Abstract
There is a very complex interaction between the brain and the cerebral vasculature to meet the metabolic demands of the brain for proper function. Preservation of cerebrovascular function and integrity has a central role in this sophisticated communication within the brain and any derangements can have deleterious acute and chronic consequences. In almost all forms of cognitive impairment, from mild to Alzheimer’s disease, there are changes in cerebrovascular function and structure leading to decreased cerebral blood flow, which may initiate and/or worsen cognitive impairment. In this focused review, we discuss the contribution of two major vasoactive pathways to cerebrovascular dysfunction and cognitive impairment in an effort to identify early intervention strategies.
Keywords: cerebral vasculature, vascular, cognitive impairment, endothelin, renin-angiotensin system
Vascular Function and Cognition
The brain is a unique organ with no energy reserve. It requires constant blood flow to perform all the complex functions ranging from cognition to regulation of cardiovascular homeostasis. Cerebral blood flow (CBF) is tightly regulated to meet the demands of the brain by providing constant flow at the right pressure as well as delivering it to where it is needed the most as determined by brain activity. The cerebrovascular network comprised of arteries, arterioles, capillaries, venules and veins plays a central role in achieving this high level of regulation as recently reviewed in the context of physiology and neurodegenerative disorders.1–4 Its importance in cognitive function is increasingly recognized as discussed in a recent scientific statement on brain health from the American Heart Association.5
Reductions in CBF are known to occur in both vascular contributions to cognitive impairment/dementia (VCID) and Alzheimer’s disease (AD) but the role this phenomenon plays in the initiation and/or progression of the dementias is not as clear. Whereas hypoxia/ischemia is thought to be the initiating event in VCID, many believe that blood flow changes in AD occur in response to the neurodegeneration.6 It remains a strong possibility, however, that vascular dysfunction is a key precipitating event in both conditions given that they share the common vascular risk factors of hypertension, diabetes and aging, amongst others.7 Progress in this area has been slow given the fact that most human investigations include a single “snap shot” in time, revealing reductions in flow co-occurring with reduced cognition.8 In a cohort of individuals with dominantly inherited AD, however, quantification of white matter hyperintensities (WMH), a measure of small vessel disease in the brain, was shown to precede symptoms of cognitive decline by almost 7 years,9 suggesting a causative role of vascular dysfunction, rather than a consequence of significant degeneration. In a separate work with the same cohort, pathologic changes in amyloid β (Aβ) in the cerebral spinal fluid (CSF) were shown to precede symptom onset by 30 years.9 Although reduction in CBF can contribute to increased accumulation of Aβ, at least in this cohort, it is not the initiating event in the onset of AD. A study from the Alzheimer’s disease Neuroimaging Initiative also demonstrated decreased CBF (measured by arterial spin labeling MRI) before changes in classical markers like Aβ or tau.10 It is likely that the reductions in CBF in AD occur as a result of vascular dysfunction due to alterations in the delicate balance of constrictor and dilator vasoactive substances.11, 12 In a post-mortem assessment of brain tissue from both AD and VCID patients, cortical perfusion (evaluated by the ratio of myelin associated glycoprotein to proteolipid protein (MAG:PLP1) as a surrogate marker of perfusion) was negatively correlated with the degree of elevation of potent vasoconstrictor endothelin-1 (ET-1) in AD. The authors concluded that, although vascular pathology including amyloid angiopathy and small vessel disease occur in AD, increased vascular contractility (with ET) is a more important contributor to the hypoperfusion characteristic of AD.12
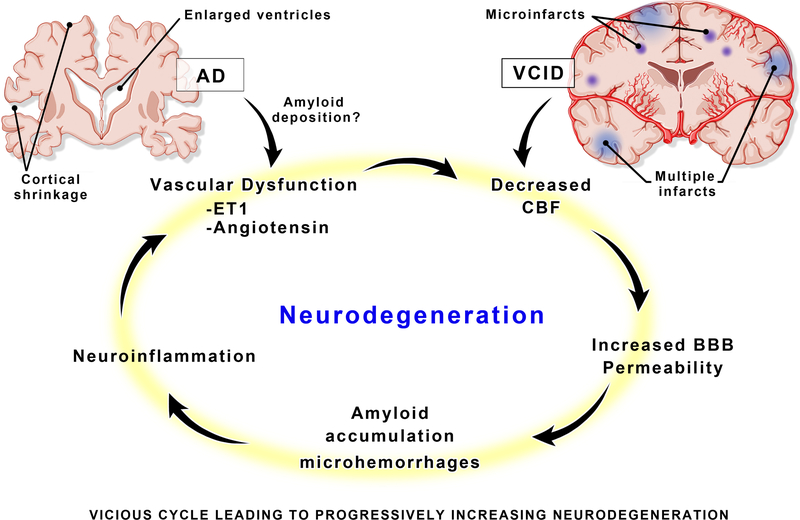
Regardless of the initiating event, experts agree that AD, VCID, and mixed dementias all incorporate aspects of vascular dysfunction, blood brain barrier (BBB) breakdown, tissue loss and neurovascular uncoupling, in a vicious cycle (Fig. 1).2–4, 6, 7, 13 While the underlying pathology of cerebrovascular dysfunction leading to cognitive impairment is multifactorial, this brief review will focus on the potential contributions of major vasoactive pathways including the renin-angiotensin (RAS) and ET systems to the disruption of cerebrovascular health leading to neurodegenerative diseases like VCID and AD.
Figure 1.
A vicious cycle of pathologic neurovascular changes in the brain, leads to a malignantly progressive burden of neurodegeneration. Although Alzheimer’s disease (AD) and vascular cognitive impairment/dementia (VCID) may enter the cycle at different initiating points, the mediators of damage are similar and occur long before clinical symptoms present. BBB = blood–brain barrier, ET1 = endothelin 1, CBF = cerebral blood flow.
Vasoactive Pathways
The Endothelin (ET) System
The family of endothelins, consisting of three related vasoactive peptides, ET-1, ET-2, and ET-3, plays important roles in cardiovascular (patho)physiology and embryonic development.14, 15 While ET-1 was the first isoform to be isolated as the most potent vasoconstrictor peptide, ETs mediate a wide spectrum of physiological functions via autocrine and paracrine mechanisms. Hence, the ET biosynthesis is tightly regulated and involves a two-step proteolytic maturation process.15 Preproendothelins (PPET) are first processed at conserved multibasic sites by furin or a furin-like enzyme to generate big ET, which is further processed by endothelin converting enzyme (ECE) to release the biologically active peptide. ET-1 mediates its diverse effects via two distinct G protein-coupled receptor subtypes, ETA and ETB. ETA receptor, localized mainly on vascular smooth muscle cells (VSMCs) of arteries and arterioles as well as on pericytes surrounding capillaries, is responsible for the contractile and proliferative responses to ET-1. It was recently shown that this receptor could be found on brain microvascular endothelial cells.16 The role of ETB in vascular regulation is more complex. For instance, ETB receptor located on endothelial cells mediates vasodilation via the release of relaxing factors such as NO. This receptor subtype can also lead to vasoconstriction when the receptors are expressed on VSMCs, as occurs in disease conditions.15 For example, VSMC ETB receptors are upregulated in basilar and middle cerebral arteries (MCA) in diabetes and after ischemic events.17 The readers are referred to a recent review that provides detailed information on the components of the ET system within the brain.18
ET-1 and cerebrovascular dysfunction
Cerebrovascular dysfunction can be the result of dysregulation of different functional mechanisms involved including pressure-induced myogenic tone, ligand-mediated vasoreactivity, neurovascular coupling and endothelial barrier function as well as vascular structure. In this section, we will review the impact of ET-1 on each of these mechanisms in the above order. Unless otherwise noted, the reviewed information is based on animal models.
