Abstract
Negative social experiences influence both depression and cardiovascular dysfunction. Many individuals who experience negative mood states or cardiovascular conditions have limited social support. Therefore, investigation of drug treatments that may protect against the consequences of social stress will aid in designing effective treatment strategies. The current study used an animal model to evaluate the protective effect of sertraline administration on behavioral and cardiovascular consequences of social stress. Specifically, male prairie voles (Microtus ochrogaster), which are socially monogamous rodents that share several behavioral and physiological characteristics with humans, were isolated from a socially-bonded female partner, and treated with sertraline (16 mg/kg/day, ip) or vehicle during isolation. Unexpectedly, sertraline did not protect against depression-relevant behaviors, and it was associated with increased short- and long-term heart rate responses. However, sertraline administration improved heart rate variability recovery following a behavioral stressor, including increased parasympathetic regulation, and altered long-term neuronal activity in brain regions that modulate autonomic control and stress reactivity. These results indicate that sertraline may partially protect against the consequences of social stressors, and suggest a mechanism through which sertraline may beneficially influence neurobiological control of cardiac function.
Keywords: Autonomic, Depression, Fos, Prairie vole, Serotonin, Sertraline
1. Introduction
The disruption of social bonds can adversely influence both emotional and cardiovascular health, and therefore may play an important role in the relationship between mood disorders and cardiovascular disease (Cacioppo et al., 2010; Gore, 1978; Shankar et al., 2011). Individuals with decreased levels of social engagement or who feel lonely experience an increased risk for depressive disorders, as well as mortality from cardiovascular and other related disorders (Cacioppo et al., 2010; Ramsay et al., 2008). For example, higher levels of social isolation in humans are associated with an increased risk of death from all causes and specifically from cardiovascular disease (Kaplan et al., 1988). Similarly, studies with animal models demonstrate that social isolation and other forms of social stress are associated with depressive behaviors as well as cardiovascular and autonomic dysfunction, contributing evidence to the hypothesis that negative social experiences may mediate the association of depression and cardiovascular disease (Bosch et al., 2009; Shively et al., 2009). Social stress may negatively influence the relationship between behavioral and physiological functions through deleterious changes in common underlying neurobiological pathways (Roos et al., 2017; Sasagawa et al., 2017; Spasojevic et al., 2012).
In order to better understand the neurobiological changes involved in the responses to social stress, previous studies have utilized the prairie vole (Microtus ochrogaster) as a laboratory model for the investigation of social behavior and social bonding [e.g., (Aragona et al., 2003; Cushing et al., 2003; DeVries et al., 1995)], and dysfunction associated with the disruption of social bonds [e.g., (Bosch et al., 2009; Sun et al., 2014)]. Prairie voles engage in several social behaviors that mirror those of humans, including living in family groups, cooperative rearing of offspring, and the development of long-term male-female bonds (Carter & Getz, 1993; Young & Wang, 2004). Moreover, when exposed to short- or long-term social stressors, prairie voles display disruptions consistent with depressive disorders and cardiovascular disease, including behavioral changes such as helplessness and reduced exploration, heart rate (HR) and rhythm abnormalities, autonomic imbalance, and central nervous system disruptions in regions associated with stress and emotion (Bales et al., 2006; Bosch et al., 2004; Bosch et al., 2009; Grippo et al., 2007b). These previous findings indicate that the prairie vole is a valuable translational model for the investigation of social and neural mechanisms involved in behavioral and cardiovascular disorders.
One potential neurobiological system that may influence the interactions of social behavior, emotion, and cardiovascular function is the serotonin system. Serotonergic projections that originate from the raphe nuclei modulate functions of cortical, limbic, and hindbrain regions, influencing a wide variety of physiological and behavioral processes (Gershon & Tack, 2007; Jacobs & Fornal, 1991; Mohammad-Zadeh et al., 2008). Several types of serotonin receptors are located throughout these cortical and subcortical regions, creating a coordinated system for modulating cardiovascular function, mood, and social behavior (Jacobs & Fornal, 1991; Lesch, 2007; Thayer & Brosschot, 2005). For example, the serotonergic system interacts with the hypothalamic-pituitary-adrenal (HPA) axis, with prolonged exposure to stress impairing function in both systems (Andrews & Matthews, 2004; Duval et al., 2002; Leonard, 2006; Mahar et al., 2014; Wood, 2014). The dorsal raphe nuclei (DRN) send serotonergic projections to the hypothalamic paraventricular nucleus (PVN), resulting in an activation of stress responses [see review by (Myers et al., 2017)]. As such, the PVN is an important site for the integration of stress- and autonomic-related signals (Sladek et al., 2015; Swanson & Sawchenko, 1980). Further, regions of the amygdala also interact with the PVN and are responsive to stress, and thus may play a role in the modulation of physiological and behavioral stress reactivity (Clinard et al., 2015; Herman et al., 2016; Lukkes et al., 2012; Wang et al., 2012). Dysfunction in this circuit has been linked to affective disorders as well as cardiovascular disease (Chiou et al., 2009; Liu et al., 2009; Nalivaiko & Blessing, 2001; Silberman & Winder, 2013). Consequently, antidepressant drugs that influence serotonin, such as serotonin reuptake inhibitors (SRIs), not only improve mental health (Flament et al., 1999; Papakostas & Fava, 2008), but also can improve physiological functions and quality of life in patients with cardiovascular disease (Glassman et al., 2002; Roose et al., 1998; Shapiro et al., 1999).
Although SRIs may improve emotion and cardiovascular function, the influence of these drugs on behavioral, neural, and cardiovascular responses to social stressors is not fully understood. Previous evidence suggests that SRIs protect against dysfunctional behavioral and cardiovascular outcomes in humans and animal models, and alter the function of stress- and autonomic-related brain regions (Dale et al., 2015; Finnell & Wood, 2016), which may have relevance to social behaviors and responses to social stressors. For example, long-term use of the SRI paroxetine improved depressive symptoms and was associated with the re-establishment of diurnal cortisol cycles in humans (Ruhe et al., 2015). Additionally, short-term use of the SRI escitalopram attenuated amygdala reactivity to fearful faces in depressed patients (Godlewska et al., 2012). The use of SRIs also alleviated depressive- and anxiety-like behaviors in animal models (Bourke et al., 2014; Hestermann et al., 2014; Liu et al., 2012), possibly through acting directly on the HPA axis (Jensen et al., 1999), or indirectly via the amygdala (Flandreau et al., 2013). Furthermore, a reduction of HR was observed in depressed female cynomolgus monkeys after an 18-month treatment with the SRI sertraline (Groban et al., 2014).
