Abstract
Recent reports indicate that, in addition to treating hypertension, renal denervation (RDN) also mitigates renal inflammation. However, since RDN decreases renal perfusion pressure, it is unclear whether these effects are due to the direct effects of RDN on inflammatory signaling or secondary to decreased arterial pressure (AP). Therefore, this study was conducted to elucidate the contribution of renal nerves to renal inflammation in the DOCA-salt rat, a model in which RDN decreases AP and abolishes renal inflammation. In Experiment 1, we assessed the temporal changes in renal inflammation by measuring renal cytokines and AP in DOCA-salt rats. Uninephrectomized (1K) adult male Sprague Dawley rats that received surgical renal denervation (RDN) or sham (Sham) were administered DOCA (100mg, s.c.) and 0.9% saline for 21 days. AP was measured by radiotelemetry, and urinary cytokine excretion were measured repeatedly. In Experiment 2, the contribution of renal nerves in renal inflammation was assessed in a 2-kidney DOCA-salt rat to control for renal perfusion pressure. DOCA-salt treatment was administered after unilateral (U-)RDN. In Experiment 1, DOCA-salt induced increases in AP and renal inflammation (assessed by urinary cytokines) were attenuated by RDN versus Sham. In Experiment 2, GRO/KC, MCP-1, and macrophage infiltration were lower in the denervated kidney vs. the contralateral Sham kidney. No differences in T-cell infiltration were observed. Together, these data support the hypothesis that renal nerves mediate, in part, the development of renal inflammation in the DOCA-salt rat independent of hypertension. The mechanisms and cell-specificity mediating these effects require further investigation.
Keywords: Hypertension, renal nerves, renal denervation inflammation
Summary
These data demonstrate the pro-inflammatory response and hypertension in DOCA-salt rat model is primarily mediated by renal nerves, since renal nerve ablation mitigated the hypertension and prevented any increase in renal inflammatory cytokine content. Renal denervation specifically reduced macrophage infiltration and chemokine content when arterial pressure remained elevated, highlighting a direct, cell-specific mechanism.
INTRODUCTION
Hypertension remains the greatest risk factor for cardiovascular disease and is the most common cause of morbidity and mortality worldwide1. While the etiology of HTN is multifaceted, the principal role for increased sympathetic nerve activity (SNA), particularly to the kidney, is extensively documented in both clinical and experimental settings2–4. In recent years, particular attention has focused on renal sympathetic nerves because renal denervation (RDN) attenuates and/or reverses several experimental models of HTN4, 5. These findings were translated to the first clinical trials6, 7 in which catheter-based RDN decreased arterial pressure in hypertensive patients. Predictably, these trials generated a large amount of interest and excitement for this alternative approach to treat resistant HTN. In recent years, several clinical studies8–15 consistently report a significant and lasting anti-hypertensive response, most notably in the SPYRAL HTN-OFF MED, SPYRAL HTN-ON MED, and RADIANCE trials16, 17.
The mechanisms mediating the anti-hypertensive effects of RDN remains undefined but initially were believed to result from loss of neural control of renin release, renal vascular resistance, and tubular sodium reabsorption5. An alternate hypothesis has emerged which points to renal nerves as primary mediators of immune cell trafficking, infiltration and activation in the kidney. This results in renal inflammation and concomitant renal dysfunction18, 19. Specifically, Xiao and colleagues first reported that T-cell infiltration following 14 days of AngII treatment in mice was ameliorated by RDN, providing strong evidence for a role of renal sympathetic nerves in mediating renal inflammation in hypertension18.
Consistent with this hypothesis, we recently reported RDN attenuates renal inflammation during the development of DOCA-salt hypertension20. Specifically, RDN abolished DOCA-salt induced increases in several pro-inflammatory cytokines in the kidney and this was correlated with a 50% reduction in arterial pressure compared to control rats20. However, in a follow-up study we found that, although RDN reduced arterial pressure in rats with established DOCA-salt hypertension, it had no effect on renal cytokines21. When combined these studies suggest that, although renal nerves are required for the development of renal inflammation in DOCA-salt rats, they play no role in maintaining inflammation once it is established.
Although these studies did not address the mechanism by which RDN influences development of renal inflammation in the DOCA-salt rat, we envision at least two general possibilities. The first is that renal nerves (afferent or efferent) may directly influence trafficking of immune cells into the kidney18, 22 independent of any other physiological response to RDN. This stimulatory action would be lost after RDN. The second is that, since increased renal perfusion pressure is a known cause of renal inflammation23, 24, loss of renal nerves via RDN may reduce renal inflammation simply by lowering systemic AP.
The primary goal of the present study therefore was to delineate the individual contributions of renal nerves and renal perfusion pressure to the pathogenesis of renal inflammation in DOCA-salt rats. Moreover, we sought to establish the feasibility of serial measurements of urinary cytokines as a biomarker of renal inflammation during the development of DOCA-salt hypertension in conscious rats.
METHODS
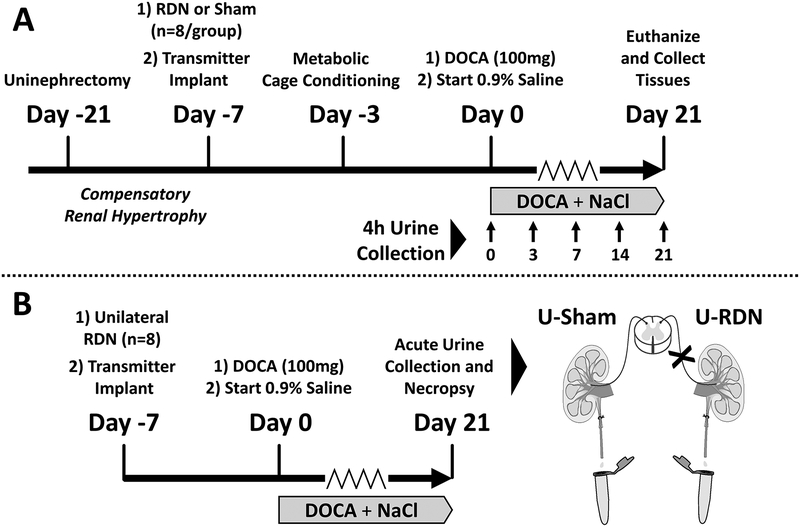
The data that support the findings of this study are available from the corresponding author upon reasonable request. All procedures were approved by the University of Minnesota IACUC and in compliance with the NIH Guide for Care and Use of Laboratory Animals. Animals were housed in a temperature- and humidity-controlled room (70–72°F; 30–35%) with a 12-hour on/off light schedule. Animals had ad libitum access to chow (Teklad 2018; Envigo, USA) and distilled water. All surgical procedures were performed under 2–3% isoflurane anesthesia (Phoenix Pharmaceutical, USA). Pharmacological prophylactic care included atropine (0.2 mg/kg, i.p.; West-Ward Pharmaceuticals, USA), ketoprofen (5 mg/kg, s.c.; Fort Dodge Animal Health, USA) and gentamicin (2.5 mg/kg, i.m.; Hospira, USA) administration prior to each surgery. These experiments were conducted using the DOCA-salt rat model, a well-established pre-clinical model that recapitulates moderate to severe salt-sensitive hypertension and renal dysfunction25. Timelines of the experimental protocols are depicted in Figure 1A–B, and further defined below.