The relative role(s) of the mechanisms listed above, in the regulation of CBF, vary along the vascular tree. For example, the myogenic response inherent to VSMCs on arteries and arterioles is an important mechanism that contributes to the regulation of cerebral autoregulation and hence brain perfusion. ET-1 increases myogenic tone of isolated pial arteries and relatively smaller parenchymal arterioles in rodents and mechanisms involve generation of reactive oxygen and nitrogen species.17, 19 In diabetes, ETB receptors on VSMC are upregulated and treatment with bosentan, a dual antagonist that blocks both ET receptor subtypes, not only prevents this increase in myogenic tone of small arteries (male rats measured by ex vivo pressurized myography20) but also reverses the myogenic dysfunction when treatment is given after vascular disease is established.17
Numerous studies in different disease models reported that the brain vasculature is an early target for potent ET-mediated vasoreactivity. For example, ET-1 mediates microvascular dysfunction by increased constriction, vascular congestion due to activation of vascular cell adhesion molecule (VCAM-1) in endothelial cells, BBB breakdown, and inflammation, leading to cognitive impairment in a model of experimental cerebral malaria in mice.21, 22 Although it has been known for a long time that ET-1-mediated inhibition of the large-conductance calcium-activated potassium (BK) channels contributes to amplified vasoconstriction by ET-1, underlying mechanisms remained unknown. A recent study showed that ET-1 mediates vasoconstriction of small cerebral arteries (middle cerebral, cerebellar, and posterior cerebral) via phosphorylation of Rab11A protein on recycling endosomes in an ETA receptor dependent manner and thereby decreasing the cell surface expression of the β1 subunit of BK channels in isolated VSMC.23 In both type 1 and type 2 rodent models of diabetes, ET-1 causes potent vasoconstriction and contributes to impaired relaxation of cerebral arteries like basilar artery and MCA even before peripheral arteries are affected.24, 25 As recently reviewed, ETA blockade by BQ-123 prevents/restores diabetes-mediated impairment of endothelium-dependent vasorelaxation of pial vessels.17
Targeted delivery of blood where it is needed in the brain is achieved by neurovascular coupling and results in an increase in CBF known as functional hyperemia. This response occurs at the capillary and precapillary level by close interaction of endothelial cells, pericytes, neurons and astrocytes. Although it is still under investigation, recent evidence suggests that the initial response in capillaries is then propagated to upstream arterioles.3 In experimental hypertension models, ET-1 has been reported to disrupt this response and cause neurovascular dysfunction, suggesting that the ET system is present and active at the capillary level.26, 27 While in vivo evidence is lacking, a study with endothelial cells and pericytes isolated from bovine brain capillaries reported that ET-1 secreted from endothelial cells causes actin cytoskeleton reorganization and morphological changes in pericytes in a paracrine manner via ETA-induced increase in intracellular calcium levels.28 Given that capillaries constitute about 85% of the cerebrovasculature, capillary endothelial cells and pericytes may have a profound effect on regulation of CBF and ET-1 is likely to be an important contributor.2–4
Another possible mechanism by which ET-1 can impact cerebrovascular function is the disruption of BBB integrity. In a rodent model of status epilepticus, vascular endothelial growth factor (VEGF) upregulation was found to activate the ETB receptor as well as downstream phosphatidylinositol 3 kinase (PI3K)/AKT signaling, resulting in vasogenic edema.29 In brain microvascular endothelial cells, inhibition of the ETB receptor was found to prevent matrix metalloprotease-9 (MMP-9) activation and alterations in tight junction proteins.30 Blockade of the ETB receptor in traumatic brain injury also promotes recovery of BBB integrity via downregulation of VEGF and MMP-9, suggesting activation of similar pathways in ET-1-mediated breakdown of BBB in different disease states and ETB receptor antagonism may be an effective therapeutic strategy.31 Additional evidence comes from endothelial specific ET-1 overexpression causing greater BBB breakdown, reactive astrogliosis, and increased caspase 3 around blood vessels after a 30 min MCA occlusion.32
There is also evidence for ET-1 involvement in dysregulation of microvascular structure. For example, in rodent models of diabetes, there is erratic neovascularization that involves pathological angiogenesis and remodeling of the cerebrovasculature, associated with decreased pericyte coverage of microvessels and cognitive deficits, even 7–8 weeks after diabetes onset.33 VEGF mediates this pathological vascularization via the downregulation of roundabout-4 (ROBO-4) guidance protein in endothelial cells.34 Proangiogenic ephrin-B2 signaling in pericytes also contributes to misguided vascularization in diabetes as evidenced by in vivo silencing of pericyte ephrin-2B preventing the development of immature vascular patterns.35 Interestingly, dual ET receptor blockade with bosentan can prevent and reverse pathological neovascularization and improve BBB integrity in diabetes.36 While the impact of ET antagonism on VEGF and pericyte ephrin-B2 signaling as underlying mechanisms of improved vascularization remain to be determined, another study demonstrated that restoration of proangiogenic properties of brain microvascular endothelial cells under diabetic conditions is associated with decreased ET-1 and upregulated ETB receptors.37 Moreover, ET-1 reduces the levels of proangiogenic and neurotrophic brain-derived neurotrophic factor (BDNF) produced by brain microvascular endothelial cells grown under diabetes mimicking and/or hypoxic conditions.38
In summary, ET-1 negatively impacts cerebrovascular function, structure, and integrity across a wide spectrum of diseases that are risk factors for VCID and AD. All these changes can contribute to the development of neuroinflammation and neurovascular degeneration in cognitive impairment by reducing blood flow and increasing BBB permeability.