Given previous evidence indicating both behavioral and cardiovascular benefits of SRI pharmacotherapy, the goal of the present study is to investigate the effects of sertraline (Zoloft) on the behavioral, cardiac, and neuronal consequences of disrupted social bonds in the prairie vole model. To this end, male prairie voles were paired with a female partner for 5 days followed by isolation from the female partner for an additional 5 days. Half of the males received sertraline daily for 15 days, while the other half received vehicle (during continued isolation from the female partner). We hypothesized that sertraline would buffer the deleterious changes in behavior, cardiac function, and long-term neural activity in brain regions associated with stress, emotion, and cardiovascular function following the social stress of partner loss.
2. Materials and Methods
2.1. Subjects
Subjects included a total of 23 male prairie voles, each paired with a female partner (total of 23 female partners). All animals used in this protocol were bred in-house by experienced investigators at Northern Illinois University. Offspring were removed from breeding pairs at 21 days of age, and housed in same-sex sibling pairs until the commencement of experimentation (only one animal from each same-sex sibling pair was used for the procedures described here). Animals were allowed ad libitum access to food (Purina Rabbit Chow, Purina, St. Louis, MO) and tap water, maintained at a room temperature of 20–21°C and a relative humidity of 40–50%, under a standard 14:10 light/dark cycle (lights on at 0630). All experimental protocols were approved by the Northern Illinois University Institutional Animal Care and Use Committee and followed National Institute of Health guidelines as stated in the Guide for the Care and Use of Laboratory Animals.
2.2. General experimental design
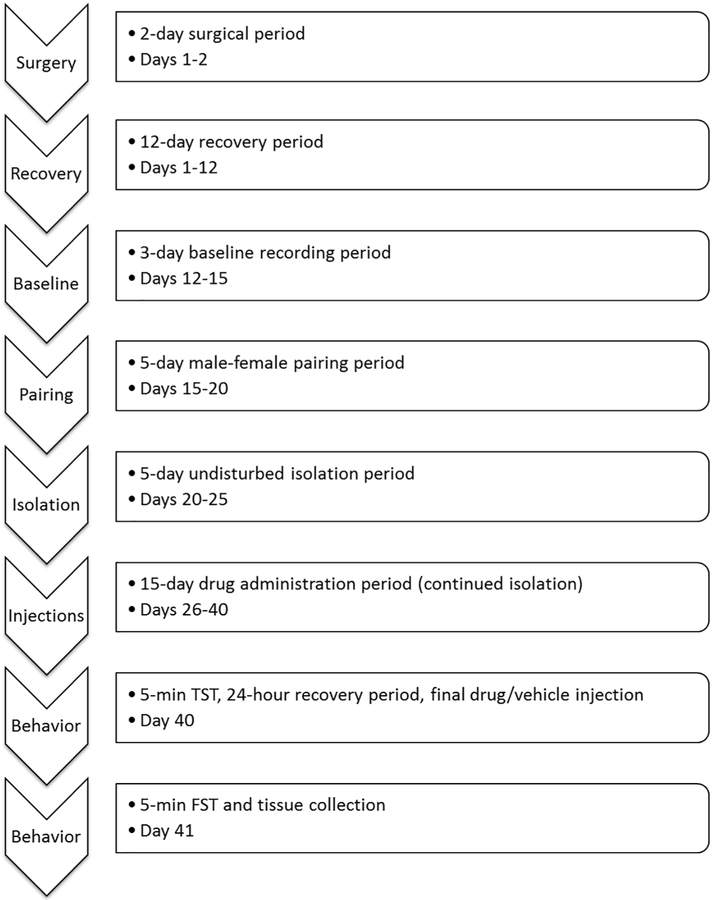
The general experimental design is described here (also see Figure 1), with specific procedures described in the following sections. A radiotelemetry transmitter was implanted into each male prairie vole (2 days). Following a period of surgical recovery (10-12 days) and baseline cardiovascular recordings (3 days), all males were removed from the home cage and housed with an unrelated female (5 days). Following this male-female pairing period, all female partners were removed and the male experimental animals were housed individually, undisturbed, for an additional 5 days. Following this isolation period, the experimental males received either daily sertraline injections (16 mg/kg, ip) or a volume-matched vehicle control injection for 15 days, during continued isolation (20 total days of isolation). On the final day of the drug administration period, all experimental animals underwent a 5-min tail-suspension test (TST), followed by the final sertraline or vehicle injection, and a 22-h recovery period. 24 hours following the TST, all experimental animals underwent a 5-min forced swim test (FST). Ten minutes following the FST, all experimental animals were euthanized, followed by brain extraction.
Figure 1:
Schedule of procedures used in the current protocol. All short-term procedures were conducted during the light period, 3-6 hours following light onset.
2.3. Transmitter implantation
Wireless radiofrequency transmitters (model TA10ETA-F10; Data Sciences International, St. Paul, MN) were implanted intraperitoneally into male prairie voles similar to methods described previously (Grippo et al., 2007a). Animals were anesthetized with a mixture of isoflurane (Henry Schein, Dublin, OH) and oxygen throughout the surgical procedures. Briefly, the body of the transmitter was implanted into the intraperitoneal cavity, and negative and positive leads were tunneled subcutaneously and sutured to the muscle on the exterior of the thorax, to the left and right of the heart. All incisions were closed using sterile suture. Following immediate recovery from anesthesia, animals were housed for 5 days in custom-designed divided cages that allowed limited interactions between the instrumented animal and its respective sibling, while permitting adequate healing of suture wounds (Grippo et al., 2007a). A heat lamp was made available (covering approximately 1/3 of the cage) for the first 2 nights after the surgical procedures, to allow the animal to self-regulate its body temperature. A solution of 2% sucrose was made available to the animal - in addition to food and water – for the first 5 days following the surgical procedures, to ensure adequate fluid intake. All animals were then returned to standard cages (with the respective siblings) to recover for an additional 5-7 days. Animals were assessed for the following characteristics of recovery: (a) visible signs of eating and drinking, (b) adequate urination and defecation, (c) adequate activity level [approximately 2 counts per minute (cpm) or higher; and shifts in activity approximately every 2-3 hours], (d) adequate body temperature (approximately 37.5 °C), and (e) stabilization of HR across the recovery period.
2.4. Quantification of electrocardiographic and activity recordings
Electrocardiographic (ECG) signals were collected via radiotelemetric recordings (sampling rate 5 kHz, 12-bit precision digitizing). A gross measure of activity was monitored via the receiver (sampling rate 256 Hz). The data were recorded at timed intervals throughout all experimental protocols (at hourly intervals during the baseline, pairing, isolation, and drug administration periods; and at 15-minute intervals following the TST), with the exception of during the TST and FST – during which time the data were recorded continuously.
