Abstract
Throughout early neurodevelopment, myelination helps provide the foundation for brain connectivity and supports the emergence of cognitive and behavioral functioning. Early life nutrition is an important and modifiable factor that can shape myelination and, consequently, cognitive outcomes. Differences in the nutritional composition between human breast and formula milk may help explain the functional and cognitive disparity often observed between exclusively breast versus formula-fed children. However, past cognitive and brain imaging studies comparing breast and formula feeding are often: cross-sectional; performed in older children and adolescents relying on parental recall of infant feeding; and generally treat formula-fed children as a single group despite the variability between formula compositions. Here we address some of these weakness by examining longitudinal trajectories of brain and neurocognitive development in children who were exclusively breastfed versus formula-fed for at least 3 months. We further examine development between children who received different formula compositions. Results reveal significantly improved overall myelination in breastfed children accompanied by increased general, verbal, and non-verbal cognitive abilities compared to children who were exclusively formula-fed. These differences were found to persist into childhood even with groups matched for important socioeconomic and demographic factors. We also find significant developmental differences depending on formula composition received and that, in particular, long-chain fatty acids, iron, choline, sphingomyelin and folic acid are significantly associated with early myelination trajectories. These results add to the consensus that prolonged and exclusive breastfeeding plays an important role in early neurodevelopment and childhood cognitive outcomes.
Keywords: Infant brain development, Cognitive development, Brain MRI, Myelination
Introduction
Infancy and early childhood are sensitive and rapid periods of brain growth that coincide with the emergence of nearly all cognitive, behavioral, and social-emotional functions (Johnson, 2001). Throughout this period, the brain’s eloquent networks are shaped and refined through processes that include myelination, dendritic arborisation and synaptogenesis, and synaptic pruning. These adaptive processes are modulated by neural activity and are responsive to environmental, genetic, hormonal, and other influences (Stiles and Jernigan, 2010). The development and pattern of myelination follows a well-described neuroanatomical arc (Brody et al., 1987), progressing in a posterior-to-anterior and centre-outwards spatiotemporal pattern that corresponding to maturing cognitive functions (McGee et al., 2005; Markham and Greenough, 2004). That is, there is a strong overlap in the emergence of a specific cognitive function and the myelination of brain regions and networks subserving that function (Fornari et al., 2007; Van der Knaap et al., 1991; Pujol et al., 2006). Beyond this temporal association, prior studies have further shown the importance of white matter and cortical myelination to cognitive development and brain plasticity (McGee et al., 2005; Pujol et al., 2004, 2006; Fields, 2008; Fornari et al., 2007), and altered myelination and white matter maturation in a variety of intellectual, behavioral, and psychiatric disorders (Bartzokis et al., 2003; Flynn et al., 2003; Davison and Dobbing, 1966; Wolff et al., 2012). We have further shown that early trajectories of myelination are associated with cognitive abilities and outcomes (O’Muircheartaigh et al., 2014; Deoni et al., 2014).
The assembly and maintenance of the myelin sheath requires a carefully orchestrated delivery of nutrients, including lipids and fatty acids, proteins, minerals, and other micronutrients (Dobbing, 1964). Long-chain polyunsaturated fatty acids (LC-PUFAs), choline, iron, zinc, cholesterol, phospholipids, and sphingomyelin play essential roles in myelin elaboration, as key components of the myelin sheath and/or energy sources (Oshida et al., 2003; Saher et al., 2005; Hadley et al., 2016; Chang et al., 2009). Deficiencies in these nutrients throughout infancy can significantly alter myelin content, composition, and morphology, potentially disrupting normal brain function and impairing cognitive outcomes.
Compositionally, human breastmilk provides many of the nutritional building blocks that support healthy physical growth, immune system development, and brain maturation (Kramer et al., 2008; Jacobi and Odle, 2012; Hoi and McKerracher, 2015; M’Rabet et al., 2008; Reynolds, 2001). This includes micro and macro-nutrients, short and long-chain PUFAs, phospholipids, neurotrophic factors, biofactors, and hormones that are important for myelination. While many of these nutrients are also provided by infant formula, their concentration often varies considerably from human milk, and does not mimic the changing nutritional composition of human milk across an individual feed (from foremilk to hindmilk), or from colostrum to mature milk (Ballard and Morrow, 2013). It is possible, therefore that given the importance of these nutritional components to brain development, nutritional differences between breast milk and infant formula, or between formula, may inluence trajectories of brain myelination and, subsequently, affect cognitive development.
With specific reference to brain myelination, breastmilk is an important source of long-chain PUFAs, including docosahexaenoic and arachidonic acid (DHA and ARA), the together comprise more than 20% of the brain’s fatty acid content (Chang et al., 2009), and phospholipids such as phosphatidylcholine that make up 10% of the lipid weight of myelin. Approximately 40% of the lipid content of mature human milk is sphingomyelin (Blaas et al., 2011), a sphingolipid that plays a critical role in development of the myelin sheath (Oshida et al., 2003; Jana and Pahan, 2010). Breastmilk is also an important source of cholesterol, which is essential for myelin synthesis (Saher et al., 2005). Even in otherwise healthy children, prolonged deficits in these and other nutrients have been associated with developmental abnormalities and cognitive impairments. For example, prolonged essential fatty acid deficiency or low blood levels of ARA and DHA have been associated with learning disorders, ADHD, dyslexia, and autism spectrum disorder (Hadley et al., 2016).
The nutritional composition differences between breast and infant formula milk may help to explain some of the observed difference in overall cognitive functioning and ability between breast and formula fed infants (Horwood and Fergusson, 1998). Even controlling for important confounds such as birth weight, pregnancy length, parent education level, and family socioeconomic status and demographics, the general consensus from prior studies is that children and adolescents breastfed as infants show improved performance on tests of cognitive functioning (Horwood and Fergusson, 1998; Anderson et al., 1999; Kramer et al., 2008; Mortensen et al., 2002; Huang et al., 2014). These results are also generally supported by brain imaging studies, which have shown increased white matter volume, total gray matter volume, and regional cortical thickness increases in association with breastfeeding duration and percentage of breastmilk in a infant’s diet. These neuroimaging finds have further been associated with improved cognitive function as measured by IQ (Ou et al., 2015; Isaacs et al., 2010; Kafouri et al., 2013; Luby et al., 2016). Although these studies have been performed predominately in older children and adolescents, our group’s prior work (Deoni et al., 2013a,b) extended these findings to infants, showing cross-sectional differences in early brain myelination between exclusively breastfed, exclusively formula-fed, and mixed-fed infants and toddlers. These differences were found to present prior to one year of age and extend throughout childhood, and were associated with duration of breastfeeding.
An important limitation of past neuroimaging (MRI) studies, however, has been their cross-sectional nature with children pooled across large age-ranges, making it difficult to draw causative conclusions. In addition, formula-fed children are often treated as a single group without consideration of the potential differences in formula composition. These limitations generally stem from the retrospective nature of most studies, with nutritional composition information often not remembered or readily available. Though infant formula is tightly regulated (e.g., http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm048694.htm), there exists measurable differences in micronutrient, PUFA, and phospholipid content across different infant formulas.
To investigate nutritional influences on longitudinal infant and child brain development in a naturalistic setting, we longitudinally characterised myelination in a large group (n = 150, 57 females) of healthy and neurotypically developing children from 3 months to 9 years of age. A total of 452 total MRI and neurocognitive datasets were acquired on these children. These children were drawn from a larger study of normal brain development and were selected since knowledge of infant feeding habits, duration of exclusive breastfeeding, and main infant formula composition was known. Brain myelination was quantified using a multi-component relaxometry (MCR) technique termed mcDESPOT (Deoni et al., 2008), which decomposes the measured MRI signal into contributions from distinct sub-voxel anatomical water pools (MacKay et al., 1994). Through the acquisition of multiple T1 weighted and T1/T2 weighted spoiled and fully-balanced steady-state images with different flip angles, mcDESPOT applies a 3-pool tissue model (Deoni et al., 2013a, b) to quantify the T1 and T2 relaxation and volume fraction properties for water pools associated with intra- and extra-cellular water, water trapped within the lipid bilayers of the myelin sheath, and a non-exchange free water pool (i.e., cerebral spinal fluid). The volume fraction of the myelin-associated water, termed the myelin water fraction (MWF) is used as a surrogate measure of myelin volume, and has been verified via comparisons with histology (Wood et al., 2016), and used previously to investigate trajectories of early brain maturation (Deoni et al., 2012), myelin-function relationships throughout childhood (O’Muircheartaigh etal., 2013; O’Muircheartaigh et al., 2014), and myelin loss in adults with multiple sclerosis and other demyelinating disorders (Kolind et al., 2013, 2012, 2015). For children up to 5 years and 8 months of age, cognitive function and development was measured using the Mullen Scales of Early Learning (MSEL) (Mullen, 1995), a population-normed tool that provides standardised measures of fine and gross motor control, expressive and receptive language, and visual processing. In addition to domain specific scores, computed early learning composite (ELC) and verbal and non-verbal development quotients (VDQ and NVDQ) composite values relect overall cognitive, verbal, and non-verbal functioning. Each of these age normalized composite values has a mean of 100 and standard deviation of 15.