Figure 1.
Protocol Timelines for Experiments 1 (Panel A) and 2 (Panel B).
Experiment 1: Temporal Relationship between Renal Inflammation and Arterial Pressure in DOCA-Salt Rat
The timeline for this protocol is shown in Figure 1A. Sixteen uninephrectomized male Sprague Dawley rats (weight: 275–300g; age: 10–12 weeks) were anesthetized (2–3% isoflurane) and randomly assigned to one of two treatment groups: 1K-DOCA-salt + renal denervation (RDN; n=8); 1K-DOCA-salt + sham denervation (Sham; n=8). RDN was achieved by a two-step procedure, as follows: (1) surgical ablation of visible renal nerves bundles along the renal artery and vein, 2) chemical ablation by perivascular application of 10% phenol (in 100% ethanol). Sham surgeries were treatment with 0.9% saline application20. All animals were also instrumented with radiotelemeters (Model HD-S10, Data Sciences International, USA) at the time of RDN/Sham surgery, where catheters were introduced to the abdominal aorta via femoral artery cannulation.
One week following RDN/Sham, each group began a three-week DOCA-salt treatment as we have previously published20, 26, 27. Animals were briefly anesthetized, and a silicone pellet containing 100mg DOCA was implanted subcutaneously and drinking water was replaced with 0.9% saline.
Arterial pressure (AP) and heart rate (HR) were measured over 24 hours on protocol days 0, 3, 7, 14, and 21. On the same day as AP measurements urine was collected for analysis of inflammatory cytokines. To attain these samples, the rats were transferred to a metabolic cage and urine was collected for 4 hours over ice (0800h-1200h). Rats were then returned to their home cages and 24-hour AP and HR were collected (1200h-1200h). Rats were conditioned to this procedure three days prior to the start of the experimental protocol. After 21 days of treatment, animals were acutely anesthetized (5% isoflurane) and euthanized by decapitation. Allometric data was recorded, and urine and renal tissue samples were collected and snap frozen for further analysis.
Experiment 2: Effect of Unilateral Renal Denervation on Renal Inflammation in 2K-DOCA-salt Rats
The timeline for this protocol is shown in Figure 1B. This experiment was designed to assess the inflammatory profile of a DOCA-salt kidney exposed to increased renal perfusion pressure in the presence and absence of renal nerves. Therefore, in contrast to Experiment 1 that was conducted in uninephrectomized (1K) DOCA-salt rats, this experiment used the two-kidney DOCA-salt (2K-DOCA) model, thereby enabling us to compare an intact kidney to a denervated kidney from the same animal. Importantly, in this setting both kidneys are exposed to the same perfusion pressure throughout the protocol even though typically AP does not increase as much as observed in the one-kidney model.
Ten male Sprague Dawley rats (Weight: 275–300g; Age: 10–12 weeks) underwent a unilateral RDN (U-RDN) as described above. The contralateral kidney (right) underwent a sham procedure (U-Sham). A radiotelemetry transmitter was implanted at this time to measure AP and HR. Next, rats received 100mg DOCA implants (s.c.) and 0.9% saline for drinking water, and were then monitored for 21 days.
Following the 21-day DOCA-salt treatment, each rat underwent an acute surgical preparation to collect urine from each kidney, followed by necropsy. Animals were anesthetized and maintained with 2–3% isoflurane, and each ureter was cannulated (PE10; BD Intramedic, USA). Following a 10-minute stabilization period, urine samples from each ureter were collected simultaneously on ice over 30 minutes. Rats were then euthanized by exsanguination and pneumothorax. Allometric data was recorded, and tissue samples were dissected and snap frozen for further analysis.
Biochemical and Histological Analysis of Renal Inflammation
The inflammatory profiles for Experiments 1 and 2 were determined by measuring several pro-inflammatory chemo- and cytokines in renal tissue and urine. Specifically, we measured IL-1β, IL-2, IL-6, MCP-1, and GRO/KC. IL-1β = Interleukin-1-Beta; IL-2 = Interleukin-2; IL-6 = Interleukin-6; MCP-1 = Monocyte chemoattractant protein-1; GRO/KC = Growth-related oncogene/keratinocyte chemoattractant. These inflammatory cytokines and chemokines have previously been shown to be elevated in DOCA-salt kidneys20, 21. Renal tissue samples (mixed cortex and medulla) were homogenized in phosphate-buffered saline containing EDTA-free protease inhibitor cocktail (cOmplete Mini; Item: #11836170001; Roche Diagnostics, USA). Inflammatory cytokines were measured by Luminex assay according to manufacturer’s instructions (MILLIPLEX Custom Assay, MilliporeSigma, USA). Data are expressed in picograms of analyte per milligrams of total protein (tissue) or milligrams of creatinine (urine). Total protein from tissue and urine samples was measured by a bicinchoninic acid kit (Thermo Scientific, USA). Urine creatinine was quantified by commercial kit according to manufacturer’s directions (Cayman Chemical, USA).