ET-1 and cognitive impairment
There is clinical and preclinical evidence implicating a potential role for ET-1 in AD and VCID.39 Clinically, ET-1 and endothelin converting enzymes (ECE-1 and 2) activities are upregulated in postmortem temporal cortex specimens from AD patients, and ECE-2 expression and ET-1 release are upregulated by Aβ42 in vitro.40, 41 Leptomeningeal blood vessels isolated from postmortem brains of AD patients also show greater ECE-1 activity and ET-1 levels despite decreased ECE-1 protein levels. This has been proposed to be specific to AD since there is no specific change in vessels from VCID patients.42 On the other hand, Barker and colleagues reported that while ET-1 is decreased in the white matter of AD patients, there is a trend for elevated ET-1 in VCID patients.11 Based on this finding, authors speculated that ET-1 may be contributing to abnormal regulation of white matter perfusion in VCID. Interestingly, in the same study, they reported that ET-1 levels correlated positively with brain perfusion as assessed by the MAG:PLP1 ratio, a surrogate marker of perfusion which decreases in chronically hypoperfused tissue. The authors speculated that lower ET-1 levels and MAG:PLP1 ratio in AD brains may reflect a compensatory protective mechanism. However, this finding is quite controversial as we have commented on earlier in this review, the same group reported that ET-1 levels in autopsied tissue, correlated negatively with perfusion and concluded that ET-1-mediated vasoconstriction may be a critical contributor to hypoperfusion in AD brains.12
ET-1-mediated vascular dysfunction and ensuing cognitive deficits have been reported in experimental models including the transforming growth factor-beta 1 (TGF-β1) transgenic mouse that mimics the vascular pathology of AD,43, 44 intracerebral Aβ infusion in Dahl salt sensitive rats as well as under water deprivation conditions,45, 46 but studies in familial (5×FAD, APPSWE and Tau) or sporadic (APOE2-4) AD models are lacking with the exception of one study that showed restoration of aorta and carotid artery relaxation with dual ET receptor blockade in the APPSWE model.47
To determine a causal role of ET-1 in cognitive impairment in different disease models, selective or dual ET receptor blockers have been used in a limited number of studies. ETA receptor antagonists, BQ-123 or BMS182874, prevented cognitive dysfunction in a model of AD in which disease is induced by injection of Aβ1–40 in the lateral cerebral ventricle,48 whereas, the dual ETA/ETB receptor antagonist TAK-044 had no effect in this model. Interestingly, the dual receptor blocker, bosentan, improved learning and memory as well as mitochondrial and carotid artery endothelial function in a different model of AD. In this study, cognitive impairment was induced by combined administration of a single intracerebroventricular infusion of Aβ and chronic oral administration of L-methionine, resulting in hyperhomocysteinemia.49 Bosentan also improved cognition in a two-kidney, one-clip model of hypertension.50 On the contrary, stimulation of ETB receptor with intracerebral injection of an ETB specific agonist, ILR1620, has been reported to decrease the progression of AD in an experimental model in which cognitive deficits were induced by an Aβ1–40 injection into the brain.51 Similarly, this ETB receptor activation has been reported to prevent cerebral oxidative stress and improve cognitive function in type 1 diabetic rats that received intracerebroventricular Aβ1–40 injections along with IRL1620 and the ETB selective antagonist BQ-788.52 These conflicting results suggest that ET effects on cognition may be complex and need to be evaluated within the neurovascular unit platform. ET-1 and its receptors are found on different brain cells, as recently reviewed.18 In addition to its effects on vascular reactivity and structure, ET-1 is considered to be a proinflammatory peptide.18, 53 While ET-1 is not detectable on astrocytes under physiological conditions, ET-1 and ETB receptors are upregulated in pathological conditions and associated with astrogliosis.53 ET-1-mediated cognitive deficits in the cerebral malaria model were associated with increases in proinflammatory cytokines.21, 22 ET-1 may also be directly involved in neuronal loss as several studies reported that inhibition of ET-1 signaling prevents retinal neurodegeneration.18, 54 Unlike RAS modulators as discussed below, there is no human data on cognitive outcomes with the use of ET receptor antagonists. Unfortunately, a recent Study Of Diabetic Nephropathy With Atrasentan (SONAR), which included cognitive function as a secondary endpoint, was halted by the sponsor, before meaningful results could be obtained.
The Renin-Angiotensin System (RAS)
RAS is a complex and interconnected system of peptide hormones and their receptors are involved in many physiological functions such as water/electrolyte balance, and renal/hemodynamic homeostasis. Angiotensin II (Ang II) is the main RAS peptide and it is produced from angiotensin I (Ang I) by the action of angiotensin converting enzyme (ACE). Angiotensin I itself is produced from the parent protein, angiotensinogen, by the action of the enzyme, renin. Ang II mediates its actions through binding to its cognate receptor, angiotensin type 1 (AT1) receptor. In addition to the AT1 receptor, Ang II can stimulate other receptors either directly (angiotensin type 2 receptor, AT2) or indirectly (Mas receptor) after its conversion to angiotensin 1–7 (Ang-(1–7)) via the angiotensin converting enzyme 2 (ACE2). Ang II can also be converted to Ang IV, which binds to the angiotensin type 4 receptor (AT4). AT2, AT4 and Mas receptors are generally thought to mediate opposing actions to the AT1 receptor, thus constituting a protective axis under disease conditions. The overall outcome of the RAS in a certain organ or tissue depends on the receptor expression, the ligand availability, and their relative affinity for the receptors, as well as the interaction between them, either leading to potentiation or inhibition of their actions. The different RAS components within the brain have been reviewed in detail in recent reviews from our group and others.55–57 Therefore, we will focus on the role of RAS in cerebrovascular dysfunction and dementias, including AD and VCID.
RAS and cerebrovascular dysfunction
The RAS system has been described and studied in the brain vasculature. Brain endothelial cells express both AT1 and AT2 receptors.58, 59 While brain arteries from immature rats express more AT2 receptor, AT1 is more expressed in adult brain arteries,60 but AT2 expression can be upregulated under pathological conditions.56 The readers are referred to our recent review for more details on the expression of different RAS components within the brain.55
ACE is associated with the luminal surface of endothelial cells. ACE-mediated conversion of Ang I to Ang II leads to vasoconstriction of the cerebral vessels via activation of the AT1 receptor on neighboring smooth muscle cells. ACE also degrades bradykinin, which is an active vasodilator, necessary for CBF regulation.60 Ang II/AT1 axis stimulates VSMC hypertrophy, proliferation and migration, along with collagen synthesis and deposition, which collectively lead to vascular remodeling, fibrosis and stiffness. On the other hand, activation of the protective axis of RAS counteracts the actions of Ang II/AT1 and leads to vasodilation and increased CBF.55 The critical role of the RAS in cerebrovascular dysfunction becomes obvious in the setting of hypertension and diabetes. Ang II impairs cerebrovascular autoregulation and induces endothelial dysfunction and BBB breakdown.61, 62 Inhibition of ACE or AT1 blockade reverses hypertension-induced cerebrovascular dysfunction and remodeling, independent of the effect on blood pressure.63, 64 Blockade of AT1 improves the proangiogenic and barrier function of endothelial cells via the activation of AT2 signaling.58, 59 Furthermore, ACEI and ARBs reversed brain functional capillary rarefaction in diabetic hypertensive rats.65 Similarly, diabetes is associated with Ang II mediated cerebral endothelial dysfunction, which is ameliorated with ACE inhibition or AT1 blockade.66, 67 In addition, AT1 blockade protects against diabetes-induced cerebrovascular remodeling and myogenic dysfunction.68
RAS and cognitive impairment
Overactivation of the RAS by pharmacological or genetic upregulation of angiotensin II levels in rodents causes cognitive impairment via AT1 stimulation.69–71 Interruption of Ang II/AT1 canonical axis using renin inhibition, ACE inhibition or AT1 blockade has been shown to ameliorate cognitive impairment in experimental models. Stimulation of the protective axis of RAS through activation of ACE2, Ang-(1–7)/Mas, AT2, or Ang IV/AT4 have been shown to achieve similar protective effects.57, 72
The protective effects of RAS modulation have been shown in many different models of cognitive impairment including chronic cerebral hypoperfusion, AD models achieved by injection or overexpression of Aβ, diabetic or hypertensive animals and post-stroke cognitive impairment (PSCI) models.57, 72 In the next section, we will discuss the experimental and clinical evidence of the role of RAS in AD and VCID.