In addition to comparisons of brain and cognitive development trajectories associated with exclusive breast and formula-fed children, we further stratified the formula-fed children based on the main formula composition they received over the first 3 months of life and examined developmental differences between them. This analysis allowed us to more specifically investigate the role of nutritional composition on early brain growth. Finally, we extended this analysis to investigate the influence of individual nutrients on developmental myelin trajectories by examining the associations between specific formula nutrient levels and growth curve parameters.
Overall, we find that compared to exclusive breastfeeding for 3 months, children who exclusively received formula milk have lower overall neurodevelopment, including both neuroimaging measures of myelination and measures of cognitive performance that persist into later childhood, even with groups matched for important socioeconomic and demographic factors. In addition, significant deviations in development are evident across children who received different formula compositions. Further, individual nutrient analysis suggests an important role for DHA, ARA, folic acid, sphingomyelin, iron, and phosphatidylcholine in brain development. These results further stress the importance of proper early nutrition for optimal brain development and, by consequence, cognitive outcomes in healthy children.
Materials & methods
Infant participants
Infants included in this study were drawn from a large and ongoing longitudinal study of normal brain and behavioral development: the Brown university Assessment of Myelination and Behavior Across Maturation (BAMBAM) study (Deoni et al., 2012). BAMBAM currently includes more than 500 children recruited between birth and 5 years of age, and combines neuroimaging (MRI) measures, comprehensive observational and parent report measures of cognitive and behavioral development, on-going medical history information, biospecimen collection, and anthropometry. To obtain longitudinal measures of development, children are scanned and cognitive assessed at 6-month increments from time of recruitment until 2 years of age, and yearly thereafter. As a general study of neurotypical development, infants and young children with major risk factors for developmental, behavioral, or developmental disorders are excluded during enrolment. These risk factors included in utero exposure to alcohol, tobacco, or illicit recreational drugs; premature (<37 weeks gestation) or multiple birth; abnormalities on fetal ultrasound; complicated pregnancy (e.g., preeclampsia, gestational diabetes); 5 min APGAR scores < 8; NICU admission; history of neurological disorder or trauma (e.g., head injury, epilepsy); psychiatric or developmental disorders in first-degree relatives (including maternal depression requiring medication). Ongoing screenings, including the modified checklist for autism (MCAT) and child behavior checklist (CBCL) (Bilenberg, 1999; Chlebowski et al., 2013), and updated medical history information have been used to remove enrolled children with clinically concerning behaviors, diagnosed medical conditions, or head trauma following initial enrolment.
A combination of retrospective and prospective infant nutrition data was acquired from parents using detailed medical histories and parent interview. This included type of infant formula used; percentage of breastfeeding; and length of exclusive breastfeeding. This information was updated at each study visit, which occurred approximately every 6 months for children under 2 years of age, and yearly for older children. Using this information, children were categorized as either exclusively infant formula-fed or exclusively (at least 90 days) breastfed. Children who were fed a combination of breastmilk and formula were excluded from this analysis. Infants within the exclusively formula-fed group were further sub-divided based on parental reports of the main formula composition they received in at least 80% of feedings throughout the child’s first 3 months. All infant formulas consumed by children in this study were commercially available in the US.
Using these criteria, 88 (34 female) exclusively formula-fed infants and young children were selected into group #1. This number included 21 (9 female) children who received formula #1; 28 (10 female) who received formula #2; and 39 (15 female) who received formula #3. A sample of 62 (23 female) exclusively breast-fed infants were also selected and matched to the overall formula-fed children with regards to mean age at scans (p = .24), pregnancy length (p = .39), birth weight (p = .52) and length (p = .09), male:female ratio (p = .85), parent marital status (p = .66), maternal and paternal education levels (p = .9 and p = .9, respectively), family size (p = 1), and the mean inter-scan interval (time between each set of repeat scans, p = .29). Group demographics are provided in Table 1. There were no significant differences in these demographic characteristics between the individual formula groups, mal-e:female ratio (p = .26), gestation (p = .17), birth weight (p = .08), birth length (p = .5), maternal or paternal education (p = .64), family size (p = .85), or marital status (p = .98). Two-tailed student t-tests were used to compare group mean age, pregnancy length/gestation duration, birth weight, birth length, and inter-scan interval. Chi-squared tests were used to compare group parental education level, marital status, and family size.
Table 1.
Participant demographics for the breast and each formula-fed groups. Maternal and paternal education was scaled according to the Hollingshead scales of socioeconomic status, with 5 corresponding to at least 1 year of college education; 6 being a college or university graduate; and 7 holding a graduate or professional degree.
| Breastfed | All Formula | Formula #1 | Formula #2 | Formula #3 | ||
|---|---|---|---|---|---|---|
| Gender | Male (n) | 39 | 54 | 12 | 18 | 24 |
| Female (n) | 23 | 34 | 9 | 10 | 15 | |
| Age Range (days) | 86–3384 | 88–3409 | 103–3409 | 98–3308 | 88–3384 | |
| Mean Gestation (days) | 278 ± 17 | 281 ± 19 | 278 ± 6 | 275 ± 11 | 285 ± 12 | |
| Mean Birth Weight (g) | 3376 ± 675 | 3313 ± 540 | 3164 ± 589 | 3179±555 | 3446 ± 508 | |
| Mean Birth Height (inches) | 20 ± 3.6 | 21 ± 3.5 | 20 ± 0.5 | 20 ± 1.8 | 21 ± 4.9 | |
| Mean Maternal Education | 6 ± 1.3 | 5.6 ± 1.5 | 5.8 ± 1.2 | 5.4 ± 1.1 | 5.6 ± 1.8 | |
| Mean Paternal Education | 5.9 ± 1 | 5.5 ± 1.6 | 5.8 ± 1.3 | 5.1 ± 1.1 | 5.5 ± 1.6 | |
| Mean Family Size (# Children) | 2.3 ± 1.1 | 2.3 ± 1.5 | 2 ± 2 | 2.1 ± 1.5 | 2.2±1 | |
| # Scans/Child | 3.4 ± 1.2 | 2.5 ± 1.3 | 2.2 ± 1.4 | 3 ± 1.2 | 2.1 ± 1.3 | |
| Mean Inter-Scan Period (days) | 423 ± 234 | 380 ± 250 | 439 ± 250 | 407 ± 299 | 330 ± 190 | |
| Marital Status | Married/Living Together (n) | 46 | 68 | 10 | 21 | 29 |
| Divorced/Single (n) | 16 | 20 | 3 | 7 | 9 | |
A total of 231 scans were obtained on the breastfed children, and 221 on the formula-fed children (n = 42 for formula #1; n = 81 for formula #2; and n = 98 for formula #3). A pictorial display of the longitudinal imaging points and ages for each child is provided in Fig. 1. All child ages were corrected to a 40-week gestational age by subtracting the difference between 40 weeks and the child’s actual gestation duration from the child’s age.
Fig. 1.
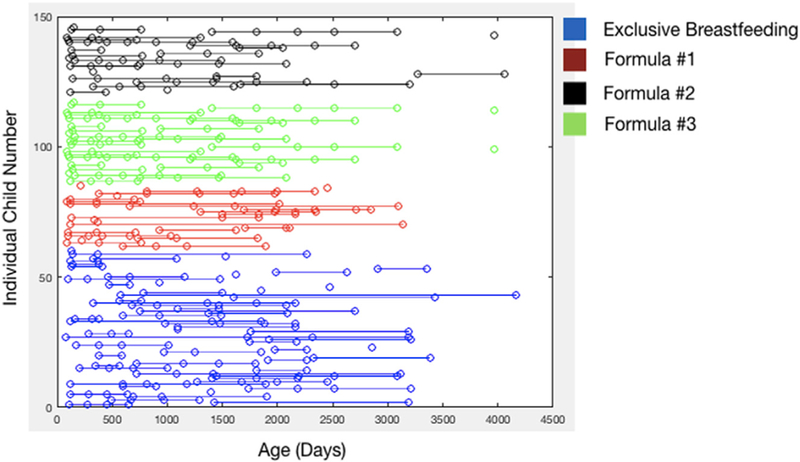
Pictorial view of the imaging time points and ages for each infant denoted by feeding group. Each row represents a single individual, with dots denoting the ages at which MRI, neuropsychological, infant feeding, and medical history data were acquired and updated.