Kidney samples were also collected for histological assessment of injury and inflammation, as previously described21. At necropsy, kidneys were excised and bisected, fixed in 10% formalin for 24–48 hours, and embedded in paraffin wax. Tissues were sectioned and stained by the University of Minnesota Veterinary Diagnostic Laboratory. Transverse sections (4μm thickness) were stained for anti-CD3 (T-cells) or anti-Iba1 (macrophage) antibodies. Primary polyclonal antibodies were used to detect T-cells (anti-CD3; #CP215; Biocare Medical, USA) and macrophages (anti-Iba1; #CP290; Biocare Medical, USA). A peroxidase-labeled polymer conjugated to goat anti-rabbit Ig secondary antibody (EnVision+HRP; Agilent/Dako Corp., USA) was used as the detection reagent, and 3-Amino-9-Ethylcarbazole (AEC) as the chromogen. Lastly, slides were counterstained with Mayer’s hematoxylin and photographed using a Nikon Eclipse outfitted with a Nikon DS-Fi1 digital color camera. Signal was quantified automatically as %area from scanned slides at 10X magnification using methods outlined by Ruifrok and Johnston28 using ImageJ Software29.
Confirmation of Renal Denervation
Renal norepinephrine (NE) content was measured to assess completeness of efferent renal sympathetic nerve ablation. Tissue homogenates were assayed by high-performance liquid chromatography analysis with electrochemical detection, as previously described30. Data are expressed as nanograms of norepinephrine per gram of tissue.
Statistical Analysis
Repeat measurements from Experiment 1 were analyzed separately by a two-way repeated-measured ANOVA with a Bonferroni post-hoc test to assess treatment effects (Sham vs. RDN) at matched time points. Additionally, time-dependent changes from baseline (vs. Day 0) within each treatment group were assessed by a one-way repeated measures ANOVA with a Dunnett post-hoc test. All non-repeated measures data were analyzed by an unpaired Student’s t-test (two-tailed). Data collected in Experiment 2 were analyzed by a paired Student t-test (two-tailed), comparing the ipsilateral treated tissues to contralateral sham controls (U-RDN vs. U-Sham). Statistical analyses were performed with GraphPad Prism 7.0 software. Statistical significance was accepted at p<.05. Data are presented as mean ± SEM.
RESULTS
Experiment 1: Temporal Relationship between Renal Inflammation and Arterial Pressure in DOCA-Salt Rat
Cardiovascular and Allometric Responses.
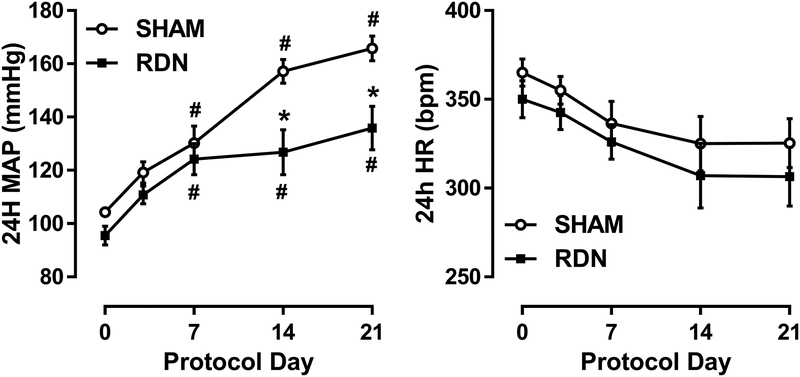
Prior to the DOCA implant, no significant differences were detected between Sham and RDN groups regarding baseline 24-hour averages of AP (105±2 vs. 96±3 mmHg) or HR (380±8 vs. 356±6 bpm). Following DOCA-salt administration, AP increased to 166±5 mmHg over three weeks in the Sham group compared to 136±8 mmHg (p<.05) in the RDN group (Figure 2A). No effect of treatment or time on HR was detected between or within Sham and RDN groups (Figure 2B). Moreover, no effect was observed between Sham and RDN in daily sodium intake throughout 3-week protocol (21.8±1.3 vs. 24.4±2.5 mmol/day).
Figure 2. Mean Arterial Pressure Response to DOCA-Salt.
Repeated measurements of 24-hour mean arterial pressure (MAP) and heart rate (HR) in Sham or RDN-treated rats over a 21-day DOCA-salt treatment. Data presented as mean±SEM. #p<.05 vs. respective baseline (Day 0); *p<.05 vs. Sham at matched timepoint; Two-way ANOVA with Bonferroni post-hoc test. Sham: n=8; RDN: n=8.
Upon protocol completion, there were no differences between groups for bodyweight or heart weight in contrast to kidney weight, which was significantly less in RDN compared to Sham rats (5.80±0.18 vs. 6.60±0.24 g/kg bodyweight).
Renal Inflammation and Urinary Cytokines.
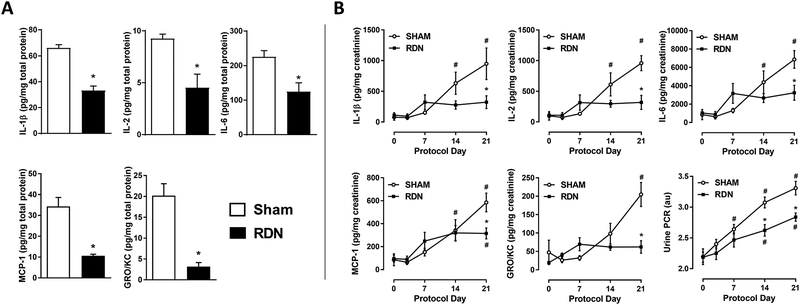
Renal inflammation was assessed biochemically and histologically. Firstly, pro-inflammatory cytokines were quantified by repeated measurements in urine throughout the 21-day treatment, as well as in renal parenchymal tissue at the end of the protocol (Figure 3A–B). Compared to Sham, at the end of the protocol all renal inflammatory cytokines measured (IL-1β, IL-2, IL-6, MCP-1, GRO/KC) were substantially reduced (↓44–84%) in RDN kidney tissue (Figure 3A) similarly to our previous study20.