RAS and AD
Preclinical studies examined the effect of RAS modulation in AD in the context of its pathophysiology, which is thought to involve aggregation and deposition of Aβ and/or tau proteins. AD models were achieved by central injection or genetic overexpression of Aβ in vivo as well as the in vitro treatment of the various cell types of the neurovascular unit with Aβ. Earlier in vitro studies showed a role of ACE in degradation of Aβ proteins, thus preventing its accumulation. While this suggested a possible detrimental role of ACEI in AD through accumulation of Aβ aggregates, this has not been corroborated with subsequent in vivo studies or clinical data.72 Treatment with BBB penetrating ACEI showed a beneficial role on cognition in AD mouse models with or without decreasing the Aβ deposition.73–75 However, these effects were not seen with an ACEI that does not cross the BBB. The beneficial effects of ACEI on cognitive function in AD were further supported with clinical studies. As compared to other anti-hypertensives, ACEI use was associated with reduced rate of cognitive decline in AD patients.76 Centrally acting ACEI showed a superior effect in terms of slowing AD progression.77
Similar to ACEI, ARBs have been associated with reduced cognitive impairment in AD, both clinically and experimentally. Using a pharmacologically induced AD mouse model, ARBs reduced cognitive impairment through improving cerebrovascular dysfunction and decreasing oxidative stress.69 Clinically, ARB use was associated with reduced incidence and progression of AD with a possible superior effect of ARB over ACEI.78, 79 Direct targeting of the protective axis of RAS in AD animal models achieved a similar effect to Ang II/AT1 pathway blockade. Stimulation of the AT2 with compound 21 (C21) prevented cognitive decline in a pharmacologically induced AD mouse model.80 Furthermore, combining C21 with the cognition enhancing medication, memantine, achieved a synergistic effect.81 Similar to AT2 agonism, stimulation of the Ang-(1–7)/Mas82 and Ang IV/AT483 axes were shown to improve cognition in AD mouse models. Medications to target these receptors are still in clinical development and therefore the efficacy of AT2, AT4, and Mas receptors modulation is yet to be tested in AD patients.84, 85
RAS and VCID
VCID, which includes vascular cognitive impairment and PSCI, is the leading cause of Alzheimer’s Disease Related Dementias.86 Animal models to study VCID include chronic cerebral hypoperfusion and stroke-induced cognitive impairment. Using the bilateral carotid artery stenosis (BCAS) model of chronic cerebral hypoperfusion, modulation of different RAS components was shown to be protective against cognitive impairment and dementia. Centrally active ACEI and ARBs improved cognition in chronic cerebral hypoperfusion induced dementia.87, 88 Using the same model, cognitive enhancement was achieved with administration of either Ang-(1–7), the renin inhibitor, aliskiren, or the AT2 agonist, C21.89,90,91 Our group has recently shown a protective role of AT2 stimulation on progressive cognitive decline after stroke in hypertensive as well as aged rats, an effect that was seen even with a delayed treatment that did not affect the acute stroke outcome.92, 93
Similar to their beneficial effects in AD patients, ACEI and ARB use was associated with slowed cognitive decline in patients with vascular dementia compared to other antihypertensive medications, with ARBs suggested to be the most effective drug class.94
Several mechanisms may underlie RAS modulation-mediated cognitive effects. These mechanisms include a direct effect on the vasculature involving normalization of cerebrovascular function, increased blood flow and oxygen/nutrient supply to the brain, and improved BBB integrity as well as direct neuroprotection.57 Hypertension is the top co-morbidity associated with VCID and AD. While the protective effects of RAS modulators can be attributed to their blood pressure lowering effects, several studies have shown that treatment paradigms with sub-hypotensive doses were able to reduce cognitive decline. Furthermore, while blood pressure lowering is beneficial, other anti-hypertensive agents were not associated with a similar magnitude of reduced cognitive decline shown with ACEI/ARBs.56 Since the brain has its local RAS, it is logical that the RAS modulating drugs that aim to target cognitive impairment should have good CNS penetration. Centrally acting ACEI and ARBs show superior efficacy in terms of cognitive enhancement and reduced impairment. However, drugs like C21 with poor BBB penetration improve cognitive impairment as well in animal models. This could be due to local direct actions on the vascular endothelium or systemic immune cells together with a possible penetration after BBB breakdown in conditions associated with VCID.56 However, the latter assumption is challenged by a possible detrimental effect of BBB disruption on drug delivery. A number of studies have pointed to the accumulation of blood-derived debris and cells into enlarged perivascular spaces after BBB breakdown which hinders normal diffusion of drugs into the CNS.4
At the molecular level, RAS modulation is linked to decreased oxidative stress and inflammation, activation of peroxisome proliferator-activated receptors (PPAR), nitric oxide (NO) production, and upregulation of neurotrophic factors such as BDNF. Our studies have shown that direct or indirect AT2 stimulation via AT1 blockade leads to BDNF upregulation which plays a critical role in RAS-mediated cerebrovascular angiogenesis.56 Furthermore, RAS modulation has been shown to increase acetylcholine in the brain and interfere with the Aβ cascade through decreased production, aggregation and deposition, as well as increased clearance.55–57
Future Considerations
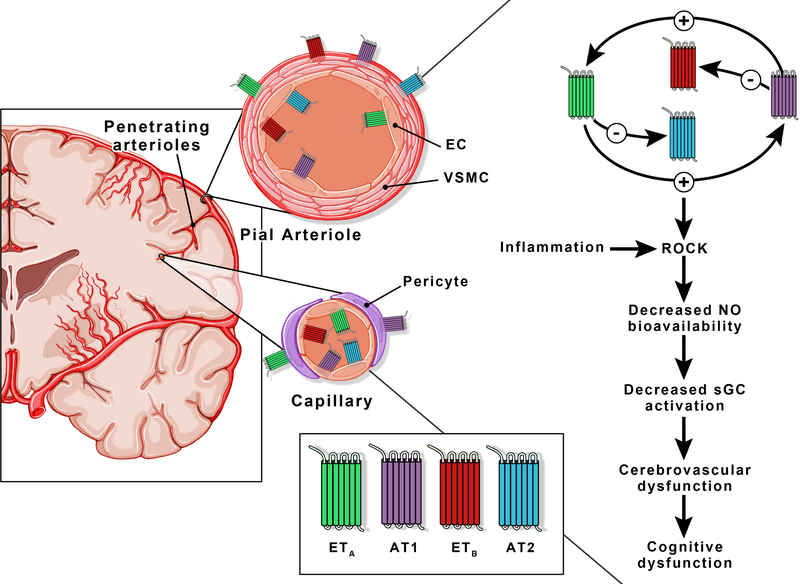
Studies suggest that there is crosstalk between these major vasoactive pathways (Fig. 2). Hypertension-mediated neurovascular dysfunction involves activation of both ETA and AT1 receptors in the cerebrovasculature.27 Cerebral ischemia leads to upregulation of smooth muscle ETA and ETB as well as AT1 receptors in the cerebral vasculature and combined inhibition of ETA and AT1 receptors reduces neurovascular injury, whereas individual inhibition does not have an effect.95 Another study reported that ET-1-induced contraction of basilar arteries is lower in the presence of AT1 blockade by losartan and this involves modulation of endothelial ETB receptor.96 A more recent study extended these findings into a subarachnoid hemorrhage model demonstrating that beneficial effects of AT1 blockade involves upregulation of endothelial ETB receptor.97 ET-1 can also impact angiotensin receptor expression and signaling. ET receptor antagonism can restore increased AT1 and reduced AT2 expression back to control levels in the hearts of spontaneously hypertensive rats.98 The interaction of these two powerful systems in regulating cerebrovascular function in the context of cognitive impairment remains to be determined.