Imaging methods and analysis
A multimodal imaging protocol was performed to assess brain morphology and myelination. mcDESPOT (multicomponent Driven Equilibrium Single Pulse Observation of T1 and T2)(Deoni et al., 2008) was used to quantify the myelin water fraction (MWF), a surrogate marker of myelin content or volume, throughout the brain. All infants were scanned during natural and non-sedated sleep using acoustically-reduced mcDESPOT imaging protocols described previously (Deoni et al., 2012) that comprise 8 T1-weighted spoiled gradient recalled echo (SPGR) images; 2 inversion (IR-) prepared SPGR images; and 16 T1/T2 weighted steady-state free precession (SSFP) images. Total imaging times ranged from 16 min for the youngest infants to 24 min for the older 4-year old and older children.
All data were acquired on a Siemens 3T Tim Trio scanner equipped with a 12-channel head RF array. To minimize intra-scan motion, children were swaddled with a pediatric MedVac vacuum immobilization bag (CFI Medical Solutions, USA) and foam cushions. Scanner noise was reduced by lessening the peak gradient amplitudes and slew-rates, and using a noise-insulating scanner bore insert (Quiet Barrier HD Composite, UltraBarrier, USA). MiniMuff pediatric ear covers and electrodynamic headphones (MR Confon, Germany) were also used (Dean et al., 2014). Children were continuously monitored with a pediatric pulse-oximetry system and infrared camera. Data used for this analysis had no visible motion-artefacts present in their acquired data, however, 12 datasets (5 from the breastfed group and 7 across the formula-fed groups) were rejected for either incomplete data (2) or visible ghosting and ringing artefacts (10).
Following data acquisition and inspection for image artefacts, conventional mcDESPOT preprocessing was performed consisting of image alignment (Jenkinson et al., 2002), non-brain signal removal (Smith, 2002), and correction for main and transmit magnetic field (B0 and B1) inhomogeneities (Deoni, 2011). A three-pool tissue signal model (the myelin-associated water; intra-extra axonal water; and a non-exchanging free-water pool) was then fit to the mcDESPOT data to derive voxel-wise MWF maps (Deoni et al., 2013a,b) using a stochastic region contraction approach (Deoni and Kolind, 2015).
Each child’s map was then non-linearly aligned to an existing study specific template using the Advanced Normalization Tools software package (Avants et al., 2011) using a previously described procedure (Deoni et al., 2012). Briefly, the high flip angle T1 weighted SPGR image from each child was non-linearly aligned to one of 14 age-specific templates (constructed at 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 64 and greater than 60 months), which have similar image size and tissue contrast. This transformation was then applied to the child’s quantitative MWF image. An overall study template in approximate MNI space was also previously constructed from these age templates, with pre-computed transformations between it and each age template and this transformation was then applied to the MWF image.
White matter masks, corresponding to 5 bilateral regions (frontal, temporal, occipital, parietal, and cerebellar WM) as well as the body, genu, and splenium of the corpus callosum were generated from the JHU white matter atlas (Oishi et al., 2011), registered to the study template, and superimposed onto each child’s MWF map. Mean values for each region were calculated for each child and used for subsequent developmental analysis and trajectory modeling.
Analysis of myelination trajectories
To examine group-wise developmental differences between the breast and formula-fed infants, a non-linear mixed effects modeling approach was used to fit a modified Gompertz growth model (example shown in Fig. 2)(Dean et al., 2015) to the regional MWF data, with the form:
Fig. 2.
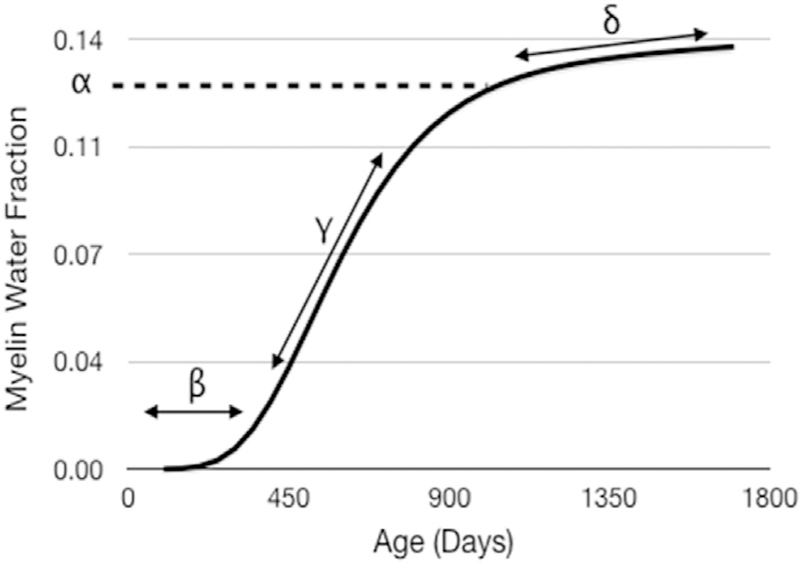
The modified Gompertz growth model used for all brain growth analysis labelled with relevant model parameters. Here, beta defines the onset of myelination; gamma is the initial rate of myelination; alpha is the MWF value at the shoulder point, or transition from rapid to slower myelination; and delta is the secondary slower rate of myelination.
As shown previously, the modified Gompertz model provides the most robust and reliable fit to developmental MWF data compared to other models (Dean et al., 2015). Each of the 4 Gompertz curve parameters were compared between the breast and formula-fed groups using an unpaired t-test with significance defined as p < .001 (p < .05 corrected for the 32 regional and parameter comparisons).
Examining this data further, we fit Gompertz growth models independently to children exclusively fed each of the three formula compositions (details provided below). Each model parameter was compared using an analysis of variance followed by a post-hoc Tukey test to determine which of the infant formula groups differed. Significance for these analysis was defined as p < .00052 (p < .05 corrected for the 96 comparisons performed).
Cognitive assessments and analysis
Alongside MR imaging, general cognitive ability and skills were evaluated in each child under 5 years and 8 months of age within 7 days of scanning using the Mullen Scales of Early Learning (Mullen, 1995). For older children, the Wechsler Intelligence Scale for Children, 5th Edition (WISC-V) was used. Due to the difference in cognitive assessment tool, we restricted our analysis here to only the MSEL data. The MSEL is a population-normed tool that provides domain-level assessment of fine and gross motor control, receptive and expressive language, and visual reception. In addition to age-normalized T-scores for each domain, the early learning composite (ELC) and verbal and non-verbal development quotients (VDQ and NVDQ, respectively) composite scores may be calculated that relect overall cognitive ability, and verbal and non-verbal functioning. Longitudinal group differences (breast vs. all infant formula-fed, and between each formula brand) in ELC were examined using mixed effects modeling assuming a linear trend with age.
Formula nutrient analysis and analysis
To examine the potential relationship between specific nutrients to aspects of development, the nutritional composition of each infant formula composition was determined. Alpha-lactalbumin, Beta-lactoglobulin, ARA, DHA, Calcium, Phosphorus, Sodium, Potassium, Copper, Magnesium, Vitamin B12, and Folic Acid were measured in the analytical laboratories of Asure Quality, Auckland, New Zealand. The phospholipid profile (Phosphatidylcholine, Phophatidylinositol, Phosphatidylserine, Phosphatidylethanolamine, Sphingomyelin) of each product was determined at the analytical laboratories of Neotron, Italy using the method of Giuffrida et al. (2013). This method was validated for the quantification of Phosphatidylcholine, Phosphatidylethanolamine and Sphingomyelin. The method for sphyngolmyelin had a quantification limit of <200 mg/kg.
Nutrients that differed substantively (with a difference greater than 25% in concentration between the minimum and maximum value) between the 3 infant formulas were identified as: ARA, DHA, folic acid, phosphatidylcholine, and sphingomyelin (Table 2). Associations between these nutrient values and aspects of development were investigated by constructing a series of 4 general linear models (GLMs) that modeled each Gompertz model parameter as an outcome variable, and each nutrient value as a predictor variable. For the Gompertz parameters, all children were included in the same mixed-effects model.
Table 2.