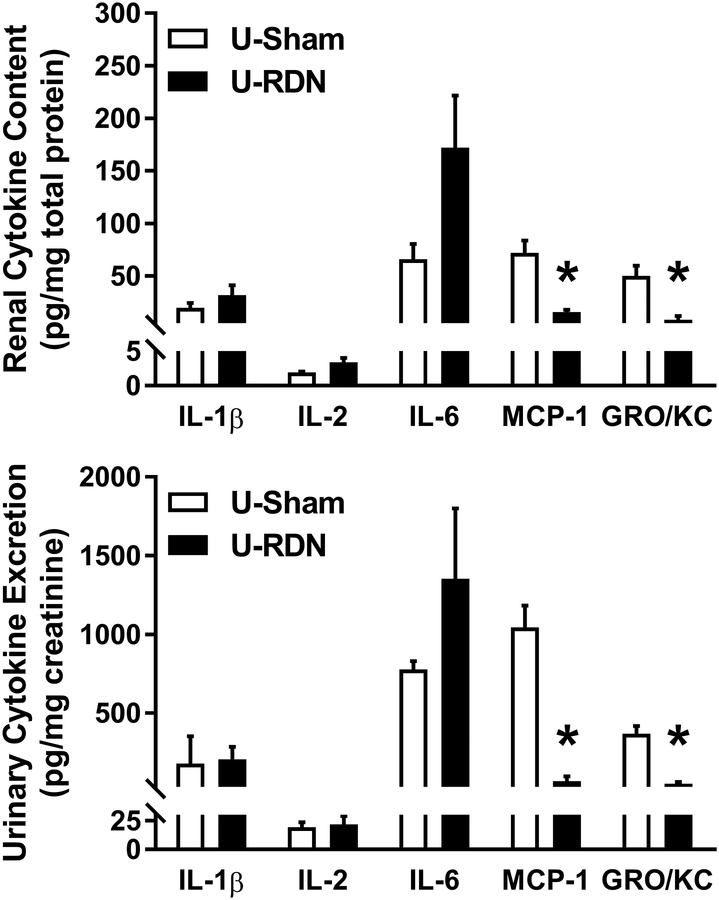
Figure 3. Urinary and Renal Cytokine Content Response.
(Panel A) All measured renal pro-inflammatory cytokine contents after three weeks of DOCA-salt were lower in rats pretreated with RDN vs. Sham (*p<.05; Student’s unpaired t-test). (Panel B) Urinary cytokine excretion was reduced at Protocol Day 21 in RDN vs. Sham. Urine protein-to-creatinine ratio (UPCR) was lowered at Protocol Day 14 and 21 (#p<.05 vs. respective baseline (Day 0); *p<.05 vs. Sham at matched timepoint; two-way ANOVA with Bonferroni post-hoc test). Sham: n=8; RDN: n=8. Data presented as mean±SEM.
A novel aspect of this study was the measurement of urinary cytokines as a real-time biomarker of renal inflammation in conscious rats (Figure 3B). Repeated measurements of urinary cytokine excretion over the three-week protocol revealed a time-dependent increase in urinary cytokine excretion in response to DOCA-salt treatment in Sham rats. In contrast, there was no significant increase in urinary cytokine excretion in RDN rats in response to DOCA-salt treatment with the exception of MCP1 at Day 21. Importantly, the magnitude of differences in urinary cytokine excretion on Day 21 between Sham and RDN rats (IL-1β ↓66%; IL-2 ↓67%; IL-6 ↓53%; MCP-1 ↓46%; GRO/KC ↓70%) were similar to that measured in renal tissue (IL-1β ↓50%; IL-2 ↓52%; IL-6 ↓44%; MCP-1 ↓69%; GRO/KC ↓84%). Urinary protein-to-creatinine ratio (UPCR), a measurement of renal injury, increased over time in both groups compared to their respective baseline, but UPCR was significantly lower in RDN at Protocol Days 14 and 21 (Figure 3B).
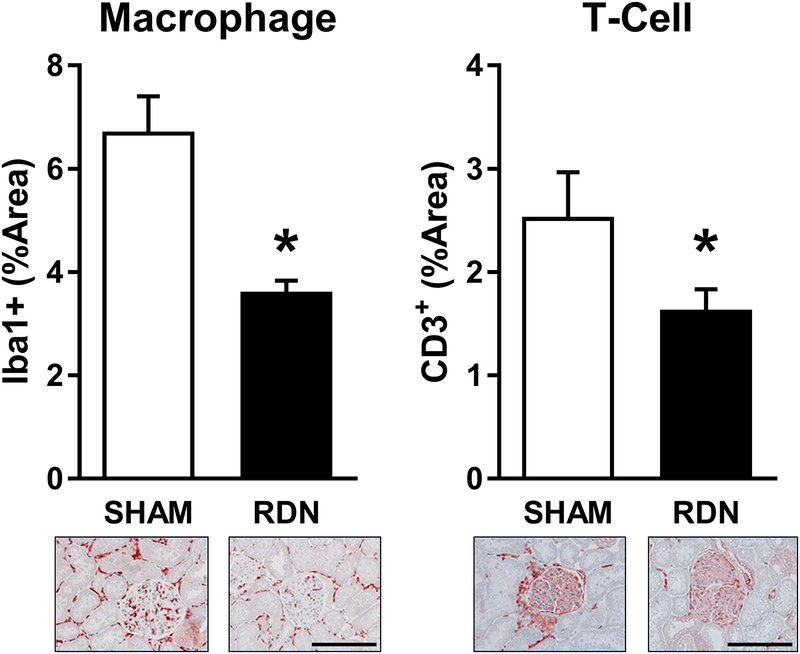
Immunohistochemical detection of immune cell markers was performed to quantify immune cell (T-cell and macrophage) infiltration of Sham and RDN kidneys after 21 days of DOCA-salt treatment (Figure 4). Both macrophage (Iba1+) and T-cell (CD3+) infiltration were markedly lower in RDN compared to Sham samples.
Figure 4. Histological Assessment of Renal Immune Cell Infiltration.
Both T-cell and macrophage infiltration of the renal cortex were reduced in RDN vs. Sham (*p<.05; Student’s unpaired t-test). Representative images of cortical tissue are presented at 10X magnification. Scale bar = 100μm. Data presented as mean±SEM.
Confirmation of Renal Denervation.
Renal norepinephrine (NE) content was quantified to determine the efficacy of RDN treatment. Compared to Sham (81.2±6.8 ng/g tissue), RDN renal NE content was reduced by more than 90% (7.5±2.1 ng/g). These data confirm that a majority of efferent sympathetic nerves were successfully ablated by RDN.
Experiment 2: Effect of Unilateral Renal Denervation on Renal Inflammation in 2K-DOCA-Salt Rats
Cardiovascular and Allometric Responses.