Figure 2.
ET and Ang II receptor localization on the cerebrovascular networks. Both ET (ETA and ETB) and Ang II (AT1 and AT2) receptors are present on endothelial and smooth muscle cells of the pial vessels. A distinction at the capillary level is the co-presence of ETA and ETB receptors on endothelial cells. There is a bidirectional crosstalk between the ET system and RAS: ET-1 upregulates deleterious AT1 and downregulates protective AT2 receptor expression while Ang II upregulates deleterious ETA and downregulates protective ETB receptor expression. AT1 and ETA receptor signaling activates ROCK, decreases NO bioavailability and sGC signaling ultimately resulting in reduced CBF and cognitive deficits. EC = endothelial cells, VSMC = vascular smooth muscle cells, ROCK = Rho-associated protein kinase, NO = nitric oxide, sGC = Soluble guanylyl cyclase.
ET-1 has been shown to cause cerebral microvascular dysfunction via decreasing NO bioavailability through the activation of the Rho-associated protein kinase (ROCK).45 A recent study demonstrated that a high salt diet, which activates the ET system, causes neurovascular and cognitive dysfunction via limiting NO bioavailability.99 Interestingly, ETA receptor antagonism did not improve the reduced CBF and endothelial dysfunction observed in this model. On the other hand, neutralization of circulating interleukin (IL-) 17 or inhibition of ROCK, ameliorated neurovascular and cognitive dysfunction. These studies suggest that the involvement of RAS and ET systems in neurovascular and cognitive function may differ under physiological and pathophysiological conditions. These studies also identify inflammatory pathways as well as downstream signaling pathways like Rho kinase as potential therapeutic targets for the prevention and/or treatment of cognitive impairment.
The importance of the restoration of cerebrovascular dysfunction to improve cognitive function was recognized in the recently completed Lacunar Intervention Trial 1 (LACI-1), which was the first step of studies to determine the impact of endothelial dysfunction on prevention and progression of small vessel disease (SVD) and ultimately VCID. Results showed that isosorbide mononitrate (ISMN) and cilostazol, two commonly prescribed drugs to improve vascular function in heart and peripheral arterial diseases, are well-tolerated in patients with lacunar strokes, laying the foundation for phase III LACI-2 Trial, which will assess the safety and efficacy of these drugs in the prevention of lacunar strokes and progression of SVD. Both of these drugs target mediators downstream of Ang II and ET-1.
In conclusion, preservation and/or restoration of cerebrovascular function and integrity is fundamental for cognitive function. Modulation of major vasoactive pathways and their downstream signaling can identify therapeutic opportunities to intervene early in the disease process. However, one important consideration is the impact of biological variables such as age and sex. Just within the context of this focused review, there are reports that the relative abundance and activity of RAS and ET system components may differ between males and females.55 Despite similar upregulation of receptors in both sexes in cultured human cerebral arteries, vessels from women were significantly less responsive to Ang II or ET-1 than men.100 This issue is highly likely to be applicable to various systems and needs to be further investigated.
Supplementary Material
HIGHLIGHTS:
Cerebrovascular dysfunction plays a central role in cognitive impairment.
Both the angiotensin and endothelin system have been implicated in cerebrovascular dysfunction and cognitive decline.
The contribution of these vasoactive pathways to cognitive impairment and underlying mechanisms are discussed in this review.
Interventions to modulate these pathways and limit cognitive impairment are presented and reviewed from both clinical and experimental evidence.
Acknowledgments:
The authors would like to thank the medical illustrator Colby Zahn in the Department of Neurosurgery at the Augusta University for producing the figures in this manuscript.
Sources of Funding: This work was supported in part by a Veterans Affairs (VA) Merit Award (BX000347), VA Senior Research Career Scientist Award and National Institutes of Health (NIH) awards (R01NS083559) to Adviye Ergul, R01NS104573 multi-PI grant to Susan C. Fagan and Adviye Ergul, and American Heart Association (AHA) postdoctoral fellowship (18POST34060036) to Abdelrahman Y. Fouda.
abbreviations:
- Aβ
Amyloid β
- ACE
Angiotensin converting enzyme
- ACEI
Angiotensin converting enzyme inhibitors
- AD
Alzheimer’s disease
- Ang II
Angiotensin II
- Ang-(1–7)
Angiotensin 1–7
- AT1
Angiotensin type 1 receptor
- AT2
Angiotensin type 2 receptor
- Aβ
Amyloid β
- BBB
Blood-brain barrier
- BCAS
Bilateral carotid artery stenosis
- BDNF
Brain-derived neurotrophic factor
- C21
Compound 21
- CBF
Cerebral blood flow
- CSF
Cerebral spinal fluid
- ECE
Endothelin converting enzyme
- ET
Endothelin
- ETA
Endothelin receptor type A
- ETB
Endothelin receptor type B
- ISMN
Isosorbide mononitrate
- MAG:PLP1
Ratio of myelin associated glycoprotein to proteolipid protein
- MCA
Middle cerebral artery
- MMP-9
Matrix metalloprotease-9
- NO
Nitric oxide
- PI3K
Phosphatidylinositol 3 kinase
- PPAR
Peroxisome proliferator-activated receptors
- PPET
Preproendothelins
- PSCI
Post-stroke cognitive impairment
- RAS
Renin-angiotensin system
- ROBO-4
Roundabout-4
- ROCK
Rho-associated protein kinase
- SVD
Small vessel disease
- TGF-β1
Transforming growth factor-beta 1
- VCAM-1
Vascular cell adhesion molecule-1
- VCID
Vascular contributions to cognitive impairment/dementia
- VGEF
Vascular endothelial growth factor
- VSMC
Vascular smooth muscle cells
- WMH
White matter hyperintensities
Footnotes
Disclosures: Adviye Ergul is a Senior Research Career Scientist at the Ralph H. Johnson Veterans Affairs Medical Center in Charleston, SC. The contents do not represent the views of the Department of Veterans Affairs or the US Government.