Multiple segments of 1 or 5 min of stable, continuous data were used to evaluate HR, heart rate variability (HRV) and activity level. Multiple 1-minute segments of data were used to evaluate cardiac and activity variables on the final day of each the baseline, pairing, isolation, and drug administration periods; and following the TST. The segments of data resulted in approximately 5-15 minutes of accumulated data for HR, HRV, and activity level for each time point. The entire 5-minute segment of data was used to evaluate cardiac and activity variables during the TST and FST (binned into separate 1-minute segments). Cardiac variables were assessed via ECG signals recorded using the vendor software (Data Sciences International). HR was evaluated using the number of beats per unit time (beats per minute, bpm). HRV was evaluated using two indices: (a) standard deviation of normal-to-normal intervals (SDNN index), which is hypothesized to represent the convergence of sympathetic and parasympathetic efferent signals on the cardiac pacemaker; and (b) root mean square of successive differences between normal heart beats (RMSSD), which is hypothesized to reflect beat-to-beat variance in HR as an estimation of parasympathetically-mediated function (Shaffer & Ginsberg, 2017; Shaffer et al., 2014;Task Force of the European Society of Cardiology & North American Society of Pacing and Electrophysiology, 1996). Activity was assessed with the vendor software (Data Sciences International), and was evaluated using the number of counts per unit time (cpm).
All data segments were manually inspected to ensure that the vendor software appropriately detected each R-wave used to calculate HR, SDNN index, and RMSSD. Manual corrections were made in the case of a missed R-wave or an incorrectly-detected wave [e.g., if the software included a P-wave or a non-wave (noise) in its calculations]. Sections of ECG data that included artifact from animal movement or poor signal-to-noise ratio were excluded from the analyses.
2.5. Baseline measurement period
Following recovery from radiotelemetry transmitter implantation, cardiac and activity data were recorded hourly during a 3-day undisturbed baseline period. Activity levels are high in prairie voles and occur in approximately 2-3-hour ultradian rhythms throughout the light and dark periods (Grippo et al., 2007a). Therefore, resting parameters were derived from cardiac data sampled during a period of minimal activity (5 cpm or lower, during which time the animal may have been sitting, resting quietly, exhibiting minimal movement, or sleeping).
2.6. Male-female pairing period
Following the baseline period, each male animal was separated from its respective sibling and placed in a clean, neutral cage with an unrelated female of approximately the same age and body weight, for 5 days. During this period, male-female prairie vole pairs were allowed to behave naturally within the confines of the laboratory cage. Previous investigations have shown that 5 days is a sufficient time period for male and female prairie voles to form a pair bond (Bosch et al., 2009; Williams et al., 1992), and that social behaviors are normally distributed after the animals are paired (McNeal et al., 2014). ECG and activity data were recorded hourly throughout this period.
2.7. Isolation period
Following the pairing period, each male prairie vole was removed from its female partner and housed in isolation, in a clean, neutral cage, for 5 additional undisturbed days (without visual, olfactory, or auditory cues from the previous female partner). Previous investigations have demonstrated that this time course is sufficient to induce depression-relevant changes in behavior and cardiac function in prairie voles (Bosch et al., 2009; McNeal et al., 2014). ECG and activity data were recorded hourly throughout this period.
2.8. Drug administration period
Five days into the isolation period, each male prairie vole was randomly assigned to one of two groups: sertraline or vehicle. The sertraline group received sertraline hydrochloride (16 mg/kg/day, ip; generously donated by Pfizer Inc., New York, NY) for a total of 15 days (while remaining isolated from the female partner; therefore the total isolation period was 20 days). Sertraline hydrochloride was chosen as the SRI for use in the current study for several reasons, including: (a) its demonstrated efficacy in improving behavioral and cardiovascular outcomes in patients with depression and/or cardiovascular disease and in relevant animal models; (b) its demonstrated efficacy in the context of social isolation; and (c) its potential for superior efficacy over cognitive-behavioral therapy and other SRIs in improving psychological and physiological health outcomes (Berkman et al., 2003; Flament et al., 1999; Glassman et al., 2002; Groban et al., 2014; Papakostas & Fava, 2008; Ramanathan et al., 2003; Shapiro et al., 1999). The drug dose and time course were selected to be consistent with therapeutically-effective dosing in humans and other animal models (Glassman et al., 2002; Sanders-Bush et al., 1989; Sitges et al., 2012; Yildirim et al., 2012). Sertraline was dissolved in distilled water vehicle each day (4mg/ml), and sonicated for at least 5 minutes. The vehicle group was administered distilled water (ip), which was also sonicated daily to ensure consistency of temperature between the sertraline and vehicle solutions. A solution of 2% sucrose was made available to all animals during this period (regardless of group) – in addition to food and water – to minimize body weight loss observed during the administration of sertraline (Nielsen et al., 1992). ECG and activity data were recorded hourly throughout this period.
2.9. Behavioral assessments
General.
Two behavioral tests, the TST and FST, were used to evaluate depression-relevant behaviors in each animal. These tests characterize adaptive coping strategies, including active movements, relative to a maladaptive response of immobility or lack of movement; and are hypothesized to serve as useful operational measures of depression-relevant behaviors in rodents based on previous studies of validity and reliability (Cryan et al., 2005a; Cryan et al., 2005b; Steru et al., 1985). Both the TST and FST have been validated for use in prairie voles, and are suggested to serve as useful operational indices of depression-relevant behaviors and short-term stress-coping behaviors in this model (Bosch et al., 2009; McNeal et al., 2017). However, although these tests both focus on a state of inactivity (immobility), they are not simply dry- and wet-context representations of the same behavioral state (Cryan et al., 2005a). Hence, value may be gained from conducting both behavioral tests in the same paradigm. Preliminary tests of validity, coupled with published studies, indicate that a time period of 24 hours between behavioral tests is sufficient to avoid carry-over effects on the display of behavioral and physiological dependent variables; and that the order in which the tests are presented (when separated by 24 hours) does not influence behavioral, cardiovascular, or other physiological outcome measures for any individual test (Grippo et al., 2008; McNeal et al., 2017). Therefore, all animals in the present study were exposed to these behavioral tests in the same order.
The TST and FST behavioral trials were digitally video-recorded and scored for specific behaviors (as noted in the following sections) by at least 2 experimentally-blind, trained raters. For each behavioral category, raters were required to reach at least 90% inter-rater reliability. If the agreement between 2 raters did not reach 90% inter-rater reliability for a specific behavioral category, a third rater would score the entire file, followed by a discussion among all raters to determine whether there was a systematic reason why the initial raters did not agree, and/or to devise a strategy to improve agreement among the raters (for example, one or both raters might re-score the entire file). For all files, the scores between 2 raters (or among 3 raters, as relevant) were averaged.
