning Grant 5P20CA183640-02, NAPTA: Optimizing clinical trial design and delivery of particle therapy for cancer, and Contract No. DE-AC02-05CH11231 with the U.S. Department of Energy.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: No conflicts of interest disclosures from any authors.
References:
- 1.Barringer B Radium in the treatment of carcinoma of bladder and prostate: review of one year’s work. JAMA 68, 1227–1230 (1917). [Google Scholar]
- 2.Mishra MV et al. Establishing Evidence-Based Indications for Proton Therapy: An Overview of Current Clinical Trials. Int J Radiat Oncol Biol Phys 97, 228–235, doi: 10.1016/j.ijrobp.2016.10.045 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Kamada T et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 16, e93–e100, doi: 10.1016/S1470-2045(14)70412-7 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Schulz-Ertner D The clinical experience with particle therapy in adults. Cancer J 15, 306–311, doi: 10.1097/PPO.0b013e3181b01922 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Castro JR et al. Treatment of cancer with heavy charged particles. Int J Radiat Oncol Biol Phys 8, 2191–2198 (1982). [DOI] [PubMed] [Google Scholar]
- 6.Uhl M, Herfarth K & Debus J Comparing the use of protons and carbon ions for treatment. Cancer J 20, 433–439, doi: 10.1097/PPO.0000000000000078 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Mishra KK et al. Long-term Results of the UCSF-LBNL Randomized Trial: Charged Particle With Helium Ion Versus Iodine-125 Plaque Therapy for Choroidal and Ciliary Body Melanoma. Int J Radiat Oncol Biol Phys 92, 376–383, doi: 10.1016/j.ijrobp.2015.01.029 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Durante M, Orecchia R & Loeffler JS Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol 14, 483–495, doi: 10.1038/nrclinonc.2017.30 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Peeters A et al. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol 95, 45–53, doi: 10.1016/j.radonc.2009.12.002 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Jakel O, Land B, Combs SE, Schulz-Ertner D & Debus J On the cost-effectiveness of Carbon ion radiation therapy for skull base chordoma. Radiother Oncol 83, 133–138, doi: 10.1016/j.radonc.2007.03.010 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Tobias CA & Todd PW Heavy charged particles in cancer therapy. National Cancer Institute monograph 24, 1–21 (1967). [PubMed] [Google Scholar]
- 12.Nowakowski VA et al. Charged particle radiotherapy of paraspinal tumors. Int J Radiat Oncol Biol Phys 22, 295–303 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Castro JR et al. Experience in charged particle irradiation of tumors of the skull base: 1977–1992. Int J Radiat Oncol Biol Phys 29, 647–655 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Linstadt D et al. Long-term results of helium ion irradiation of uveal melanoma. Int J Radiat Oncol Biol Phys 19, 613–618 (1990). [DOI] [PubMed] [Google Scholar]
- 15.Linstadt DE, Castro JR & Phillips TL Neon ion radiotherapy: results of the phase I/II clinical trial. Int J Radiat Oncol Biol Phys 20, 761–769 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Tsujii H et al. Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother Oncol 73 Suppl 2, S41–49 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Matsufuji N Selection of carbon beam therapy: biophysical models of carbon beam therapy. Journal of radiation research, doi: 10.1093/jrr/rry014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon SJ, McNamara AL, Schuemann J, Paganetti H & Prise KM A general mechanistic model enables predictions of the biological effectiveness of different qualities of radiation. Sci Rep 7, 10790, doi: 10.1038/s41598-017-10820-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebner DK & Kamada T The Emerging Role of Carbon-Ion Radiotherapy. Frontiers in oncology 6, 140, doi: 10.3389/fonc.2016.00140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogata T et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer research 65, 113–120 (2005). [PubMed] [Google Scholar]
- 21.Mattke M et al. High control rates of proton-and carbon ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer, doi: 10.1002/cncr.31298 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Adeberg S et al. Treatment of meningioma and glioma with protons and carbon ions. Radiation oncology 12, 193, doi: 10.1186/s13014-017-0924-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fossati P et al. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Phys Med Biol 57, 7543–7554, doi: 10.1088/0031-9155/57/22/7543 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Roach M 3rd et al. New Clinical and Research Programs in Particle Beam Radiation Therapy: The University of California San Francisco Perspective. Int J Part Ther 2, 471–473, doi: 10.14338/IJPT-15-00025.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prayongrat A et al. Present developments in reaching an international consensus for a model-based approach to particle beam therapy. Journal of radiation research, doi: 10.1093/jrr/rry008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glowa C et al. Carbon ion radiotherapy: impact of tumor differentiation on local control in experimental prostate carcinomas. Radiation oncology 12, 174, doi: 10.1186/s13014-017-0914-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glowa C et al. Carbon ion radiotherapy decreases the impact of tumor heterogeneity on radiation response in experimental prostate tumors. Cancer Lett 378, 97–103, doi: 10.1016/j.canlet.2016.05.