Nutritional differences between individual infant formulas.
| Units | Formula #1 | Formula #2 | Formula #3 | % Difference (Min-Max) | |
|---|---|---|---|---|---|
| ARA | mg/L | 173 | 238 | 255 | 32 |
| DHA | mg/L | 62.2 | 117 | 120.6 | 48 |
| Folic Acid | mcg/L | 304 | 232 | 146.2 | 52 |
| Phosphatidylcholine | mg/L | 85 | 58 | 60 | 29 |
| Sphingomyelin | mg/L | 28.1 | 62 | 28.1 | 55 |
| Iron | mg/100 g | 10.6 | 8.42 | 11.65 | 28 |
| Choline | mg/100 g | 170 | 92.5 | 144 | 46 |
Results
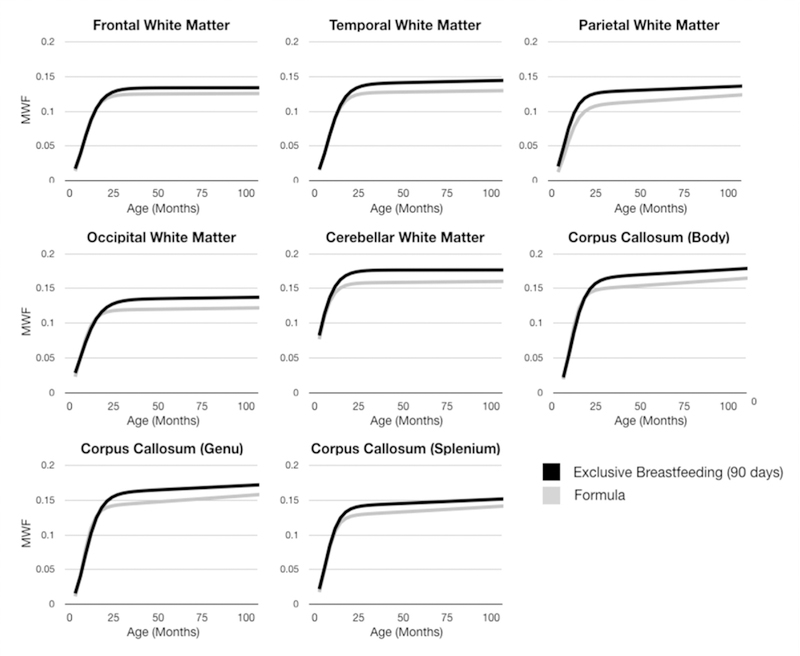
Fig. 3 contains the group-mean longitudinal MWF trajectories for the exclusively breast and all formula fed infants. In all investigated brain regions, we find differential patterns of development, with breastfed children qualitatively exhibiting a prolonged period of rapid development between 500 and 750 days of age, with an overall increase in myelin content by 2 years of age that persists throughout childhood. While the formula-fed group appears to show increased MWF before 1 year of age, they suffer a slower initial rate of MWF development between 1 and 2 years of age, and fail to reach the overall MWF magnitude of the breastfed group. Exploring these trajectory differences quantitatively (Table 3), in each brain region, there are statistically significant differences between all Gompertz growth model parameters in the frontal, temporal, and occipital white matter, and the body and genu of the corpus callosum. In the remaining regions (parietal and cerebellar white matter, splenium of the corpus callosum) there were significant differences between the α (asymptotic MWF value) and β (initial MWF onset) Gompertz terms; δ (secondary rate of MWF development) was found to be significantly different in parietal white matter and splenium. In each of these regions, γ (initial rate of MWF development) was not found to significantly differ between the groups.
Fig. 3.
Longitudinal growth curves for each investigated brain region between the exclusively breast and formula-fed infants. In each region, we see significant differences in development, as further quantified in Table 3.
Table 3.
Comparison of the individual Gompertz growth model parameters in each of the 8 brain regions between the exclusively breast and all formula-fed infants. Bold p values denote significant differences at the p < .0016 level (p < .05 corrected for the 32 independent comparisons).
| Region | Parameter | Breast-Fed |
Formula-Fed |
p Value | ||
|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | |||
| Frontal WM | α | 1.38E-01 | 3.92E-03 | 1.18E-01 | 5.54E-03 | <.0001 |
| β | 1.5E+00 | 8.01E-02 | 1.32E+00 | 1.71E-01 | <.0001 | |
| γ | 4.8E-03 | 2.73E-04 | 5.3E-03 | 6.69E-04 | <.0001 | |
| δ | 1.51E-04 | 1.26E-05 | 5.04E-05 | 2.31E-05 | <.0001 | |
| Temporal WM | α | 1.4E-01 | 4.04E-03 | 1.25E-01 | 5.46E-03 | <.0001 |
| β | 1.57E+00 | 9.33E-02 | 1.42E+00 | 1.77E-01 | <.0001 | |
| γ | 5.22E-03 | 3.2E-04 | 5.71E-03 | 6.85E-04 | <.0001 | |
| δ | 7.65E-05 | 1.32E-05 | 4.46E-05 | 2.18E-05 | <.0001 | |
| Parietal WM | α | 1.28E-01 | 3.03E-03 | 1.17E-01 | 4.75E-03 | <.0001 |
| β | 1.62E+00 | 8.85E-02 | 1.36E+00 | 1.72E-01 | <.0001 | |
| γ | 6.16E-03 | 3.36E-04 | 6.24E-03 | 7.45E-04 | .43 | |
| δ | 1.61E-05 | 1.12E-05 | 5.45E-05 | 2.06E-05 | <.0001 | |
| Occipital WM | α | 1.3E-01 | 3.25E-03 | 1.17E-01 | 4.76E-03 | <.0001 |
| β | 1.46E+00 | 8.6E-02 | 1.22E+00 | 1.69E-01 | <.0001 | |
| γ | 5.76E-03 | 3.6E-04 | 6.26E-03 | 7.97E-04 | <.0001 | |
| δ | 1.94E-05 | 1.19E-05 | 7.3E-05 | 2.04E-05 | <.0001 | |
| Cerebellar WM | α | 1.72E-01 | 3.99E-03 | 1.66E-01 | 5.02E-03 | <.0001 |
| β | 6.06E-01 | 6.85E-02 | 4.65E-01 | 1.16E-01 | <.0001 | |
| γ | 5.57E-03 | 4.46E-04 | 5.96E-03 | 7.98E-04 | .01 | |
| δ | 1.11E-08 | 1.13E-05 | 4.51E-08 | 1.59E-05 | .98 | |
| Corpus Callosum (Body) | α | 1.62E-01 | 3.88E-03 | 1.44E-01 | 5.9E-03 | <.0001 |
| β | 1.67E+00 | 8.82E-02 | 1.46E+00 | 1.82E-01 | <.0001 | |
| γ | 6E-03 | 3.24E-04 | 6.54E-03 | 7.72E-04 | <.0001 | |
| δ | 2.89E-05 | 1.11E-05 | 4.96E-05 | 2.09E-05 | <.0001 | |
| Corpus Callosum (Genu) | α | 1.6E-01 | 4.13E-03 | 1.41E-01 | 6.26E-03 | <.0001 |
| β | 1.71E+00 | 9.36E-02 | 1.52E+00 | 1.98E-01 | <.0001 | |
| γ | 5.38E-03 | 3.08E-04 | 5.93E-03 | 7.39E-04 | <.0001 | |
| δ | 1.14E-04 | 1.2E-05 | 4.36E-05 | 2.24E-05 | <.0001 | |
| Corpus Callosum (Splenium) | α | 1.38E-01 | 3.04E-03 | 1.29E-01 | 5.13E-03 | <.0001 |
| β | 1.78E+00 | 9.87E-02 | 1.49E+00 | 1.86E-01 | <.0001 | |
| γ | 7.04E-03 | 3.87E-04 | 7.14E-03 | 8.5E-04 | .39 | |
| δ | 1.51E-05 | 1.06E-05 | 4.46E-05 | 2.06E-05 | <.0001 | |
Examining differences between children who received different formula compositions, we find significant qualitative (Fig. 4) and quantitative (Table 4) differences in developmental patterns throughout the brain. In general, results from the ANOVA analyses revealed significant differences across the majority of brain regions examined and in almost all Gompertz model parameters. In particular, we note that children who received formula compositions with higher levels of DHA, ARA, choline, and sphingolipids (formulas #2 and #3) showed increased levels of myelin development. Of note, Formula #1, which showed to slowest myelin development, has the lowest concentration of DHA, ARA, and sphingomyelin, but has the highest concentration of iron and vitamin B12. Iron deficiency has previously been associated with cognitive impairments in older children (Lozoff and Georgieff, 2006).