Tail-suspension test.
On the final day of the drug injection period, the TST was used to evaluate depression-relevant behaviors in each animal (Steru et al., 1985). The animal was suspended by its tail using adhesive tape attached to a metal bar (5 mm in diameter), and hung in the middle of an opaque plastic box (32 × 28 × 29 cm), approximately 25 cm above the apparatus floor, for 5 minutes. Care was taken to ensure that the animal’s tail was not damaged during placement or removal of the tape. The animal was administered its final drug injection (sertraline or vehicle) following the TST and immediately replaced in the home cage. The apparatus was cleaned with a 10% bleach solution between each trial.
ECG and physical activity data were recorded continuously throughout the behavioral test, and at 15-minute intervals for 22 hours following the test. Trials were digitally video-recorded and scored by experimentally-blind reviewers for the duration of immobility exhibited by each animal (i.e., no movement besides those required for respiration), versus the duration of active behaviors (i.e., active movements characterized by either contortions of the body and/or flailing of the limbs). An increase in the duration of inactivity (i.e., immobility) during this behavioral test is hypothesized to be an operational index of a maladaptive response, relevant to the construct of depression in humans (Cryan et al., 2005a).
Forced swim test.
Twenty-four hours following the TST (and therefore 24 hours following the final drug injection), the FST was used as an additional index of depression-relevant behaviors in each animal (Cryan et al., 2005c). The apparatus was a clear Plexiglas cylinder (20 × 45 cm) filled with 18 cm of 25 ± 1°C tap water. Subjects could neither touch the bottom of the tank nor climb out. Each animal was placed into the tank for 5 minutes. Following the FST, animals were returned to the home cage and allowed access to a heat lamp for 10 minutes. The apparatus was cleaned with a 10% bleach solution between each trial.
ECG and physical activity data were recorded continuously throughout the behavioral test. Trials were digitally video-recorded and scored by experimentally-blind reviewers for the duration of active (i.e., swimming, struggling, or climbing) versus passive (i.e., floating or immobility) behavioral responses. Behaviors were scored according to the following criteria: (a) struggling, defined as movements during which the forelimbs broke the water surface; (b) climbing, defined as movements during which the forelimbs broke the water surface and were in direct contact with the wall of the apparatus; (c) swimming, defined as movements of the fore and hind limbs resulting in directed motion, without breaking the water surface; and (d) floating, defined as idle floating or treading water - the behavior during which the animal used limb movement to maintain its equilibrium without any directed movement of the trunk. An increase in the duration of floating (i.e., immobility) during this behavioral test is hypothesized to represent a maladaptive state of helplessness, relevant to depression in humans (Cryan et al., 2005b; Cryan et al., 2005c).
2.10. Brain extraction
Ten minutes following the end of FST (therefore approximately 24 hours following the final drug injection), each experimental animal was anesthetized with a combination of ketamine (67 mg/kg, sc; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, sc; NLS Animal Health). The animal was anesthetized within 1 minute of being removed from the home cage. Each animal was then euthanized under anesthesia, and brains were removed for the analysis of delta (Δ) FosB-immunoreactivity in the PVN, basolateral amygdala (BLA), and central amygdala (CeA). The brains were processed with a passive perfusion (i.e., spin immersion) technique described previously (Cushing et al., 2003). Briefly, brains were immersed in a fixative solution consisting of 4% paraformaldehyde containing 5% acrolein, and gently agitated for a total of 4 hours. Brains were postfixed for 24 hours in 4% paraformaldehyde, and sunk in 25% sucrose. Tissue was stored in 25% sucrose at 4° C until it was sectioned at 40 μm on a cryostat. Sliced serial brain sections were stored in wells at −20° C, in cryoprotectant antifreeze solution.
2.11. ΔFosB Immunohistochemistry
Serial brain slices (40 μm) were assayed for the expression of ΔFosB as an index of accumulated, long-term neural activation, using standard double-label avidin:biotinylated enzyme complex (ABC) immunohistochemistry (Nestler, 2015). Anti-FosB (Santa Cruz Biotechnology Inc., Santa Cruz, CA; rabbit polyclonal IgG) was used at a concentration of 1:2500, and the target was visualized using nickel-diaminobenzadine (Ni-DAB) dissolved in 0.1 M Tris buffer.
All immunohistochemistry procedures were performed at room temperature, unless otherwise noted. Free-floating sections were rinsed 3 times during a 30-minute period with potassium phosphate-buffered saline (KPBS). Sections were then incubated in 3% hydrogen peroxide for 15 minutes. After 3 washes in KPBS, tissues were incubated in KPBS + 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO) + 3% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 hour. Sections were then incubated in primary antibody for ΔFosB (1:2500) diluted in KPBS + blocking reagent (Roche Diagnostics Co., Mannheim, Germany) + 0.3% Triton X-100 + 1% normal goat serum at 4° C for 72 hours. Following this incubation period, tissues were rinsed 3 times during a 30-minute period with KPBS, and then tissues were incubated in biotinylated secondary antibody (1:200; Vector Laboratories, anti-rabbit polyclonal IgG BA-1000) diluted in KPBS + 0.05% Triton X-100 for 1.5 hours. Sections were washed 3 times in KPBS and then incubated in A/B solution (45 μl A, 45 μl B per 10 ml KPBS + 0.4% Triton X-100; Vectastain Elite PK-6100; Vector Laboratories) for 1 hour. Following this incubation period, tissues were rinsed twice in KPBS and then twice in 0.1 M Tris buffer (pH 7.5). ΔFosB was visualized by incubation in DAB solution with nickel intensification (168 μl buffer stock solution, 200 μl DAB stock solution, 160 μl, H2O2 solution, 120 μl nickel solution per 10 ml distilled water; DAB Peroxidase Substrate Kit SK-4100; Vector Laboratories) for 7 minutes, after which tissues were washed 3 times in KPBS.
Stained sections were mounted on electrostatically-charged slides, air-dried, dehydrated in a series of ethanol dilutions, cleared with Histoclear (National Diagnostics, Atlanta, GA), and then protected with coverslips using Histomount mounting medium (National Diagnostics).
2.12. Quantification of ΔFosB-Immunoreactivity
General procedures.