References:
- 1.Iadecola C The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: From physiology to disease and back. Physiological reviews. 2019;99:21–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nature neuroscience. 2018;21:1318–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in alzheimer disease and other neurodegenerative disorders. Nature reviews. Neurology 2018;14:133–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: A presidential advisory from the american heart association/american stroke association. Stroke. 2017;48:e284–e303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rius-Perez S, Tormos AM, Perez S, Talens-Visconti R. Vascular pathology: Cause or effect in alzheimer disease? Neurologia. 2018;33:112–120 [DOI] [PubMed] [Google Scholar]
- 7.Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of alzheimer’s disease--a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiology of disease. 2015;82:593–606 [DOI] [PubMed] [Google Scholar]
- 8.Du AT, Jahng GH, Hayasaka S, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin KP, Miller BL, Weiner MW, Schuff N. Hypoperfusion in frontotemporal dementia and alzheimer disease by arterial spin labeling mri. Neurology. 2006;67:1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Viqar F, Zimmerman ME, et al. White matter hyperintensities are a core feature of alzheimer’s disease: Evidence from the dominantly inherited alzheimer network. Annals of neurology. 2016;79:929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC. Early role of vascular dysregulation on late-onset alzheimer’s disease based on multifactorial data-driven analysis. Nature communications. 2016;7:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker R, Ashby EL, Wellington D, Barrow VM, Palmer JC, Kehoe PG, Esiri MM, Love S. Pathophysiology of white matter perfusion in alzheimer’s disease and vascular dementia. Brain : a journal of neurology. 2014;137:1524–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas T, Miners S, Love S. Post-mortem assessment of hypoperfusion of cerebral cortex in alzheimer’s disease and vascular dementia. Brain : a journal of neurology. 2015;138:1059–1069 [DOI] [PubMed] [Google Scholar]
- 13.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. American journal of physiology. Heart and circulatory physiology. 2017;312:H1–H20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415 [DOI] [PubMed] [Google Scholar]
- 15.Ergul A Development of endothelin receptor antagonists as potential therapeutic agents. Exp. Opin. Ther. Patents 2003;13:33–44 [Google Scholar]
- 16.Abdul Y, Ward R, Dong G, Ergul A. Lipopolysaccharide-induced necroptosis of brain microvascular endothelial cells can be prevented by inhibition of endothelin receptors. Physiological research. 2018;67:S227–S236 [DOI] [PubMed] [Google Scholar]
- 17.Li W, Abdul Y, Ward R, Ergul A. Endothelin and diabetic complications: A brain-centric view. Physiological research. 2018;67:S83–S94 [DOI] [PubMed] [Google Scholar]
- 18.D’Orleans-Juste P, Akide Ndunge OB, Desbiens L, Tanowitz HB, Desruisseaux MS. Endothelins in inflammatory neurological diseases. Pharmacology & therapeutics. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: Role of et-1-induced peroxynitrite generation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumont AS, Dumont RJ, McNeill JH, Kassell NF, Sutherland GR, Verma S. Chronic endothelin antagonism restores cerebrovascular function in diabetes. Neurosurgery. 2003;52:653–660; discussion 659–660 [DOI] [PubMed] [Google Scholar]
- 21.Freeman BD, Martins YC, Akide-Ndunge OB, Bruno FP, Wang H, Tanowitz HB, Spray DC, Desruisseaux MS. Endothelin-1 mediates brain microvascular dysfunction leading to long-term cognitive impairment in a model of experimental cerebral malaria. PLoS pathogens. 2016;12:e1005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins YC, Freeman BD, Akide Ndunge OB, Weiss LM, Tanowitz HB, Desruisseaux MS. Endothelin-1 treatment induces an experimental cerebral malaria-like syndrome in c57bl/6 mice infected with plasmodium berghei nk65. The American journal of pathology. 2016;186:2957–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai X, Leo MD, Jaggar JH. Endothelin-1 stimulates vasoconstriction through rab11a serine 177 phosphorylation. Circ Res. 2017;121:650–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachidanandam K, Harris A, Hutchinson J, Ergul A. Microvascular versus macrovascular dysfunction in type 2 diabetes: Differences in contractile responses to endothelin-1. Exp Biol Med (Maywood). 2006;231:1016–1021 [PubMed] [Google Scholar]
- 25.Hardigan T, Ward R, Ergul A. Cerebrovascular complications of diabetes: Focus on cognitive dysfunction. Clin Sci (Lond). 2016;130:1807–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, Davisson RL, Iadecola C. Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension. 2012;60:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin ii hypertension. J Neurosci. 2012;32:4878–4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehouck MP, Vigne P, Torpier G, Breittmayer JP, Cecchelli R, Frelin C. Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1997;17:464–469 [DOI] [PubMed] [Google Scholar]
- 29.Kim JE, Kang TC. Trpc3- and etb receptor-mediated pi3k/akt activation induces vasogenic edema formation following status epilepticus. Brain research. 2017;1672:58–64 [DOI] [PubMed] [Google Scholar]
- 30.Kim JY, Ko AR, Hyun HW, Kang TC. Etb receptor-mediated mmp-9 activation induces vasogenic edema via zo-1 protein degradation following status epilepticus. Neuroscience. 2015;304:355–367 [DOI] [PubMed] [Google Scholar]
- 31.Michinaga S, Kimura A, Hatanaka S, Minami S, Asano A, Ikushima Y, Matsui S, Toriyama Y, Fujii M, Koyama Y. Delayed administration of bq788, an etb antagonist, after experimental traumatic brain injury promotes recovery of blood-brain barrier function and a reduction of cerebral edema in mice. Journal of neurotrauma. 2018;35:1481–1494 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Yeung PK, McAlonan GM, Chung SS, Chung SK. Transgenic mice over-expressing endothelial endothelin-1 show cognitive deficit with blood-brain barrier breakdown after transient ischemia with long-term reperfusion. Neurobiology of learning and memory. 2013;101:46–54 [DOI] [PubMed] [Google Scholar]
- 33.Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, Johnson M, Fagan SC, Ergul A. Enhanced cerebral but not peripheral angiogenesis in the goto-kakizaki model of type 2 diabetes involves vegf and peroxynitrite signaling. Diabetes. 2012;61:1533–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelsaid M, Coucha M, Hafez S, Yasir A, Johnson MH, Ergul A. Enhanced vegf signalling mediates cerebral neovascularisation via downregulation of guidance protein robo4 in a rat model of diabetes. Diabetologia. 2017;60:740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coucha M, Barrett AC, Elgebaly M, Ergul A, Abdelsaid M. Inhibition of ephrin-b2 in brain pericytes decreases cerebral pathological neovascularization in diabetic rats. PLoS One. 2019;14:e0210523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdelsaid M, Ma H, Coucha M, Ergul A. Late dual endothelin receptor blockade with bosentan restores impaired cerebrovascular function in diabetes. Life Sci. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelsaid M, Williams R, Hardigan T, Ergul A. Linagliptin attenuates diabetes-induced cerebral pathological neovascularization in a blood glucose-independent manner: Potential role of et-1. Life sciences. 2016;159:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward R, Abdul Y, Ergul A. Endothelin-1 inhibition improves the mbdnf/probdnf ratio in endothelial cells and ht22 neurons under high glucose/palmitate growth conditions. Physiological research. 2018;67:S237–s246 [DOI] [PubMed] [Google Scholar]