Fig. 4.
Longitudinal growth curves for each investigated brain region between each of the three formula brands. In each region, we see significant differences in development, as further quantified in Table 4. Breastfeeding data (blue curve) is shown only for reference.
Table 4.
Comparison of the individual Gompertz growth model parameters in each of the 8 brain regions between each of the formula groups. Bold p values denote significant differences at the p < .0005 level (p < .05 corrected for the 96 comparisons).
| Region | Parameter | Formula 1 |
Formula 2 |
Formula 3 |
p Value | Differing Formulas | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | ||||
| Frontal WM | α | 1.15E-01 | 8.3E-03 | 1.24E-01 | 1.06E-02 | 1.37E-01 | 1.03E-02 | <.0001 | All |
| β | 1.46E+00 | 1.9E-01 | 1.21E+00 | 2.28E-01 | 1.11E+00 | 2.44E-01 | <.0001 | 1&2, 1&3 | |
| γ | 5.75E-03 | 7.88E-04 | 4.86E-03 | 9.71E-04 | 3.72E-03 | 7.79E-04 | <.0001 | All | |
| δ | 4.66E-05 | 3E-05 | 2.45E-05 | 4.25E-05 | 1.85E-06 | 3.11E-05 | <.0001 | 1&3 | |
| Temporal WM | α | 1.31E-01 | 9.53E-03 | 1.31E-01 | 1.04E-02 | 1.26E-01 | 6.59E-03 | .1 | |
| β | 1.41E+00 | 1.73E-01 | 1.37E+00 | 2.48E-01 | 1.42E+00 | 3.31E-01 | .7 | ||
| γ | 5.42E-03 | 7.19E-04 | 5.32E-03 | 1.01E-03 | 5.52E-03 | 1.1E-03 | .7 | ||
| δ | 1.16E-05 | 3.07E-05 | 1.78E-05 | 4.06E-05 | 4.68E-05 | 2.33E-05 | .0005 | 1&3 | |
| Parietal WM | α | 1.13E-01 | 7.85E-03 | 1.26E-01 | 9.45E-03 | 1.2E-01 | 5.24E-03 | <.0001 | 1&2 |
| β | 1.42E+00 | 2.02E-01 | 1.22E+00 | 2.25E-01 | 1.57E+00 | 3.35E-01 | <.0001 | 2&3 | |
| γ | 6.48E-03 | 9.44E-04 | 5.46E-03 | 1.04E-03 | 6.56E-03 | 1.15E-03 | .0001 | 1&2 | |
| δ | 5.54E-05 | 3.12E-05 | 2.38E-05 | 3.85E-05 | 4.72E-05 | 2.02E-05 | .0005 | 1&2 | |
| Occipital WM | α | 1.23E-01 | 8.19E-03 | 1.27E-01 | 9E-03 | 1.19E-01 | 5.88E-03 | .003 | |
| β | 1.76E+00 | 2.15E-01 | 1.05E+00 | 2.01E-01 | 1.62E+00 | 4.16E-01 | <.0001 | 1&2, 2&3 | |
| γ | 7.67E-03 | 7.88E-04 | 5.21E-03 | 9.89E-04 | 7.87E-03 | 1.64E-03 | <.0001 | 1&2, 2&3 | |
| δ | 8.92E-06 | 3.06E-05 | 4.34E-05 | 3.58E-05 | 6.88E-05 | 2.3E-05 | <.0001 | 1&2, 1&3 | |
| Cerebellar WM | α | 1.59E-01 | 7.18E-03 | 1.75E-01 | 9.86E-03 | 1.57E-01 | 6.84E-03 | <.0001 | 1&2, 2&3 |
| β | 6.24E-01 | 2.01E-01 | 2.93E-01 | 1.46E-01 | 1.36E+00 | 4.41E-01 | <.0001 | 1&2, 1&3, 2&3 | |
| γ | 7.38E-03 | 1.55E-03 | 4.7E-03 | 1.02E-03 | 1.04E-02 | 2.87E-03 | <.0001 | 1&2, 1&3, 2&3 | |
| δ | 1.7E-05 | 2.48E-05 | 9.48E-09 | 2.89E-05 | 1.45E-05 | 2.18E-05 | <.0001 | ||
| Corpus Callosum (Body) | α | 1.39E-01 | 9.69E-03 | 1.55E-01 | 1.16E-02 | 1.47E-01 | 6.13E-03 | <.0001 | 1&2 |
| β | 1.51E+00 | 2.11E-01 | 1.31E+00 | 2.36E-01 | 1.63E+00 | 3.32E-01 | <.0001 | 2&3 | |
| γ | 6.71E-03 | 9.53E-04 | 5.7E-03 | 1.06E-03 | 6.83E-03 | 1.15E-03 | .0001 | 1&2 | |
| δ | 4.95E-05 | 3.06E-05 | 1.46E-05 | 3.87E-05 | 4.52E-05 | 1.94E-05 | .0001 | 1&2 | |
| Corpus Callosum (Genu) | α | 1.36E-01 | 9.85E-03 | 1.47E-01 | 1.2E-02 | 1.51E-01 | 8.34E-03 | <.0001 | 1&2, 1&3 |
| β | 1.61E+00 | 2.19E-01 | 1.42E+00 | 2.69E-01 | 1.43E+00 | 3.14E-01 | .005 | ||
| γ | 6.21E-03 | 8.69E-04 | 5.51E-03 | 1.08E-03 | 5.13E-03 | 1E-03 | .0002 | ||
| δ | 4.08E-05 | 2.98E-05 | 1.45E-05 | 4.2E-05 | 1.84E-05 | 2.47E-05 | .003 | ||
| Corpus Callosum (Splenium) | α | 1.22E-01 | 8.39E-03 | 1.41E-01 | 1E-02 | 1.27E-01 | 5.81E-03 | <.0001 | 1&2, 2&3 |
| β | 1.55E+00 | 2.41E-01 | 1.33E+00 | 2.42E-01 | 1.77E+00 | 3.38E-01 | <.0001 | 2&3 | |
| γ | 7.76E-03 | 1.23E-03 | 6.12E-03 | 1.14E-03 | 7.66E-03 | 1.21E-03 | <.0001 | 1&2, 2&3 | |
| δ | 6.29E-05 | 3.29E-05 | 1.74E-05 | 3.75E-05 | 4.84E-05 | 2.14E-05 | <.0001 | 1&2 | |
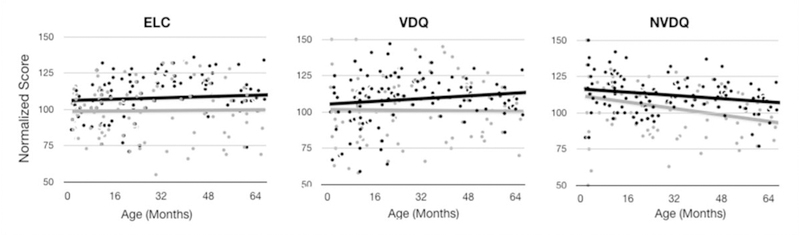
To determine if differences in cognitive maturation were also present in our sample of children, a linear mixed effects model was fit to the repeated Mullen composite scores (ELC, VDQ, and VNDQ). Results (Fig. 5 and Table 5) support our own prior results (Deoni et al., 2013a,b) as well as those of numerous cognitive studies comparing breast and formula-fed children (Horwood and Fergusson, 1998; Anderson et al., 1999; Kramer et al., 2008; Mortensen et al., 2002; Huang et al., 2014). Specifically, we find that while the mean trend for both groups fell within the normative range (85–115), there were statistically significant differences in the rate of cognitive change with age (slope) and a general increase in the mean (intercept) between the breast and formula-fed groups. Breastfed children exhibited an overall increase in ELC, VDQ, and NVDQ scores, and increased rates of development in ELC and VDQ, and an attenuated decrease in NVDQ with age. Thus, early differences in cognitive function were found to persist, and in the case of VDQ and NVDQ increase, into childhood.
Fig. 5.
Comparison of longitudinal cognitive maturation (Mullen’s ELC, VDQ, and NVDQ) curves between the exclusive breast (dark dots and line) and formula-fed (gray dots and line) children.
Table 5.