Images were captured using a Nikon Eclipse E 800 microscope, Sensi-cam camera and IPLab software (Scanalytics, Inc., Fairfax, VA). For all brain regions, the density of ΔFosB-immunoreactive cell bodies was determined with a 10x objective; cells were considered to be ΔFosB-positive cells if they exhibited black and round/oval shape characteristics demonstrated in previous studies (Garcia-Perez et al., 2012; Nishijima et al., 2013). Using ImageJ (National Institutes of Health, Bethesda, MD), ΔFosB immunoreactivity was determined with a mean optical density method similar to previous studies (Watanasriyakul et al., 2018). Background density readings were obtained from the corpus callosum of each animal and were subtracted from the density readings of the other brain regions to determine the final ΔFosB expression. The optical density values were relative to the gray values associated with the sample area (white: 0; black: 255). Density measurements for each brain region were taken from sections matched in rostral-caudal orientation to minimize variability, according to measurement coordinates listed below for each region, using information from Paxinos and Watson (Paxinos & Watson, 2005). Three to four brain sections were analyzed from each animal, and results were averaged across sections. Density measurement was performed separately for each hemisphere, and the results were averaged between hemispheres. For all animals, density measures were conducted by two trained, experimentally-blind raters, and the results were averaged between raters. Therefore, density measures were averaged across multiple brain slices, hemispheres, and raters. Damaged sections were excluded from the analyses.
Paraventricular Nucleus.
The location of the PVN was determined to be approximately Bregma − 1.56 to −1.80 mm. The PVN was further characterized by the medial-lateral position of the fornix (relative to the third ventricle) and medial and dorsal location of the optic tract (relative to more central and ventral position in more rostral sections). In ImageJ, a standardized oval shape (area: 16,700 pixels per hemisphere) was used to determine the density reading in the PVN.
Basolateral Amygdala.
The location of the BLA was approximately Bregma −2.64 to −3.00 mm, located adjacent to the external capsule, in a medial fashion. An average reading of 3 standardized squares (area: 2500 pixels) was used to determine the density reading in the BLA.
Central Amygdala.
The location of the CeA was approximately Bregma −2.64 to −3.00 mm, located medially between the BLA and the optic tract. An average reading of 3 standardized squares (area: 2500 pixels) was used to determine the density reading in the CeA.
Corpus Callosum.
The location of the corpus callosum was approximately Bregma −2.64 to −3.00 mm, and specifically the immediate area dorsal to the hippocampus and ventral to the retrosplenial dysgranular cortex. An average reading of 3 standardized squares (area: 2500 pixels) was used to determine the density reading in the corpus callosum. Corpus callosum density values were used as an indicator of background staining, and were subtracted from the density readings in the PVN, BLA, and CeA.
2.13. Data analyses
All data are presented as means ± standard error of the mean (SEM) for the analyses and figures. The data were analyzed with mixed-design analyses of variance (ANOVA) and Student's t-tests, using a Bonferroni correction for all multiple comparisons, as detailed in the following paragraphs. All tests were based on normally distributed data. A probability value of p < 0.05 was considered to be statistically significant, and effect sizes for significant pairwise comparisons are reported with Cohen’s d values for all analyses described below. Any ECG segments involving artifact from animal movement or poor signal-to-noise ratio were excluded from the analyses.
For analyses based on radiotelemetry recordings, basal measures of HR, SDNN index, RMSSD, and physical activity were assessed on the final day of the baseline, pairing, and isolation periods, and on days 5, 10, and 14 of the drug administration period, using mixed design ANOVAs with time (phase of the experiment) as the repeated factor and group (sertraline or vehicle administration) as the independent factor. Pairwise comparisons were conducted using repeated-measures (for within-group comparisons) or independent-groups Student’s t-tests (for between-group comparisons), with a Bonferroni correction applied to all multiple comparisons. All variables were analyzed using the mean of multiple segments of data recorded hourly. Data that represent the resting baseline state of HR, SDNN index, and RMSSD are inclusive of only ECG segments during periods of low activity (5 cpm or lower). Data that represent all other time points of ECG data are irrespective of activity level, and therefore may include a combination of low, medium, or high levels of activity.
For analyses conducted during the behavioral assessments, ECG, activity, and behavioral data were recorded continuously throughout the 5-minute behavioral test periods. Mean HR, SDNN index, RMSSD, physical activity, and behavioral data were assessed across the 5-minute TST and FST, using repeated-measures (for within-group comparisons) or independent-groups Student’s t-tests (for between-group comparisons), with a Bonferroni correction applied to all multiple comparisons. Specific behaviors during the TST and FST were categorized manually by at least 2 trained observers who were blind to the experimental conditions. Each digital video-recording was imported into coding and analysis software (Noldus Observer XT8.0, Noldus Information Technology, Wageningen, Netherlands). Observers were trained to produce an inter-rater reliability of at least 90% for all behavioral categories. If the initial agreement between 2 raters did not reach 90% for a behavioral category, a third rater scored the behavioral trial. Therefore, for any behavioral trial, the scores of at least 2 raters were averaged to provide a final value.
For analyses conducted following the TST, HR, SDNN index, RMSSD, and physical activity data were analyzed for 22 consecutive hours following the test, using mixed-design ANOVAs with time (hour following the TST) as the repeated factor and group (sertraline or vehicle administration) as the independent factor. Pairwise comparisons were conducted using repeated-measures (for within-group comparisons) or independent-groups Student’s t-tests (for between-group comparisons), with a Bonferroni correction applied to all multiple comparisons.
Given some unexpected findings in HR responses, that were opposite to the hypotheses of this study (as detailed below in Results), exploratory post hoc analyses were also conducted on ECG and activity data at hourly intervals on days 5, 10, and 14 of the drug administration period. These analyses were conducted to assess short-term responses following the injections, using mixed-design ANOVAs with time (hour of the day, inclusive of the 24-hour period on day 5, 10, and 14) as the repeated factor and group (sertraline or vehicle administration) as the independent factor. Pairwise comparisons were conducted using independent-groups Student’s t-tests for between-group comparisons.
3. Results
3.1. Basal cardiac function and physical activity
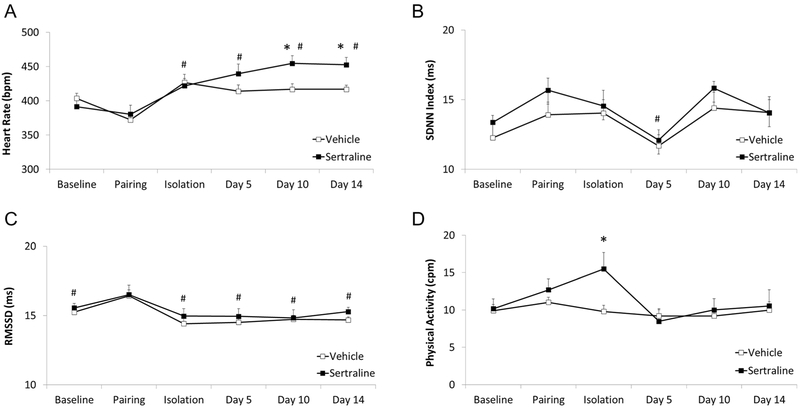
Pairing significantly increased RMSSD in both groups of prairie voles, relative to RMSSD during all other time points of the experiment. Social isolation significantly increased HR in both groups of prairie voles, relative to HR during the pairing period. Administration of sertraline led to an elevated long-term HR versus administration of vehicle, but did not significantly influence SDNN index or RMSSD. Administration of sertraline led to a slightly elevated physical activity level on the 5th day of the administration period, but did not influence activity at any other time point.