Following three weeks of DOCA-salt treatment, 24-hour mean AP increased from 102±3 (Protocol Day 0) to 136±4 mmHg (Protocol Day 21). On Protocol Day 21 rats were anesthetized and urine was collected from each kidney over ice for 30 minutes prior to euthanasia and necropsy. No significant effect on kidney weight was detected between U-Sham and U-RDN (3.85±0.23 vs. 3.76±0.18g/kg BW). Similarly, no differences in urinary flow rate (16.0±1.0 vs. 17.7±1.4 μL/min/100g BW) nor protein-to-creatinine ratio (1.92±0.27 vs. 1.73±0.17 au) were detected between U-Sham and U-RDN kidneys.
Renal Inflammation and Urinary Biomarkers.
Quantification of renal tissue lysate and urinary cytokine content revealed a differential effect of RDN on inflammatory markers (Figure 5A–B). Specifically, in both renal and urine measurements, there were no differences between U-Sham and U-RDN kidneys for IL-1β, IL-2, and IL-6 levels. However, there was a significantly lower level of chemotactic cytokines MCP-1 and GRO/KC in the U-RDN vs. the U-Sham, in both urine and renal tissue.
Figure 5. Renal and Urinary Cytokine Content After Unilateral Renal Denervation.
No effect of denervation was detected between U-Sham and U-RDN in either IL-1β, IL-2, nor IL-6 in urine or kidney lysate samples. MCP-1 and GRO/KC were significantly lower in U-RDN urine and renal samples vs. U-Sham (*p<.05; Student’s unpaired t-test). Data presented as mean±SEM; n=8/group.
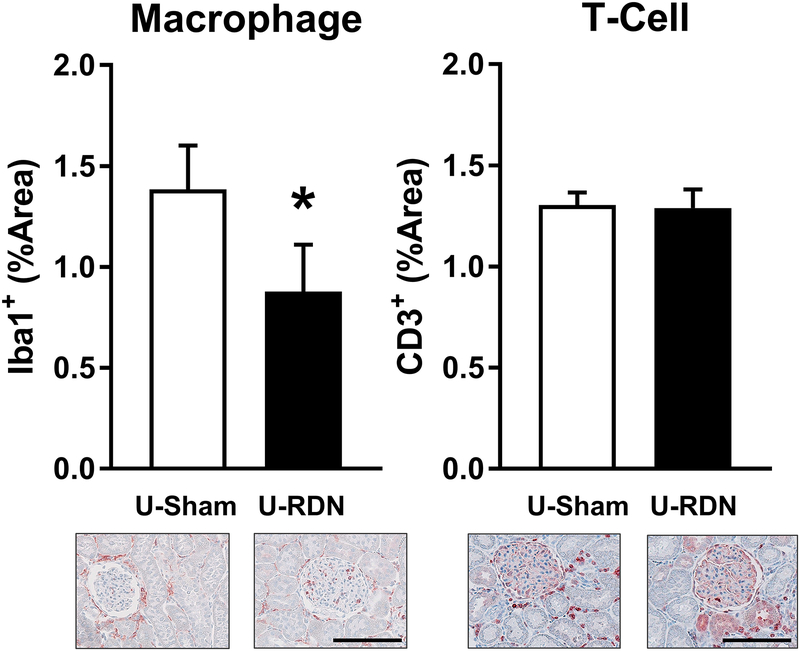
Furthermore, immunohistochemical analysis of renal macrophage and T-cell infiltration was employed to determine the selective effects of RDN, independent of the effect of changes in AP. Macrophage infiltration was significantly reduced in U-RDN kidneys compared to U-Sham (Figure 6A). This effect of RDN appears to be specific to macrophages, as no difference in T-cell infiltration was detected between U-RDN and the contralateral U-Sham kidneys (Figure 6B).
Figure 6. Renal Immune Cell Infiltration After Unilateral Renal Denervation.
Renal cortical macrophage infiltration was reduced in U-RDN samples vs. U-Sham (*p<.05; Student’s paired t-test). No effect of treatment was detected in T-cell infiltration. Representative images of cortical tissue are presented at 10X magnification. Scale bar = 100μm. Data presented as mean±SEM.
Confirmation of Renal Denervation Efficacy.
Renal norepinephrine (NE) content was quantified to determine the effectiveness of RDN treatment. Compared to U-Sham, NE content in U-RDN kidneys was lowered substantially (95.3±10 vs. 9.0±3.1 ng/g tissue). These data confirm the vast majority (>90%) of sympathetic nerves were ablated by surgical RDN.
DISCUSSION
These studies were designed to delineate the relationship between renal innervation and renal perfusion pressure in the pathogenesis of renal inflammation in the DOCA-salt rat. Firstly, the findings in this study confirm our previous report that RDN in large part prevents the development of renal inflammation in the DOCA-salt rat. Second, the present study suggests that renal nerves directly modulate renal macrophage activation and/or infiltration in contrast to T cells which are not affected by RDN. Lastly, this study also reveals urinary cytokines may serve as a real-time biomarker of renal inflammation in the rat. These findings are discussed in more detail in the following sections.
Temporal Relationship of Arterial Pressure and Renal Inflammation Responses in DOCA-Salt Rats
The cause and effect relationship between renal inflammation and hypertension is unknown, in part because renal inflammation is usually assessed only at a single time point using standard renal histopathological and/or biochemical methods31. In Experiment 1 we investigated the utility of measuring urinary cytokines as a real-time biomarker of renal inflammation in DOCA-salt rats. Urinary cytokines were measured before, 7, 14 and 21 days following the start of DOCA-salt treatment. In addition, on Day 21 of DOCA-salt we directly compared immune cell (macrophage and T-cell) infiltration and pro-inflammatory cytokine content in the kidneys to urinary cytokine excretion.
We observed that urinary cytokine excretion increased in parallel to arterial pressure in DOCA-salts rats. In addition, the proportional changes in renal tissue pro-inflammatory cytokine content observed in Experiment 1 and Experiment 2 were mirrored by urinary cytokine excretion, despite differences in the magnitudes of hypertension. Interestingly, urinary cytokine excretion (IL-1β, IL-2, IL-6, MCP-1, and GRO/KC) was markedly elevated following 21-days of DOCA-salt treatment in Sham rats but not RDN rats. In fact, apart from MCP-1, the DOCA-salt induced increases in cytokine excretion were abolished in RDN rats.