Comparison of linear trend parameters (slope and intercept) fit to longitudinal Mullen ELC, VDQ, and NVDQ values for the breast and all formula-fed children. Bold p values denote significant differences at the p < .003 level (p < .05 corrected for the 6 comparisons).
| Cognitive Score | Parameter | Breast-Fed |
Formula-Fed |
p Value | ||
|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | |||
| ELC | Slope | 0.00196 | 0.0011 | 0.00057 | 0.00021 | <.0001 |
| Intercept | 106 | 1.8 | 98.6 | 2.6 | <.0001 | |
| VDQ | Slope | 0.0041 | 0.0021 | −0.00064 | 0.0043 | <.0001 |
| Intercept | 105.2 | 2.4 | 101.7 | 3.6 | <.0001 | |
| NVDQ | Slope | −0.0048 | 0.0017 | −0.0094 | 0.0032 | <.0001 |
| Intercept | 116.6 | 1.9 | 112.2 | 3.6 | <.0001 | |
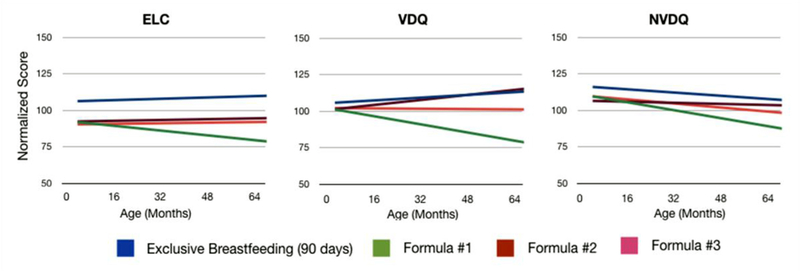
Repeating this same analysis for each infant formula, we find (Fig. 6 and Table 6) an overall correspondence between brain development profiles and trajectories of cognitive maturation. Specifically, children who received Formula #1, which shows the slowest myelination profile across the majority of brain regions, also have the most pronounced decline in cognitive function across early childhood. Formula #2, which had the closest myelination trend to breastfeeding also exhibit cognitive trends that are most consistent with breastfeeding. These results suggest not only the importance of early nutrition to brain and cognitive development, but also suggest a strong link between brain structure and cognitive performance.
Fig. 6.
Comparison of longitudinal cognitive maturation (Mullen’s ELC, VDQ, and NVDQ) profiles between children who received differing formulas. The trend for exclusive breastfeeding is provided for reference.
Table 6.
Comparison of linear trend parameters (slope and intercept) fit to longitudinal Mullen ELC, VDQ, and NVDQ values for each of the formula group. Bold p values denote significant differences at the p < .003 level (p < .05 corrected for the 18 comparisons).
| Cognitive Score | Parameter | Formula #1 |
Formula #2 |
Formula #3 |
p Value | Differing F | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | ||||
| ELC | Slope | −0.0071 | 0.005 | 0.012 | 0.006 | 0.00891 | 0.00535 | <.0001 | 1&2 1&3 |
| Intercept | 92.8 | 6 | 92.25 | 5.3 | 90.316 | 6.8 | .42 | ||
| VDQ | Slope | −0.012 | 0.0044 | 0.0074 | 0.006 | −0.00402 | 0.0046 | <.0001 | All |
| Intercept | 102.6 | 4.5 | 100.4 | 5.3 | 110.31 | 5.7 | <.0001 | 1&3 2&3 | |
| NVDQ | Slope | −0.012 | 0.0042 | −0.00032 | 0.0058 | −0.00047 | 0.0066 | <.0001 | 1&2 1&3 |
| Intercept | 111.1 | 4.5 | 106.7 | 5.2 | 102.1 | 6.9 | <.0001 | 1&3 | |
Investigating the influence of specific nutrient concentrations on the myelination model trajectory parameters (Table 7), we find that whilst each of ARA, DHA, folic acid, iron, choline, sphingomyelin, B12, and phosphatidylcholine contribute to myelination, sphingomyelin and phosphatidylcholine appear to have the most diffuse inluence throughout the brain with the remaining nutrients more associated with development in focal brain areas.
Table 7.
Analysis of correlations between identified nutrients (Table 2) and the asymptotic value from the Gompertz growth model. Bold p values denote significant differences at the p < .006 level (p < .05 corrected for the 8 analyses).
| Region | Parameter | ARA | DHA | Folic Acid | Phosphatidylcholine | Sphingomyelin | Iron | Choline |
|---|---|---|---|---|---|---|---|---|
| Frontal WM | α | .002 | .002 | .03 | <.0001 | <.0001 | .15 | .004 |
| Temporal WM | α | .04 | .03 | .17 | .004 | .002 | .34 | .26 |
| Parietal WM | α | <.0001 | <.0001 | .005 | <.0001 | <.0001 | .08 | .08 |
| Occipital WM | α | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .006 |
| Cerebellar WM | α | <.0001 | <.0001 | .013 | <.0001 | <.0001 | <.0001 | .02 |
| Corpus Callosum (Body) | α | .003 | .02 | .45 | <.0001 | <.0001 | .28 | .076 |
| Corpus Callosum (Genu) | α | .09 | .15 | .63 | <.0001 | <.0001 | .27 | .43 |
| Corpus Callosum (Splenium) | α | .05 | .02 | .76 | <.0001 | <.0001 | .56 | .86 |
Discussion
The impact of nutrition on human infant brain myelination has traditionally been indirectly investigated via studies of cognitive performance or using evoked potentials (Pivik et al., 2007), with few neuroimaging studies performed throughout infancy and early childhood (Deoni et al., 2013a,b; Luby et al., 2016). This study, therefore, adds to the existing literature examining the role of early life nutrition and feeding choice in infancy, presenting the first longitudinal neuroimaging results demonstrating differences in profiles of myelination and cognitive development between children exclusively breast or formula fed, and between children who received different infant formulas. These data suggested an important role for DHA, ARA, sphingomyelin and choline in early brain development, which was subsequently confirmed by examining the associations between these nutrients and brain growth model parameters.
On a general level, our results indicate that exclusive breastfeeding for at least 3 months is associated with improved myelination diffusely throughout the brain by 2 years of age, including early and late maturing brain regions and networks associated with a broad array of cognitive and behavioral skills. Supporting this structure-function link, we also show improved overall cognitive ability and rates of cognitive development, including verbal and non-verbal functioning, in breast-fed children versus those who received only infant formula. Examining the longitudinal trends within our data we find that these structural and cognitive differences become evident by approximately 18 months of age (depending on brain region), and persist at least into early childhood (at least 5.5 years of age). Observed differences in myelination, an essential element of the brain’s white matter structure (Fields, 2010; O’Brien and Sampson, 1965), may be predictive of previously observed white matter volume and integrity changes in older children and adolescents who were breastfed as infants (Isaacs et al., 2010). The importance of myelination to brain connectivity may also link our findings to prior reports of altered functional activation and connectivity in breastfed infants (Ou et al., 2015).
In order to more specifically link observed breast vs. formula-fed differences to nutrition, as opposed to other potential socioeconomic or demographic aspects, we also contrasted the brain and cognitive developmental profiles in children who received different formula compositions (Figs. 4 and 6). Here we found significant and consistent differences in the profiles of myelination and cognitive maturation, with children who had the lowest myelin development overall having the worst cognitive scores and vice-versa. Of note, the formula compositions associated with the highest myelin levels and cognitive scores also had the highest concentration of long-chain PUFAs (DHA and ARA), choline, folic acid, sphingolipids (sphingomyelin) and phosphatides (phosphati-dylcholine). This finding is in strong agreement with prior nutrition literature. Long-chain poly-unsaturated fatty acids (LC-PUFAs), in particular, ARA and DHA, help promote neuronal growth and white matter development (Innis, 2007). Preclinical studies of animals withheld AA and DHA via early weaning have reduced myelin basic protein expression, consistent with reduced myelin content (Kodama et al., 2008; Bruno and Tassinari, 2011). While folic acid deficiency is more often associated with neural tube defects (Bower, 1995), preclinical studies have also shown that postnatal deficiencies can negatively affect the fatty acid composition of myelin (Chida et al., 1972). Choline, a precursor to phosphatidylcholine as well as sphingomyelin, and choline-containing phosphoglycerides, comprise more than 10% of the lipid weight of myelin (Norton and Cammer, 1984). In in vitro studies of choline deficiency, significant reductions in phosphatidylcholine (–49%) and sphingomyelin (–34%) concentrations were found compared to cells grown in a choline-rich medium (Yen et al., 1999). These results are mirrored in in vivo human studies, demonstrating a 30% decrease in circulating phosphatidylcholine levels following a 3-week choline-deficient diet. Finally, both phosphatidylcholine and sphingomyelin are critical components of myelin, with dietary supplementation of sphingomyelin previously shown to improve myelination in a preclinical model (Oshida et al., 2003).