The mixed-design ANOVA for HR (Figure 2A) yielded a significant main effect of time [F(5,132) = 10.21, p = 0.001], but no main effect of group [F(1,132) = 0.32, p = 0.581], and no interaction between group and time [F(5,132) = 0.91, p = 0.481]. Using Student’s t-tests with a Bonferroni correction for multiple comparisons (such that 0.01 is the critical p value): (a) both groups exhibited a significantly higher HR during the isolation period, relative to the pairing period [t(22) = 6.93, p = 0.0001, Cohen's d = 1.27; paired t-test with sertraline and vehicle groups pooled]; and (b) both groups maintained an elevated HR on days 5, 10, and 14 of the injection period, relative to their respective pairing period HR [sertraline, day 5: t(10) = 2.97, p = 0.007; Cohen’s d = 1.31; sertraline, day 10: t(10) = 3.92, p = 0.001, Cohen’s d = 1.85; sertraline, day 14: t(10) = 3.48, p = 0.003, Cohen’s d = 1.84; vehicle, day 5: t(11) = 4.94, p = 0.0004, Cohen’s d = 1.34; vehicle, day 10: t(11) = 5.10, p = 0.0002, Cohen’s d = 1.58; vehicle, day 14: t(11) = 5.89, p = 0.0001, Cohen’s d = 1.71]; but (c) the sertraline group exhibited a significantly higher HR than the vehicle group on both days 10 [t(21) = 2.77, p = 0.006, Cohen’s d = 1.15] and 14 t(21) = 2.91, p = 0.004, Cohen’s d = 1.22] of the drug administration period.
Figure 2.
Mean (+ SEM) HR (Panel A), SDNN index (Panel B), RMSSD (Panel C), and physical activity (Panel D) of male prairie voles at baseline (pre-manipulation), and following pairing, isolation, and 5, 10, and 14 days of either sertraline (16 mg/kg/day) or vehicle administration. The data points represent multiple segments of ECG and activity data collected at hourly intervals on the final day of each experimental phase. All ECG segments were manually inspected for accuracy of R-wave detection. Manual corrections were made for incorrect or missed detection of R-waves; and data with artifact due to animal movement or poor signal-to-noise ratio were excluded. # = Significant difference relative to respective pairing period value in both groups; * = Significant difference between sertraline- and vehicle-treated group at the same time point.
The mixed-design ANOVA for SDNN index (Figure 2B) yielded a significant main effect of time [F(5,132) = 5.37, p = 0.001], but no main effect for group [F(1,132) = 0.74, p = 0.400], and no interaction between group and time [F(5,132) = 1.19, p = 0.319]. Using Student’s t-tests with a Bonferroni correction for multiple comparisons (such that 0.0125 is the critical p value), calculated on pooled sertraline- and vehicle-treated values: (a) both groups displayed a lower SDNN index on day 5 of the drug administration period, relative to the pairing period [t(22) = 3.27, p = 0.002, Cohen’s d = 0.57]; but (b) the groups did not display significantly different SDNN index values during the isolation period [t(22) = 0.56, p = 0.29], or days 10 [t(22) = 0.25, p = 0.40] or 14 [t(22) = 0.86, p = 0.20] of the drug administration period, relative to the pairing period.
The mixed-design ANOVA for RMSSD (Figure 2C) yielded a significant main effect of time [F(5,132) = 5.52, p = 0.0001], but no main effect for group [F(1,132) = 2.11, p = 0.15], and no interaction between group and time [F(5,132) = 0.14, p = 0.98]. Using Student’s t-tests with a Bonferroni correction for multiple comparisons (such that 0.01 is the critical p value), calculated on pooled sertraline- and vehicle-treated values, RMSSD during the pairing period was significantly higher than during: (a) the baseline period [t(22) = 4.21, p = 0.0002, Cohen’s d = 0.74]; (b) the isolation period [t(22) = 11.17, p = −0.0001, Cohen’s d = 1.70]; (c) day 5 of the drug injection period [t(22) = 6.49, p = 0.0001, Cohen’s d = 1.09]; (d) day 10 of the drug injection period t(22) = 5.44, p = 0.0001, Cohen’s d = 1.05]; and (e) day 14 of the drug injection period [t(22) = 4.56, p = 0.0001, Cohen’s d = 1.03].
The mixed-design ANOVA for physical activity (Figure 2D) yielded a significant main effect of time [F(5,132) = 5.48, p = 0.001], and a significant interaction between group and time [F(5,132) = 3.30, p = 0.008], but no main effect for group [F(1,132) = 0.815, p = 0.377]. The sertraline group displayed a higher physical activity level than the vehicle group during the isolation period [t(21) = 2.43, p = 0.01, Cohen’s d = 1.11]. No additional follow-up tests were conducted.
3.2. Behavioral tests
Tail suspension test.
Administration of sertraline did not significantly influence behavioral or cardiac variables during the TST, relative to administration of vehicle (Table 1, left).
Table 1.
Mean (± SEM) cardiac and behavioral responses across the 5-minute TST and FST in male prairie voles, as a function of social isolation and either sertraline (16 mg/kg/day) or vehicle administration.
| TST | FST | |||||||
|---|---|---|---|---|---|---|---|---|
| HR (bpm) | SDNN (ms) | RMSSD (ms) | Immobility (sec) | HR (bpm) | SDNN (ms) | RMSSD (ms) | Immobility (sec) | |
| Vehicle | 596.6 ± 20.1 | 20.2 ± 4.7 | 11.0 ± 0.4 | 102.4 ± 18.3 | 451.3 ± 9.0 | 19.7 ± 4.3 | 14.3 ± 0.4 | 81.5 ± 13.3 |
| Sertraline | 546.9 ± 29.8 | 23.6 ± 3.6 | 12.2 ± 0.8 | 90.8 ± 20.7 | 476.5 ± 15.9 | 14.8 ± 1.8 | 13.3 ± 0.5 | 110.9 ± 19.8 |
Independent-groups Student’s t-tests indicated that there were no significant differences between vehicle- and sertraline-treated groups across the 5-minute TST in: (a) mean HR [t(20) = 1.568, p = 0.113]; (b) mean SDNN index [t(20) = 1.57, p = 0.113]; (c) mean RMSSD [t(21) = 1.49, p = 0.08]; (d) mean physical activity level [t(16) = 0.34, p = 0.472]; or (e) manually-scored mean immobility duration [t(16) = 0.41, p = 0.689]. No follow-up tests were conducted.