Despite an elevation of ~30mmHg AP by Day 7 of DOCA-salt in Sham rats, no changes in urinary cytokines were detected until Day 14. Note, however, that AP increased an additional ~30mmHg from Days 7 through 14, and this coincided with a parallel increase in urinary cytokine excretion at Days 14 and 21. Due to the sampling rate of arterial pressure and urinary cytokines over time, we cannot conclude whether renal inflammation was the cause or consequence of increased renal perfusion pressure in this model. Further experiments with higher sampling rates are required to elucidate this sequential relationship.
There are previous reports in which urinary cytokine excretion was measured in parallel to arterial pressure but few have made repeated observations over time32. Immunosuppression therapy, targeting T-cell activation with mycophenolate mofetil (MMF), in hypertensive patients with autoimmune disease reduced systolic blood pressure and urinary TNFα excretion. The final systolic arterial pressure was also positively correlated with urinary cytokine excretion (i.e. TNFα, RANTES) following MMF treatment32. Urinary excretion of IL-1β, IL-6, and MCP-1 are elevated in adolescents with idiopathic nephrotic syndrome that also present with impaired renal function and hypertension33–35. Interestingly, repeated measurements of urinary cytokines have previously been suggested to reflect renal-specific inflammatory signaling in the nephrotic model34. Finally, urinary excretion of MCP-1 was elevated in rats undergoing 21-day DOCA-salt treatment, coinciding with increased renal immune cell infiltration and cortical inflammatory signaling36.
Differential Effect of Renal Denervation on Renal Pro-Inflammatory Cytokines
To delineate the contributions of increased renal perfusion pressure and renal nerves to renal inflammation, we conducted a study in 2K DOCA-salt rats that underwent a unilateral RDN. This experiment produced two key findings.
Firstly, macrophage infiltration was specifically reduced in the denervated kidney compared to the contralateral innervated kidney, despite the kidneys experiencing the same renal perfusion pressure. This effect was unique to macrophages, as T-cell infiltration was similar between U-RDN and U-Sham kidneys. We conclude that T-cell infiltration is likely independent of renal innervation, whereas macrophage infiltration may be partially nerve-dependent. Potential mechanisms mediating the trafficking and putative activation of these macrophage could be through direct cell-to-cell interaction with renal nerves, or through neurotransmitter paracrine signaling37–40; however, the underlying mechanisms remain to be studied.
Secondly, pro-inflammatory urinary cytokine excretion was also reduced in the denervated kidney, specifically for the chemotactic cytokines MCP-1 and GRO/KC. Since these cytokines are secreted by macrophages, and less by T-cells, we speculate the reduction in macrophage infiltration in RDN kidneys is the chief cause of the decreased cytokine levels in both the kidney and urine. Whether renal sympathetic nerves are the primary arbiters of recruitment and activation of macrophages in the kidney requires further investigation.
Inferences regarding the pathways involved in neural-mediated macrophage recruitment can be drawn from reports from Kim and Padanilam, who observed a comparable role for efferent and afferent renal nerves in the macrophage infiltration and fibrogenesis in a murine model of renal inflammation in a unilateral ureteral obstruction (UUO) and in a model of ischemia-reperfusion injury39, 40. In this UUO model, RDN obviated the inflammation and fibrosis developed after 10 days of UUO. Moreover, chronic cortical infusion of either norepinephrine (NE; efferent neurotransmitter) or calcitonin-gene related peptide (CGRP; afferent neurotransmitter) after RDN effectively restored the nephritic response to UUO40. Though the cortical infusion used in the these studies39, 40 did not control for final location of delivery and dispersion across the kidney, these data strongly support the role for efferent and afferent neurotransmitters in the recruitment and activation of inflammatory mediators such as macrophages under two different inflammatory stimuli. In addition to the development of neurogenic inflammation, Barry and Johns41 reported renal afferent nerve activation with pro-inflammatory peptide bradykinin elicited a sympatho-excitatory reflex, which suggests renal afferent nerves and inflammatory may have a reciprocal relationship. Considering that afferent renal nerve activity is elevated in the hypertension 1K DOCA-salt rat20, paired with increased renal inflammation, further studies are necessary to elucidate the mechanism and interplay between renal inflammation and afferent renal nerves.
Though the current investigation did not include normotensive rats that underwent unilateral renal denervation, we speculate the tonic sympathetic tone does not significantly contribute to basal inflammatory cytokine signaling in the kidney under normal conditions. This is based on the lack of difference observed at baseline (Day 0) in urinary cytokines excretion between the 1K-DOCA Sham and RDN rats. Similarly, Asenijevic et al.42 demonstrated unilateral RDN in healthy male Sprague Dawley rats had no significant effect on any cytokines measured in renal tissue (e.g. IL-6, IFNγ, TNF-α and CM-GSF) compared to the contralateral control kidney. Unilateral denervation reportedly increases ipsilateral pelvic pressure, putatively due an enhanced diuresis43. The diuretic effect is likely mediated by a combination of decreased renal vascular resistance and decreased sodium/water reabsorption in the proximal tubule following unilateral RDN, both of which are maintained by renal sympathetic nerve discharge44–46. In Experiment 2 we did not detect any difference in urinary flow in the two kidneys after 21-days of DOCA-salt treatment. Moreover, Shweta and colleagues reported estimated glomerular capillary pressure was slightly reduced (47mmHg vs. 50mmHg) in the denervated kidney vs. innervated kidney in spontaneously hypertensive rats following U-RDN47. Although we did not perform micropuncture measurements in the present study, we feel it is unlikely that differences in glomerular capillary pressure are responsible for differences in renal inflammation observed between the U-Sham and U-RDN kidneys.
Pressure-Dependent Renal Inflammation
Pressure-mediated inflammation and end organ damage have long been recognized as cardinal features of hypertension and renal disease48. The relationship between arterial pressure and renal pathology has been thoroughly investigated23, 24. Of note, Evans and colleagues have demonstrated that arterial pressure is a primary mediator of renal injury and T-cell infiltration in the Dahl SS hypertensive rat23. In their study renal perfusion pressure was maintained at ~125–130mmHg in one kidney by continuous servo-control during seven-days of salt-induced hypertension, whereas the contralateral kidney was exposed to steadily rising perfusion pressures (~140–160mmHg). Compared to the hypertensive kidney, inflammatory T-cell infiltration was substantially lower in the pressure-controlled kidneys. Similar effects on macrophage infiltration were also reported by the same group in a separate study24. Importantly, the servo-controlled renal perfusion pressure in these studies is nearly identical to what was achieved in the Sham and RDN animals in Experiment 1, as well as differences between the 1K- and 2K-DOCA models. With the higher pressures of 150–160mmHg of the Sham group in Experiment 1, increased urinary cytokine excretion was also significantly elevated. Combined, these data suggest that a threshold of arterial pressure above 130–140mmHg may be required to initiate or exacerbate renal inflammation.