- 39.Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and alzheimer’s disease. J Neurosci Res. 2017;95:943–972 [DOI] [PubMed] [Google Scholar]
- 40.Palmer J, Love S. Endothelin receptor antagonists: Potential in alzheimer’s disease. Pharmacol Res. 2011;63:525–531 [DOI] [PubMed] [Google Scholar]
- 41.Palmer JC, Barker R, Kehoe PG, Love S. Endothelin-1 is elevated in alzheimer’s disease and upregulated by amyloid-beta. J Alzheimers Dis. 2012;29:853–861 [DOI] [PubMed] [Google Scholar]
- 42.Palmer JC, Tayler HM, Love S. Endothelin-converting enzyme-1 activity, endothelin-1 production, and free radical-dependent vasoconstriction in alzheimer’s disease. J Alzheimers Dis. 2013;36:577–587 [DOI] [PubMed] [Google Scholar]
- 43.Papadopoulos P, Ongali B, Hamel E. Selective in vivo antagonism of endothelin receptors in transforming growth factor-beta1 transgenic mice that mimic the vascular pathology of alzheimer’s disease. Can J Physiol Pharmacol. 2010;88:652–660 [DOI] [PubMed] [Google Scholar]
- 44.Tong XK, Hamel E. Simvastatin restored vascular reactivity, endothelial function and reduced string vessel pathology in a mouse model of cerebrovascular disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraco G, Moraga A, Moore J, Anrather J, Pickel VM, Iadecola C. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension. 2013;62:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faraco G, Wijasa TS, Park L, Moore J, Anrather J, Iadecola C. Water deprivation induces neurovascular and cognitive dysfunction through vasopressin-induced oxidative stress. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elesber AA, Bonetti PO, Woodrum JE, Zhu XY, Lerman LO, Younkin SG, Lerman A. Bosentan preserves endothelial function in mice overexpressing app. Neurobiology of aging. 2006;27:446–450 [DOI] [PubMed] [Google Scholar]
- 48.Briyal S, Philip T, Gulati A. Endothelin-a receptor antagonists prevent amyloid-beta-induced increase in eta receptor expression, oxidative stress, and cognitive impairment. J Alzheimers Dis. 2011;23:491–503 [DOI] [PubMed] [Google Scholar]
- 49.Singh M, Prakash A. Possible role of endothelin receptor against hyperhomocysteinemia and beta-amyloid induced ad type of vascular dementia in rats. Brain Res Bull. 2017;133:31–41 [DOI] [PubMed] [Google Scholar]
- 50.Singh P, Gupta S, Sharma B. Antagonism of endothelin (eta and etb) receptors during renovascular hypertension-induced vascular dementia improves cognition. Current neurovascular research. 2016;13:219–229 [DOI] [PubMed] [Google Scholar]
- 51.Briyal S, Nguyen C, Leonard M, Gulati A. Stimulation of endothelin b receptors by irl-1620 decreases the progression of alzheimer’s disease. Neuroscience. 2015;301:1–11 [DOI] [PubMed] [Google Scholar]
- 52.Briyal S, Shepard C, Gulati A. Endothelin receptor type b agonist, irl-1620, prevents beta amyloid (abeta) induced oxidative stress and cognitive impairment in normal and diabetic rats. Pharmacol Biochem Behav. 2014;120:65–72 [DOI] [PubMed] [Google Scholar]
- 53.Hostenbach S, D’Haeseleer M, Kooijman R, De Keyser J. The pathophysiological role of astrocytic endothelin-1. Progress in neurobiology. 2016;144:88–102 [DOI] [PubMed] [Google Scholar]
- 54.Bogdanov P, Simo-Servat O, Sampedro J, Sola-Adell C, Garcia-Ramirez M, Ramos H, Guerrero M, Sune-Negre JM, Tico JR, Montoro B, Duran V, Arias L, Hernandez C, Simo R. Topical administration of bosentan prevents retinal neurodegeneration in experimental diabetes. International journal of molecular sciences. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: The renin angiotensin system. International journal of molecular sciences. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fouda AY, Artham S, El-Remessy AB, Fagan SC. Renin-angiotensin system as a potential therapeutic target in stroke and retinopathy: Experimental and clinical evidence. Clinical science (London, England : 1979). 2016;130:221–238 [DOI] [PubMed] [Google Scholar]
- 57.Petek B, Villa-Lopez M, Loera-Valencia R, Gerenu G, Winblad B, Kramberger MG, Ismail MA, Eriksdotter M, Garcia-Ptacek S. Connecting the brain cholesterol and renin-angiotensin (ras) systems. Potential role of statins and ras-modifying medications in dementia. Journal of internal medicine. 2018 [DOI] [PubMed] [Google Scholar]
- 58.Gallego-Delgado J, Basu-Roy U, Ty M, Alique M, Fernandez-Arias C, Movila A, Gomes P, Weinstock A, Xu W, Edagha I, Wassmer SC, Walther T, Ruiz-Ortega M, Rodriguez A. Angiotensin receptors and beta-catenin regulate brain endothelial integrity in malaria. The Journal of clinical investigation. 2016;126:4016–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alhusban A, Kozak A, Ergul A, Fagan SC. At1 receptor antagonism is proangiogenic in the brain: Bdnf a novel mediator. The Journal of pharmacology and experimental therapeutics. 2013;344:348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saavedra JM, Ito T, Nishimura Y. Review: The role of angiotensin ii at1-receptors in the regulation of the cerebral blood flow and brain ischaemia. Journal of the renin-angiotensin-aldosterone system : JRAAS. 2001;2:S102–s109 [DOI] [PubMed] [Google Scholar]
- 61.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. American journal of physiology. Heart and circulatory physiology. 2013;304:H1598–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell metabolism. 2008;7:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iadecola C, Gorelick PB. Hypertension, angiotensin, and stroke: Beyond blood pressure. Stroke. 2004;35:348–350 [DOI] [PubMed] [Google Scholar]
- 64.Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin ii in the mouse neocortex is sexually dimorphic. American journal of physiology. Heart and circulatory physiology. 2008;294:H156–163 [DOI] [PubMed] [Google Scholar]
- 65.Estato V, Obadia N, Carvalho-Tavares J, Freitas FS, Reis P, Castro-Faria Neto H, Lessa MA, Tibirica E. Blockade of the renin-angiotensin system improves cerebral microcirculatory perfusion in diabetic hypertensive rats. Microvascular research. 2013;87:41–49 [DOI] [PubMed] [Google Scholar]
- 66.Arrick DM, Sharpe GM, Sun H, Mayhan WG. Losartan improves impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type 1 diabetic rats. Brain research. 2008;1209:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trauernicht AK, Sun H, Patel KP, Mayhan WG. Enalapril prevents impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in diabetic rats. Stroke. 2003;34:2698–2703 [DOI] [PubMed] [Google Scholar]
- 68.Abdelsaid M, Kaczmarek J, Coucha M, Ergul A. Dual endothelin receptor antagonism with bosentan reverses established vascular remodeling and dysfunctional angiogenesis in diabetic rats: Relevance to glycemic control. Life Sci. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009;53:356–362 [DOI] [PubMed] [Google Scholar]
- 70.Duchemin S, Belanger E, Wu R, Ferland G, Girouard H. Chronic perfusion of angiotensin ii causes cognitive dysfunctions and anxiety in mice. Physiology & behavior. 2013;109:63–68 [DOI] [PubMed] [Google Scholar]
- 71.Tian M, Zhu D, Xie W, Shi J. Central angiotensin ii-induced alzheimer-like tau phosphorylation in normal rat brains. FEBS letters. 2012;586:3737–3745 [DOI] [PubMed] [Google Scholar]
- 72.Gebre AK, Altaye BM, Atey TM, Tuem KB, Berhe DF. Targeting renin-angiotensin system against alzheimer’s disease. Frontiers in pharmacology. 2018;9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamada K, Uchida S, Takahashi S, Takayama M, Nagata Y, Suzuki N, Shirakura S, Kanda T. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of alzheimer’s disease. Brain research. 2010;1352:176–186 [DOI] [PubMed] [Google Scholar]