Forced swim test.
Administration of sertraline did not significantly influence behavioral or cardiac variables during the FST, relative to administration of vehicle (Table 1, right).
Independent-groups Student’s t-tests indicated that there were no significant differences between vehicle- and sertraline-treated groups across the 5-minute FST in: (a) mean HR [t(20) = −1.38, p = 0.183]; (b) mean SDNN index [t(20) = 1.06, p = 0.303]; (c) mean RMSSD [t(21) = 1.58, p = 0.07]; mean physical activity [t(20) = 0.22, p = 0.832]; or manually-scored mean immobility duration [t(20) = 1.26, p = 0.221]. No follow-up tests were conducted.
3.3. Stressor recovery
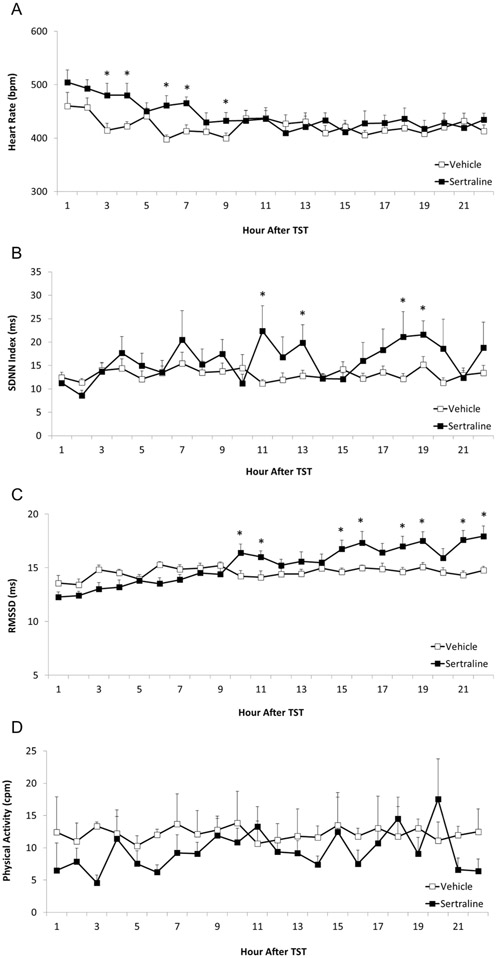
Administration of sertraline (relative to vehicle administration) was associated with a higher HR during the early portion of the recovery period after the TST; but it also was associated with higher SDNN index and RMSSD in the later portion of the recovery period. There was no between-group difference in physical activity during the TST recovery period.
The mixed-design ANOVA for HR during the TST recovery period (Figure 3A) yielded a significant main effect for time [F(21,484) = 3.12, p = 0.001], and an interaction between group and time [F(21,484) = 1.584, p = 0.050], but no main effect for group [F(1,484) = 0.97, p = 0.340]. Sertraline administration (relative to vehicle) was associated with a significantly higher HR at the following time points after the TST: (a) hour 3 [t(21) = 2.56, p = 0.009, Cohen’s d = 1.04]; (b) hour 4 [t(21) = 2.73, p = 0.006, Cohen’s d = 1.09]; (c) hour 6 [t(21) = 3.25, p = 0.002, Cohen’s d = 1.34; (d) hour 7 [t(21) = 3.27, p = 0.002, Cohen’s d = 1.39; and (e) hour 9 [t(21) = 1.83, p = 0.04, Cohen’s d = 0.74].
Figure 3.
Mean (+ SEM) HR (Panel A), SDNN index (Panel B), RMSSD (Panel C), and physical activity (Panel D) of male prairie voles following exposure to the TST, as a function of social isolation and either sertraline (16 mg/kg/day) or vehicle administration. The data points represent multiple segments of ECG and activity data collected at hourly intervals for 22 consecutive hours following the end of the TST. All ECG segments were manually inspected for accuracy of R-wave detection. Manual corrections were made for incorrect or missed detection of R-waves; and data with artifact due to animal movement or poor signal-to-noise ratio were excluded. * = Significant difference between sertraline- and vehicle-treated group at the same time point.
The mixed-design ANOVA for SDNN index (Figure 3B) yielded a significant main effect for time [F(21,484) = 2.541, p = 0.001], but no main effect for group [F(1,484) = 0.78, p = 0.391], and no interaction between group and time [F(21,484) = 1.291, p = 0.178]. Sertraline administration (relative to vehicle) was associated with a higher SDNN index at the following time points after the TST: (a) hour 11 t[21) = 2.13, p = 0.02, Cohen’s d = 0.85; (b) hour 13 [t(21) = 1.95, p = 0.03, Cohen’s d = 0.78; (c) hour 18 [t(21) = 1.70, p = 0.05, Cohen’s d = 0.69; and (d) hour 19 [t(21) = 1.91, p = 0.03, Cohen’s d = 0.85].
The mixed-design ANOVA for RMSSD (Figure 3C) yielded a significant main effect for time [F(21,484) = 6.01, p = 0.0001], a significant main effect for group [F(1,484) = 17.01, p = 0.0001], and asignificant group by time interaction [F(21,484) = 4.29, p = 0.0001]. Sertraline administration (relative to vehicle) was associated with a higher RMSSD at the following time points after the TST: (a) hour 10 [t(21) = 2.29, p = 0.02, Cohen’s d = 0.92]; (b) hour 11 [t(21) = 2.32, p = 0.02, Cohen’s d = 0.95]; (c) hour 15 [t(21) = 2.47, p = 0.01, Cohen’s d = 1.04]; (d) hour 16 [t(21) = 2.25, p = 0.02, Cohen’s d = 0.89]; (e) hour 18 [t(21) = 2.40, p = 0.01, Cohen’s d = 1.00]; (f) hour 19 [t(21) = 2.59, p = 0.009, Cohen’s d = 1.07]; (g) hour 21 [t(21) = 3.52, p = 0.001, Cohen’s d = 1.45]; and (h) hour 22 [t(21) = 3.23, p = 0.002, Cohen’s d= 1.30].