In the present study, as well as a previous report from our laboratory20, we found RDN markedly attenuated renal cytokine content in DOCA-salt rats. Based on the studies discussed above, it is possible this anti-inflammatory effect of RDN is simply due to a decrease in renal perfusion pressure. However, it is important to recognize arterial pressure remained elevated above control levels in RDN rats, even when urinary cytokine excretion remained unchanged from baseline. Moreover, in our previous study20 renal cytokine levels in RDN DOCA-salt rats were not different from normotensive control rats despite the fact their arterial pressure was higher (+15–20mmHg). Indeed, histological and biochemical analyses of renal cytokine content and immune cell infiltration in the current study demonstrate that RDN abated the renal inflammatory response to DOCA-salt. To this end, increased renal inflammation appears not to be obligatory for the moderate increase in arterial pressure (20–30mmHg) in the RDN rats of Experiment 1. Though the role of renal inflammation contributing to the increase of 20–30mmHg of AP in RDN rats cannot be ruled out, these studies clearly demonstrate an important role of renal nerves in the pathogenesis of DOCA-salt hypertension. Indeed, the measurements of inflammation are limited to the methods and targets used in this study, and we cannot rule out changes in other inflammatory cells and cytokines that were not measured. Additionally, direct inflammatory effects of mineralocorticoids49 and increased sodium intake50, 51 have been previously reported, and likely contribute to the inflammatory phenotype in the models employed in this study. This relationship, and the interplay between sodium, mineralocorticoids, and renal nerves remains a future avenue for investigation.
Supplementary Material
Perspectives and Significance.
These data demonstrate the importance of renal nerves in the development of renal inflammation in the rat model of DOCA-salt hypertension. Our findings particularly implicate renal nerve-mediated increases in macrophage infiltration and associated pro-inflammatory cytokine release. Together, these studies reveal a potential relationship and mechanism between renal nerves and renal inflammation under hypertensive conditions. Although it remains to be studied, these anti-inflammatory effects of RDN may extend beyond hypertension in the prophylaxis or treatment of other diseases associated with renal inflammation such as acute kidney injury or chronic kidney disease.
NOVELTY AND SIGNIFICANCE.
What is New?
Repeat measurements of urinary cytokine excretion over time and renal cytokine content collectively demonstrate renal denervation ameliorates the onset of renal inflammation in the DOCA-salt hypertensive rat.
Renal denervation contributes to renal macrophage infiltration and increased GRO/KC and MCP-1 content in the DOCA-salt rat, where reductions in T-cell infiltration and IL-1β, IL-2, and IL-6 are likely secondary to lowering arterial pressure.
What is Relevant?
Renal nerves serve a central role in the pathogenesis of hypertension and renal inflammation, and renal denervation may effectively treat both conditions.
ACKNOWLEDGEMENTS
We are grateful for the assistance from the University of Minnesota’s Cytokine Reference Laboratory in the measurement of tissue cytokines, as well as the University of Minnesota’s Veterinary Diagnostic Laboratory for their histological services.
SOURCES OF FUNDING
This work was supported by the NIH R01HL116476 (PI: J.W. Osborn; Co-PI: G.D. Fink) and NIH K99HL141650 (PI: C.T. Banek).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2.Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL, Joyal D. Effectiveness of renal denervation therapy for resistant hypertension: A systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:231–241. [DOI] [PubMed] [Google Scholar]
- 3.Schlaich MP, Hering D, Sobotka PA, Krum H, Esler MD. Renal denervation in human hypertension: Mechanisms, current findings, and future prospects. Curr Hypertens Rep. 2012;14:247–253. [DOI] [PubMed] [Google Scholar]
- 4.DiBona GF, Esler M. Translational medicine: The antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–253. [DOI] [PubMed] [Google Scholar]
- 5.Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol. 2017;7:263–320. [DOI] [PubMed] [Google Scholar]
- 6.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 7.Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the SYMPLICITY HTN-1 study. Lancet. 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 8.Gosse P, Cremer A, Pereira H, Bobrie G, Chatellier G, Chamontin B, Courand PY, Delsart P, Denolle T, Dourmap C, Ferrari E, Girerd X, Michel Halimi J, Herpin D, Lantelme P, Monge M, Mounier-Vehier C, Mourad JJ, Ormezzano O, Ribstein J, Rossignol P, Sapoval M, Vaisse B, Zannad F, Azizi M. Twenty-four-hour blood pressure monitoring to predict and assess impact of renal denervation: The DENERHTN study (renal denervation for hypertension). Hypertension. 2017;69(3):494–500. [DOI] [PubMed] [Google Scholar]
- 9.Pekarskiy SE, Baev AE, Mordovin VF, Semke GV, Ripp TM, Falkovskaya AU, Lichikaki VA, Sitkova ES, Zubanova IV, Popov SV. Denervation of the distal renal arterial branches vs. conventional main renal artery treatment: A randomized controlled trial for treatment of resistant hypertension. J Hypertens. 2017;35:369–375. [DOI] [PubMed] [Google Scholar]
- 10.Worthley SG, Wilkins GT, Webster MW, Montarello JK, Delacroix S, Whitbourn RJ, Warren RJ. Safety and performance of the second generation EnligHTN renal denervation system in patients with drug-resistant, uncontrolled hypertension. Atherosclerosis. 2017;262:94–100. [DOI] [PubMed] [Google Scholar]
- 11.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Bohm M. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390(10108):2160–2170. [DOI] [PubMed] [Google Scholar]
- 12.Kampmann U, Mathiassen ON, Christensen KL, Buus NH, Bjerre M, Vase H, Moller N, Kaltoft A, Poulsen PL. Effects of renal denervation on insulin sensitivity and inflammatory markers in nondiabetic patients with treatment-resistant hypertension. J Diabetes Res. 2017;2017:6915310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaldivia MT, Rivera J, Hering D, Marusic P, Sata Y, Lim B, Eikelis N, Lee R, Lambert GW, Esler MD, Htun NM, Duval J, Hammond L, Eisenhardt SU, Flierl U, Schlaich MP, Peter K. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: An anti-inflammatory effect relevant for cardiovascular risk. Hypertension. 2017;69:323–331. [DOI] [PubMed] [Google Scholar]