- 74.Dong YF, Kataoka K, Tokutomi Y, Nako H, Nakamura T, Toyama K, Sueta D, Koibuchi N, Yamamoto E, Ogawa H, Kim-Mitsuyama S. Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2911–2920 [DOI] [PubMed] [Google Scholar]
- 75.AbdAlla S, Langer A, Fu X, Quitterer U. Ace inhibition with captopril retards the development of signs of neurodegeneration in an animal model of alzheimer’s disease. International journal of molecular sciences. 2013;14:16917–16942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soto ME, van Kan GA, Nourhashemi F, Gillette-Guyonnet S, Cesari M, Cantet C, Rolland Y, Vellas B. Angiotensin-converting enzyme inhibitors and alzheimer’s disease progression in older adults: Results from the reseau sur la maladie d’alzheimer francais cohort. Journal of the American Geriatrics Society. 2013;61:1482–1488 [DOI] [PubMed] [Google Scholar]
- 77.O’Caoimh R, Healy L, Gao Y, Svendrovski A, Kerins DM, Eustace J, Kehoe PG, Guyatt G, Molloy DW. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2014;40:595–603 [DOI] [PubMed] [Google Scholar]
- 78.Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: Prospective cohort analysis. BMJ (Clinical research ed.). 2010;340:b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhuang S, Wang HF, Wang X, Li J, Xing CM. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and alzheimer’s disease: A meta-analysis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2016;33:32–38 [DOI] [PubMed] [Google Scholar]
- 80.Jing F, Mogi M, Sakata A, Iwanami J, Tsukuda K, Ohshima K, Min LJ, Steckelings UM, Unger T, Dahlof B, Horiuchi M. Direct stimulation of angiotensin ii type 2 receptor enhances spatial memory. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iwanami J, Mogi M, Tsukuda K, Jing F, Ohshima K, Wang XL, Nakaoka H, Kan-no H, Chisaka T, Bai HY, Min LJ, Horiuchi M. Possible synergistic effect of direct angiotensin ii type 2 receptor stimulation by compound 21 with memantine on prevention of cognitive decline in type 2 diabetic mice. European journal of pharmacology. 2014;724:9–15 [DOI] [PubMed] [Google Scholar]
- 82.Uekawa K, Hasegawa Y, Senju S, Nakagata N, Ma M, Nakagawa T, Koibuchi N, Kim-Mitsuyama S. Intracerebroventricular infusion of angiotensin-(1–7) ameliorates cognitive impairment and memory dysfunction in a mouse model of alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2016;53:127–133 [DOI] [PubMed] [Google Scholar]
- 83.Albiston AL, Fernando R, Ye S, Peck GR, Chai SY. Alzheimer’s, angiotensin iv and an aminopeptidase. Biological & pharmaceutical bulletin. 2004;27:765–767 [DOI] [PubMed] [Google Scholar]
- 84.Albiston AL, Diwakarla S, Fernando RN, Mountford SJ, Yeatman HR, Morgan B, Pham V, Holien JK, Parker MW, Thompson PE, Chai SY. Identification and development of specific inhibitors for insulin-regulated aminopeptidase as a new class of cognitive enhancers. British journal of pharmacology. 2011;164:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wright JW, Kawas LH, Harding JW. The development of small molecule angiotensin iv analogs to treat alzheimer’s and parkinson’s diseases. Progress in neurobiology. 2015;125:26–46 [DOI] [PubMed] [Google Scholar]
- 86.Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, Pahigiannis K, Waddy SP, Koroshetz W. The science of vascular contributions to cognitive impairment and dementia (vcid): A framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cellular and molecular neurobiology. 2016;36:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada K, Horita T, Takayama M, Takahashi S, Takaba K, Nagata Y, Suzuki N, Kanda T. Effect of a centrally active angiotensin converting enzyme inhibitor, perindopril, on cognitive performance in chronic cerebral hypo-perfusion rats. Brain research. 2011;1421:110–120 [DOI] [PubMed] [Google Scholar]
- 88.Washida K, Ihara M, Nishio K, Fujita Y, Maki T, Yamada M, Takahashi J, Wu X, Kihara T, Ito H, Tomimoto H, Takahashi R. Nonhypotensive dose of telmisartan attenuates cognitive impairment partially due to peroxisome proliferator-activated receptor-gamma activation in mice with chronic cerebral hypoperfusion. Stroke. 2010;41:1798–1806 [DOI] [PubMed] [Google Scholar]
- 89.Fuchtemeier M, Brinckmann MP, Foddis M, Kunz A, Po C, Curato C, Dirnagl U, Farr TD. Vascular change and opposing effects of the angiotensin type 2 receptor in a mouse model of vascular cognitive impairment. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong YF, Kataoka K, Toyama K, Sueta D, Koibuchi N, Yamamoto E, Yata K, Tomimoto H, Ogawa H, Kim-Mitsuyama S. Attenuation of brain damage and cognitive impairment by direct renin inhibition in mice with chronic cerebral hypoperfusion. Hypertension. 2011;58:635–642 [DOI] [PubMed] [Google Scholar]
- 91.Xie W, Zhu D, Ji L, Tian M, Xu C, Shi J. Angiotensin-(1–7) improves cognitive function in rats with chronic cerebral hypoperfusion. Brain research. 2014;1573:44–53 [DOI] [PubMed] [Google Scholar]
- 92.Ahmed HA, Ishrat T, Pillai B, Fouda AY, Sayed MA, Eldahshan W, Waller JL, Ergul A, Fagan SC. Ras modulation prevents progressive cognitive impairment after experimental stroke: A randomized, blinded preclinical trial. Journal of neuroinflammation. 2018;15:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmed HA, Ishrat T, Pillai B, Bunting KM, Vazdarjanova A, Waller JL, Ergul A, Fagan SC. Angiotensin receptor (at2r) agonist c21 prevents cognitive decline after permanent stroke in aged animals -a randomized double- blind pre-clinical study. Behavioural brain research. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levi Marpillat N, Macquin-Mavier I, Tropeano AI, Bachoud-Levi AC, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: A network meta-analysis. Journal of hypertension. 2013;31:1073–1082 [DOI] [PubMed] [Google Scholar]
- 95.Stenman E, Jamali R, Henriksson M, Maddahi A, Edvinsson L. Cooperative effect of angiotensin at(1) and endothelin et(a) receptor antagonism limits the brain damage after ischemic stroke in rat. European journal of pharmacology. 2007;570:142–148 [DOI] [PubMed] [Google Scholar]
- 96.Konczalla J, Wanderer S, Mrosek J, Schuss P, Platz J, Guresir E, Seifert V, Vatter H. Crosstalk between the angiotensin and endothelin-system in the cerebrovasculature. Current neurovascular research. 2013;10:335–345 [DOI] [PubMed] [Google Scholar]
- 97.Wanderer S, Mrosek J, Vatter H, Seifert V, Konczalla J. Crosstalk between the angiotensin and endothelin system in the cerebrovasculature after experimental induced subarachnoid hemorrhage. Neurosurgical review. 2018;41:539–548 [DOI] [PubMed] [Google Scholar]
- 98.Jesmin S, Sakuma I, Togashi H, Yoshioka M, Hattori Y, Kitabatake A, Miyauchi T. Effects of endothelin receptor antagonist on expression of at1 and at2 receptors in the heart of shr-sp. Journal of cardiovascular pharmacology. 2004;44 Suppl 1:S59–63 [DOI] [PubMed] [Google Scholar]
- 99.Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, Buendia I, Santisteban MM, Segarra SG, Koizumi K, Sugiyama Y, Murphy M, Voss H, Anrather J, Iadecola C. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated th17 response. Nature neuroscience. 2018;21:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahnstedt H, Cao L, Krause DN, Warfvinge K, Saveland H, Nilsson OG, Edvinsson L. Male-female differences in upregulation of vasoconstrictor responses in human cerebral arteries. PloS one. 2013;8:e62698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.