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habl G et al. Acute Toxicity and Quality of Life in Patients With Prostate Cancer Treated With Protons or Carbon Ions in a Prospective Randomized Phase II Study--The IPI Trial. Int J Radiat Oncol Biol Phys 95, 435–443, doi: 10.1016/j.ijrobp.2016.02.025 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Nomiya T et al. A multi-institutional analysis of prospective studies of carbon ion radiotherapy for prostate cancer: A report from the Japan Carbon ion Radiation Oncology Study Group (J-CROS). Radiother Oncol 121, 288–293, doi: 10.1016/j.radonc.2016.10.009 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Maruyama K et al. Five-year quality of life assessment after carbon ion radiotherapy for prostate cancer. Journal of radiation research 58, 260–266, doi: 10.1093/jrr/rrw122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasuya G et al. Cancer-specific mortality of high-risk prostate cancer after carbon-ion radiotherapy plus long-term androgen deprivation therapy. Cancer Sci 108, 2422–2429, doi: 10.1111/cas.13402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parodi K The biological treatment planning evolution of clinical fractionated radiotherapy using high LET. International journal of radiation biology, 1–4, doi: 10.1080/09553002.2018.1427904 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Combs SE et al. Clinical response and tumor control based on long-term follow-up and patient-reported outcomes in patients with chemodectomas of the skull base and head and neck region treated with highly conformal radiation therapy. Head & neck 36, 22–27, doi: 10.1002/hed.23274 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Seok J et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America 110, 3507–3512, doi: 10.1073/pnas.1222878110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takao K & Miyakawa T Genomic responses in mouse models greatly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America 112, 1167–1172, doi: 10.1073/pnas.1401965111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlen M et al. A pathology atlas of the human cancer transcriptome. Science 357, doi: 10.1126/science.aan2507 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Xie W et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol 35, 3097–3104, doi: 10.1200/JCO.2017.73.9987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nyman J et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 121, 1–8, doi: 10.1016/j.radonc.2016.08.015 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Motta A & Roach M 3rd. Stereotactic body radiation therapy (SBRT) for high-risk prostate cancer: Where are we now? Pract Radiat Oncol, doi: 10.1016/j.prro.2017.11.008 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Inaniwa T et al. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys Med Biol 60, 3271–3286, doi: 10.1088/0031-9155/60/8/3271 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Kanematsu N & Inaniwa T Biological dose representation for carbon-ion radiotherapy of unconventional fractionation. Phys Med Biol 62, 1062–1075, doi: 10.1088/1361-6560/62/3/1062 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Bashkirov VA et al. Novel scintillation detector design and performance for proton radiography and computed tomography. Med Phys 43, 664–674, doi: 10.1118/1.4939255 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding A et al. VirtualDose: a software for reporting organ doses from CT for adult and pediatric patients. Phys Med Biol 60, 5601–5625, doi: 10.1088/0031-9155/60/14/5601 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Bednarz B, Hancox C & Xu XG Calculated organ doses from selected prostate treatment plans using Monte Carlo simulations and an anatomically realistic computational phantom. Phys Med Biol 54, 5271–5286, doi: 10.1088/0031-9155/54/17/013 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivin del Campo E, Thomas K, Weinberg V & Roach M 3rd. Erectile dysfunction after radiotherapy for prostate cancer: a model assessing the conflicting literature on dose-volume effects. International journal of impotence research 25, 161–165, doi: 10.1038/ijir.2013.28 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Murray J, D. J, Mossop H et al. in Proceedings of American Society Clinical Oncology (Elsevier). [Google Scholar]
- 47.Hamstra DA et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase III trial. Pract Radiat Oncol, doi: 10.1016/j.prro.2017.07.008 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Marvaso G et al. Phase II multi-institutional clinical trial on a new mixed beam RT scheme of IMRT on pelvis combined with a carbon ion boost for high-risk prostate cancer patients. Tumori 103, 314–318, doi: 10.5301/tj.5000587 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Mahajan NP et al. Blockade of ACK1/TNK2 To Squelch the Survival of Prostate Cancer Stem-like Cells. Sci Rep 8, 1954, doi: 10.1038/s41598-018-20172-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keam SP et al. The Transcriptional Landscape of Radiation-Treated Human Prostate Cancer: Analysis of a Prospective Tissue Cohort. Int J Radiat Oncol Biol Phys 100, 188–198, doi: 10.1016/j.ijrobp.2017.09.037 (2018). [DOI] [PubMed] [Google Scholar]
- 51.D’Amico AV et al. Surrogate endpoints for prostate cancer-specific mortality after radiotherapy and androgen suppression therapy in men with localised or locally advanced prostate cancer: an analysis of two randomised trials. The lancet oncology 13, 189–195, doi: 10.1016/S1470-2045(11)70295-9 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Cury FL et al. Prostate-specific antigen response after short-term hormone therapy plus external-beam radiotherapy and outcome in patients treated on Radiation Therapy Oncology Group study 9413. Cancer 119, 1999–2004, doi: 10.1002/cncr.28019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morikawa LK & Roach M 3rd. Pelvic nodal radiotherapy in patients with unfavorable intermediate and high-risk prostate cancer: evidence, rationale, and future directions. Int J Radiat Oncol Biol Phys 80, 6–16, doi: 10.1016/j.ijrobp.2010.11.074 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Sadrozinski HF et al. Development of a Head Scanner for Proton CT. Nuclear instruments & methods in physics research. Section A, Accelerators, spectrometers, detectors and associated equipment 699, 205–210, doi: 10.1016/j.nima.2012.04.029 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oike T, Sato H, Noda SE & Nakano T Translational Research to Improve the Efficacy of Carbon Ion Radiotherapy: Experience of Gunma University. Frontiers in oncology 6, 139, doi: 10.3389/fonc.2016.00139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