- 14.Persu A, Gordin D, Jacobs L, Thijs L, Bots ML, Spiering W, Miroslawska A, Spaak J, Rosa J, de Jong MR, Berra E, Fadl Elmula FEM, Wuerzner G, Taylor AHM, Olszanecka A, Czarnecka D, Mark PB, Burnier M, Renkin J, Kjeldsen SE, Widimsky J, Elvan A, Kahan T, Steigen TK, Blankestijn PJ, Tikkanen I, Staessen JA. Blood pressure response to renal denervation is correlated with baseline blood pressure variability: A patient-level meta-analysis. J Hypertens. 2017;36(2):221–229. [DOI] [PubMed] [Google Scholar]
- 15.Rosa J, Widimsky P, Tousek P, Petrak O, Curila K, Waldauf P, Bednar F, Zelinka T, Holaj R, Strauch B, Somloova Z, Taborsky M, Vaclavik J, Kocianova E, Branny M, Nykl I, Jiravsky O, Widimsky J Jr. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: Six-month results from the PRAGUE-15 study. Hypertension. 2015;65:407–413. [DOI] [PubMed] [Google Scholar]
- 16.Osborn JW, Banek CT. Catheter-based renal nerve ablation as a novel hypertension therapy: Lost, and then found, in translation. Hypertension. 2018;71:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the spyral htn-on med proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 18.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin ii-induced hypertension. Circ Res. 2015;117:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731–767. [DOI] [PubMed] [Google Scholar]
- 20.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: Elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension. 2016;68:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banek CT, Gauthier MM, Baumann DC, Van Helden D, Asirvatham-Jeyaraj N, Panoskaltsis-Mortari A, Fink GD, Osborn JW. Targeted afferent renal denervation reduces arterial pressure but not renal inflammation in established doca-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2018; 314(6):R883–R891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal t-cell infiltration in the dahl salt-sensitive rat. Hypertension. 2017;70:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008;19:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer A, Chan V, Brown L. The doca-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev. 2010;6:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in doca-salt model in rats: A telemetric approach. Am J Physiol Heart Circ Physiol. 2005;289:H1519–1529. [DOI] [PubMed] [Google Scholar]
- 28.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci. 2010;154:66–73. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Johnson AC, Sasser JM, Williams JM, Solberg Woods LC, Garrett MR. Spontaneous one-kidney rats are more susceptible to develop hypertension by doca-nacl and subsequent kidney injury compared with uninephrectomized rats. Am J Physiol Renal Physiol. 2016;310:F1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–225. [DOI] [PubMed] [Google Scholar]
- 33.Al-Eisa AA, Al Rushood M, Al-Attiyah RJ. Urinary excretion of il-1beta, il-6 and il-8 cytokines during relapse and remission of idiopathic nephrotic syndrome. J Inflamm Res. 2017;10:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira Wde F, Brito-Melo GE, Guimaraes FT, Carvalho TG, Mateo EC, Simoes e Silva AC. The role of the immune system in idiopathic nephrotic syndrome: A review of clinical and experimental studies. Inflamm Res. 2014;63:1–12. [DOI] [PubMed] [Google Scholar]
- 35.Souto MF, Teixeira AL, Russo RC, Penido MG, Silveira KD, Teixeira MM, Simoes ESAC. Immune mediators in idiopathic nephrotic syndrome: Evidence for a relation between interleukin 8 and proteinuria. Pediatr Res. 2008;64:637–642. [DOI] [PubMed] [Google Scholar]
- 36.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. Tnf-alpha inhibition reduces renal injury in doca-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, Neuhuber W, Tiegs G. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol. 2008;19:1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu XX, Goldmuntz EA, Brosnan CF. The effect of norepinephrine on endotoxin-mediated macrophage activation. Journal of neuroimmunology. 1991;31:35–42. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015;87:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013;24:229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barry EF, Johns EJ. Intrarenal bradykinin elicits reno-renal reflex sympatho-excitation and renal nerve-dependent fluid retention. Acta Physiol (Oxf). 2015;213:731–739. [DOI] [PubMed] [Google Scholar]
- 42.Arsenijevic D, Cajot JF, Fellay B, Dulloo AG, Van Vliet BN, Montani JP. Uninephrectomy-induced lipolysis and low-grade inflammation are mimicked by unilateral renal denervation. Front Physiol. 2016;7:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenen A, Steinbach A, Schaper K, Zimmermann U, Miehe B, Kurt B, Rettig R, Grisk O. Effects of renal denervation on renal pelvic contractions and connexin expression in rats. Acta Physiol (Oxf). 2016;216:240–253. [DOI] [PubMed] [Google Scholar]
- 44.Bello-Reuss E, Colindres RE, Pastoriza-Munoz E, Mueller RA, Gottschalk CW. Effects of acute unilateral renal denervation in the rat. J Clin Invest. 1975;56:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiBona GF, Rios LL. Renal nerves in compensatory renal response to contralateral renal denervation. Am J Physiol. 1980;238:F26–30. [DOI] [PubMed] [Google Scholar]
- 46.Verloop WL, Hubens LE, Spiering W, Doevendans PA, Goldschmeding R, Bleys RL, Voskuil M. The effects of renal denervation on renal hemodynamics and renal vasculature in a porcine model. PLoS ONE. 2015;10:e0141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shweta A, Denton KM, Kett MM, Bertram JF, Lambert GW, Anderson WP. Paradoxical structural effects in the unilaterally denervated spontaneously hypertensive rat kidney. J Hypertens. 2005;23:851–859. [DOI] [PubMed] [Google Scholar]
- 48.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension. 2004;44:595–601. [DOI] [PubMed] [Google Scholar]
- 49.Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543 [DOI] [PubMed] [Google Scholar]
- 50.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic th17 cells by inducible salt-sensing kinase sgk1. Nature. 2013;496:513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic th17 cells. Nature. 2013;496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.