In contrast, formula compositions high in iron, but lower in LC-PUFAs and sphingolipids, appear to be associated with slower and reduced overall myelination. Although the role of iron in myelin synthesis is not yet fully understood, both animal and human infant studies have revealed associations between iron deficiency and hypomyelination, reduced oligodendrocyte functioning, and decreased myelin basic protein concentrations. Iron may also play a specific role in myelin synthesis through oligodendrocyte energy metabolism, and fatty acid synthesis. Children with prolonged iron deficiency also suffer a variety of behavioral and cognitive impairments (Saloojee and Pettifor, 2001; Lozoff and Georgieff, 2006; Congdon et al., 2012). However, while iron deficiency has been well studied, little is known regarding potential outcomes associated with iron over supplementation. Breastmilk contains little iron content, and iron deficiency may arise after 4 months in exclusively breastfed infants (Kramer and Kakuma, 2012; Ballard and Morrow, 2013). However, healthy non-anaemic infants supplemented with iron exhibit reduced growth (Dewey et al., 2002) and increased fever and illness (Pasricha et al., 2013).
There are important caveats to our examination of different infant formulas, including: 1. The retrospective nature of our investigation; and 2. The high variability of these nutrients. While formula and feeding information for this study was acquired prospectively, nutritional composition analysis was performed retrospectively using a single time assay. Thus, the specific nutritional formulations may have changed in the 6 years since the earliest imaging data was acquired. It is also important to note that it is not possible, using these observational data alone, to infer which particular nutrient (or combination) is most associated with preferential myelination trajectories. Such information is likely only be provided by pre-clinical models in which individual nutrients may be carefully varied and the effects followed.
Given the temporal delay between infant feeding and the first appearance of differences in both myelination and cognition between the breast and formula-fed children, it is likely that prolonged breastfeeding durations, follow-on complementary feeding and other environmental influences (e.g., parental interaction) are important but unexamined contributors to our results. In past cross-sectional analysis examining associations between breastfeeding duration (and including complementary feeding past 6-months) and MWF, we showed prolonged breastfeeding was associated with increased myelination in the cerebellum, internal capsule, and parietal and temporal white matter (Deoni et al., 2013a,b). In addition to prolonged breast-feeding, numerous other environmental conditions have previously been associated with differences in cognitive development and outcomes in children, such as family socioeconomic status (SES) (Noble et al., 2015), parental education (Cromwell and Panksepp, 2011), parent-child interaction (Swain et al., 2007), physical activity (Best, 2010), and sleep quality (Peirano and Algarin, 2007). Although we aimed to mitigate these SES-related factors by matching children on the basis of maternal and paternal education levels, family size, and parental marital status, it is difficult to discount their contribution without accurate and detailed assessments of each.
A variety of environmental and economic conditions have previously been associated with differences in cognitive development and outcomes in children, such as family socioeconomic status (SES) (Noble et al., 2015), parental education (Cromwell and Panksepp, 2011), parent-child interaction (Swain et al., 2007), physical activity (Best, 2010), and sleep quality (Peirano and Algarin, 2007). Although we aimed to mitigate these SES-related factors by matching children on the basis of maternal and paternal education levels, family size, and parental marital status, it is difficult to discount their contribution without accurate and detailed assessments of each. However, while the effect of these socioeconomic differences to cognitive outcomes in breastmilk and formula fed children is increasingly documented (Walfisch et al., 2013), the expected differences between formula-fed children may be less.
An additional, and as yet unresolved, question related to neurocognitive outcomes associated with breastfeeding is whether they arise due to the specific nutritional, hormonal, and other constituents of breastmilk per se; if they are driven by maternal-child interaction and other environmental differences (Walfisch et al., 2013); or are a result of a combination of the two (Reynolds, 2001). Our study does not attempt to resolve this quandary, but does provide important support for the role of early nutrition, with specific emphasis on nutrients either involved in myelin synthesis or compositional components of myelin. This is particularly evidenced by the differences in development observed amongst the exclusively formula-fed children.
In this work, we have focused on de novo myelination given its fundamental role in learning and cognition (Nagy et al., 2004; Fields, 2008) and its previously demonstrated sensitivity to nutrition (Lozoff and Georgieff, 2006; Innis, 2007; Bruno and Tassinari, 2011; Kodama et al., 2008). However, other developmental processes, including synapto-genesis and synaptic pruning are also important contributors to brain connectivity, brain function, and cognition throughout this age period. Like myelination, these processes may also be differentially influenced by nutrition (Kafouri et al., 2013; Luby et al., 2016). An MR imaging measure related to synapse density is cortical thickness, commonly quantified using conventional anatomical imaging and brain segmentation (Fischl, 2012). Analysis and examination of cortical thickness differences was not performed here owing to the challenges of accurate gray-white matter segmentation in young children, particularly those under 1 year of age. A further possibility is that the increase in myelin content measured here does not reflect more myelin per axon, but more myelinated axons overall. One approach to investigating this further is the use of neurite orientation dispersion and density imaging (NODDI) (Zhang et al., 2012) and myelin g-ratio imaging (Dean et al., 2016), which would inform on both axonal density and mean axon myelin thickness. High resolution structural and NODDI imaging data were, unfortunately, not collected across all children included in this analysis and, thus, investigations of these additional metrics remains a topic for future investigations.
Conclusions
While the exact mechanisms that underlie the previously demonstrated brain myelination and cognitive advantages differences in children, adolescents, and adults who were breastfed as infants remain unclear, our results presented here add to the growing evidence and consensus that early and exclusive breastfeeding is associated with improved neurodevelopment, including de novo myelination, and cognitive outcomes. Our longitudinal findings further suggest that early developmental differences persist into childhood and may predict changes previously identified in adolescents and adults. Furthermore, different compositions of infant nutrition appear to result in different patterns of myelin development, with some being closer to the myelin trajectory associated with breast-fed infants than others. With respect to potential nutritional contributors, our analysis highlights the importance of known neuro-associated nutrients, including long-chain poly-unsaturated fatty acids as well as the important myelin components phosphatidylcholine and sphingomyelin to early neurodevelopment.
Acknowledgements
This work was supported by the National Institutes of Health (R01 MH087510) The NIH ECHO program (UG3OD023313) and the Bill and Melinda Gates Foundation (OPP1120016). SCLD receives salary and research support from Nestec S.A.
References
- Anderson JW, Johnstone BM, Remley DT, 1999. Breast-feeding and cognitive development: a meta-analysis. Am. J. Clin. Nutr 70 (4), 525–535. [DOI] [PubMed] [Google Scholar]
- Avants BB, et al. , 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54 (3), 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard O, Morrow AL, 2013. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am 60 (1), 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, et al. , 2003. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol. Psychiatr 53 (5), 412–421. [DOI] [PubMed] [Google Scholar]
- Best JR, 2010. Effects of physical activity on children’s executive function: contributions of experimental research on aerobic exercise. Dev. Rev 30 (4), 331–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilenberg N, 1999. The Child Behavior Checklist (CBCL) and related material 52. [DOI] [PubMed] [Google Scholar]
- Blaas N, Schüürmann C, Bartke N, 2011. Structural profiling and quantification of sphingomyelin in human breast milk by HPLC-MS/MS. J. Agric. Food Chem [DOI] [PubMed]
- Bower C, 1995. Folate and neural tube defects. Nutr. Rev [PubMed]
- Brody BA, et al. , 1987. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J. Neuropathol. Exp. Neurol 46 (3), 283–301. [DOI] [PubMed] [Google Scholar]
- Bruno KD, Tassinari MS, 2011. Essential fatty acid supplementation of DHA and ARA and effects on neurodevelopment across animal species: a review of the literature. Birth Defects Res. Part B Dev. Reprod. Toxicol [DOI] [PubMed]
- Chang CY, Ke DS, Chen JY, 2009. Essential fatty acids and human brain. Acta Neurol. Taiwan [PubMed]
- Chida N, Hirono H, Arakawa T, 1972. Effects of dietary folate deficiency on fatty acid composition of myelin cerebroside in growing rats [DOI] [PubMed]
- Chlebowski C, et al. , 2013. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics 131 (4), e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon EL, et al. , 2012. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J. Pediatr 160 (6), 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Panksepp J, 2011. Rethinking the cognitive revolution from a neural perspective: how overuse/misuse of the term ‘cognition’ and the neglect of affective controls in behavioral neuroscience could be delaying progress in understanding the BrainMind. Neurosci. Biobehav. Rev 35 (9), 2026–2035. [DOI] [PubMed] [Google Scholar]
- Davison AN, Dobbing J, 1966. Myelination as a vulnerable period in brain development [DOI] [PubMed]
- Dean DC, et al. , 2015. Characterizing longitudinal white matter development during early childhood. Brain Struct. Funct 220 (4), 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, et al. , 2014. Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatr. Radiol 44 (1), 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC III, et al. , 2016. Mapping an index of the myelin g-ratio in infants using magnetic resonance imaging. NeuroImage 132, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Dean DC, et al. , 2013a. Breastfeeding and early white matter development: a cross-sectional study. NeuroImage 82, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, 2011. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magn. Reson. Med 65 (4), 1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, et al. , 2012. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. NeuroImage 63 (3), 1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, et al. , 2014. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct. Funct [DOI] [PMC free article] [PubMed]
- Deoni SCL, Matthews L, Kolind SH, 2013b. One component? Two components? Three? the effect of including a nonexchanging ‘free’ water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn. Reson. Med 70 (1), 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S, Kolind SH, 2015. Investigating the stability of mcDESPOT myelin water fraction values derived using a stochastic region contraction approach. Magn. Reson. Med [DOI] [PMC free article] [PubMed]
- Deoni S, Rutt BK, Arun T, 2008. Gleaning multicomponent T1 and T2 information from steady-state imaging data [DOI] [PubMed]
- Dewey KG, et al. , 2002. Iron supplementation affects growth and morbidity of breastfed infants: results of a randomized trial in Sweden and Honduras. J. Nutr 132 (11), 3249–3255. [DOI] [PubMed] [Google Scholar]
- Fields RD, 2010. Change in the brain’s white matter. Science [DOI] [PMC free article] [PubMed]
- Fields RD, 2008. White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31 (7), 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, 2012. FreeSurfer. NeuroImage 62 (2), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn SW, et al. , 2003. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol. Psychiatr 8 (9), 811–820. [DOI] [PubMed] [Google Scholar]
- Fornari E, et al. , 2007. Myelination shapes functional activity in the developing brain. NeuroImage 38 (3), 511–518. [DOI] [PubMed] [Google Scholar]
- Hadley KB, et al. , 2016. The essentiality of arachidonic acid in infant development. Nutrients 8 (4), 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi AG, McKerracher L, 2015. Breastfeeding and infant growth. Evol. Med. Public Health 2015 (1), 150–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood LJ, Fergusson DM, 1998. Breastfeeding and later cognitive and academic outcomes. Pediatrics [DOI] [PubMed]
- Huang J, et al. , 2014. Breastfeeding and trajectories of children’s cognitive development. Dev. Sci 17 (3), 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis SM, 2007. Dietary (n-3) fatty acids and brain development. J. Nutr [DOI] [PubMed]
- Isaacs EB, et al. , 2010. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res 67 (4), 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi SK, Odle J, 2012. Nutritional factors influencing intestinal health of the neonate. Adv. Nut. Int. Rev. J 3 (5), 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K, 2010. Sphingolipids in multiple sclerosis. NeuroMolecular Med [DOI] [PMC free article] [PubMed]
- Jenkinson M, et al. , 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17 (2), 825–841. [DOI] [PubMed] [Google Scholar]
- Johnson MH, 2001. Functional brain development in humans. Nat. Rev. Neurosci [DOI] [PubMed]
- Kafouri S, et al. , 2013. Breastfeeding and brain structure in adolescence. Int. J. Epidemiol 42 (1), 150–159. [DOI] [PubMed] [Google Scholar]
- Van der Knaap MS, et al. , 1991. Myelination as an expression of the functional maturity of the brain. Dev. Med. Child Neurol 33 (10), 849–857. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Kikusui T, Takeuchi Y, 2008. Effects of early weaning on anxiety and prefrontal cortical and hippocampal myelination in male and female Wistar rats. Dev. Psychobiol [DOI] [PubMed]
- Kolind S, et al. , 2015. Brain and cord myelin water imaging: a progressive multiple sclerosis biomarker. Neurolmage Clin [DOI] [PMC free article] [PubMed]
- Kolind S, et al. , 2013. Myelin imaging in amyotrophic and primary lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener 14 (7), 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolind S, et al. , 2012. Myelin water imaging reflects clinical variability in multiple sclerosis. Neurolmage 60 (1), 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Aboud F, Mironova E, 2008. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch. Gen. Psychiatr [DOI] [PubMed]
- Kramer MS, Kakuma R, 2012. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev 8, CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK, 2006. Iron deficiency and brain development. Semin. Pediatr. Neurol 13 (3), 158–165. [DOI] [PubMed] [Google Scholar]
- Luby JL, et al. , 2016. Breastfeeding and childhood IQ: the mediating role of gray matter volume. J. Am. Acad. Child Adolesc. Psychiatr [DOI] [PMC free article] [PubMed]
- MacKay A, et al. , 1994. In vivo visualization of myelin water in brain by magnetic resonance. Magn. Reson. Med 31 (6), 673–677. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT, 2004. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol 1 (4), 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, et al. , 2005. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309 (5744), 2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen EL, Michaelsen KF, Sanders SA, 2002. The association between duration of breastfeeding and adult intelligence. J. Am. Med. Assoc [DOI] [PubMed]
- M’Rabet L, et al. , 2008. Breast-feeding and its role in early development of the immune system in infants: consequences for health later in life. J. Nutr 138 (9), 1782–1790. [DOI] [PubMed] [Google Scholar]
- Mullen EM, 1995. Mullen scales of early learning
- Nagy Z, Westerberg H, Klingberg T, 2004. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cognit. Neurosci 16 (7), 1227–1233. [DOI] [PubMed] [Google Scholar]
- Noble KG, et al. , 2015. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci 18 (5), 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT, Cammer W, 1984. Isolation and characterization of myelin. Myelin
- O’Brien JS, Sampson EL, 1965. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. Lipid Res [PubMed]
- Oishi K, et al. , 2011. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. NeuroImage 56 (1), 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh J, et al. , 2013. Interactions between white matter asymmetry and language during neurodevelopment. J. Neurosci 33 (41), 16170–16177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh J, et al. , 2014. White matter development and early cognition in babies and toddlers [DOI] [PMC free article] [PubMed]
- Oshida K, et al. , 2003. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res [DOI] [PubMed]
- Ou X, et al. , 2015. Voxel-based morphometry and fMRI revealed differences in brain gray matter in breastfed and milk formula-fed children. Am. J. Neuroradiol 37 (4), 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasricha S-R, et al. , 2013. Effect of daily iron supplementation on health in children aged 4–23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Global Health 1 (2), e77. [DOI] [PubMed] [Google Scholar]
- Peirano PD, Algarin CR, 2007. Sleep in brain development. Biol. Res [PubMed]
- Pivik RT, et al. , 2007. The influence of infant diet on early developmental changes in processing human voice speech stimuli: ERP variations in breast and milk formula-fed infants at 3 and 6 months after birth. Dev. Neuropsychol 31 (3), 279–335. [DOI] [PubMed] [Google Scholar]
- Pujol J, et al. , 2004. Delayed myelination in children with developmental delay detected by volumetric MRI. NeuroImage 22 (2), 897–903. [DOI] [PubMed] [Google Scholar]
- Pujol J, et al. , 2006. Myelination of language-related areas in the developing brain. Neurology 66 (3), 339–343. [DOI] [PubMed] [Google Scholar]
- Reynolds A, 2001. Breastfeeding and brain development. Pediatr. Clin. North Am [DOI] [PubMed]
- Saher G, et al. , 2005. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci 8 (4), 468–475. [DOI] [PubMed] [Google Scholar]
- Saloojee H, Pettifor JM, 2001. Iron deficiency and impaired child development. BMJ (Clin. Re. Ed.) 323 (7326), 1377–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction 17 (3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL, 2010. The basics of brain development. Neuropsychol. Rev 20 (4), 327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, et al. , 2007. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J. Child Psychol. Psychiatry Allied Discip 48 (3), 262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfisch A, et al. , 2013. Breast milk and cognitive development-the role of confounders: a systematic review. BMJ Open 3 (8), e003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, et al. , 2012. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatr 169 (6), 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TC, et al. , 2016. Whole-brain ex-vivo quantitative MRI of the cuprizone mouse model. PeerJ [DOI] [PMC free article] [PubMed]
- Yen CL, Mar MH, Zeisel SH, 1999. Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB. J 13 (1), 135–142. [PubMed] [Google Scholar]
- Zhang H, et al. , 2012. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61 (4), 1000–1016. [DOI] [PubMed] [Google Scholar]