The mixed-design ANOVA for physical activity (Figure 3D) yielded no main effect for group [F(1,484) = 0.12, p = 0.741] or time [F(21,484) = 0.82, p = 0.693], and no group by time interaction [F(21,484) = 0.55, p = 0.945]. No follow-up tests were conducted.
3.4. ΔFosB immunoreactivity
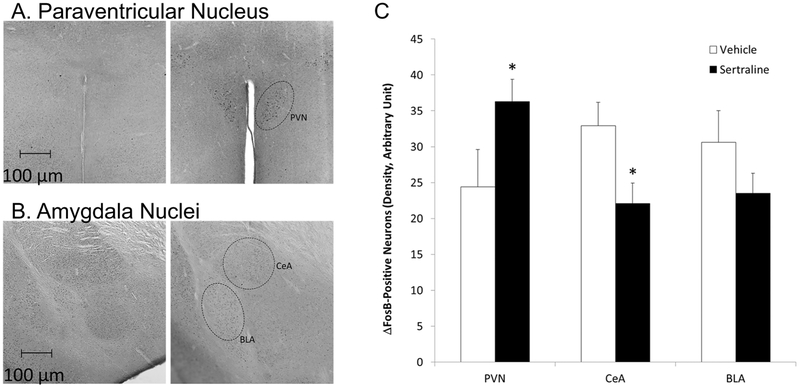
Administration of sertraline (versus vehicle) was associated with greater ΔFosB immunoreactivity in the PVN, lower immunoreactivity in the CeA, and no significant change in immunoreactivity in the BLA (Figure 4).
Figure 4.
Images, captured at 10x magnification, showing raw ΔFosB immunoreactivity in the PVN (Panel A) and amygdala nuclei (CeA and BLA; Panel B) in a representative male isolated prairie vole as a function of vehicle (left 2 images) or sertraline administration (16 mg/kg/day; right 2 images); and mean (+ SEM) ΔFosB immunoreactivity in the PVN, CeA, and BLA in each group (Panel C). * = Significant difference between sertraline- and vehicle-treated group.
An independent-groups Student’s t-test indicated that the sertraline group (versus vehicle) exhibited a significantly higher level of ΔFosB immunoreactivity in the PVN [t(17) = 1.86, p = 0.04, Cohen’s d = 0.88]; a significantly lower level of ΔFosB immunoreactivity in the CeA [t(17) = 2.23, p = 0.02; Cohen’s d = 0.82]; but no significant change in AFosBΔ immunoreactivity in the BLA [t(18) = 1.36, P = 0.1].
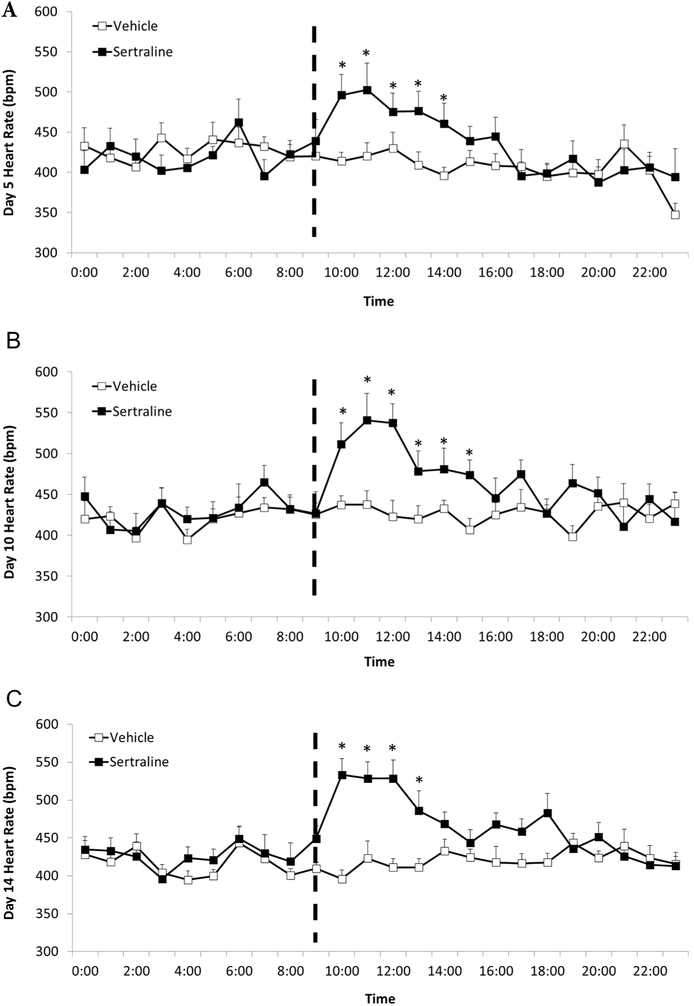
3.5. Exploratory post-injection heart rate and activity changes
Post hoc exploratory analyses were conducted to further characterize the unexpected elevation in HR in the sertraline group. Changes in HR and physical activity were investigated at hourly increments on the 5th, 10th, and 14th days of the drug injection period. Area under the curve was calculated for the 24 data from each animal, followed by independent-groups Student’s t-tests to determine specific pairwise comparisons. The sertraline group displayed a higher HR versus the vehicle group following the drug injections on days 5, 10, and 14, which was not associated with a change in physical activity.
The mixed-design ANOVA for mean daily area under the curve HR on the 5th (Figure 5A), 10th (Figure 5B), and 14th days (Figure 5C) of the drug injection period yielded a significant main effect for group [F(1, 84) = 12.60, p = 0.002], and a significant interaction between group and time [F(4,84) = 3.39, p = 0.03], but no main effect for time [F(4,84) = 1.97, p = 0.15]. Independent-groups Student’s t-tests confirmed that the sertraline group exhibited a significantly higher HR than the vehicle group for: (a) the first 5 hours following the drug injections on day 5 [hour 1: t(21) = 4.10, p = 0.0001, Cohen’s d = 1.69; hour 2: t(21) = 3.06, p= 0.003, Cohen’s d = 1.26; hour 3: t(21) = 1.97, p = 0.03, Cohen’s d = 0.82; hour 4: t(21) = 2.57, p = 0.009, Cohen’s d = 1.12; hour 5: t(21) = 2.16, p = 0.02; Cohen’s d = 0.89]; (b) the first 6 hours following the drug injections on day 10 [hour 1: t(21) = 4.69, p = 0.0001, Cohen’s d = 1.94; hour 2: t(21) = 4.93, p = 0.0001, Cohen’s d = 2.08; hour 3: t(21) = 3.96, p = 0.0004, Cohen’s d = 1.66; hour 4: t(21) = 3.00, p = 0.004, Cohen’s d = 1.23; hour 5: t(21) = 2.40, p = 0.01; Cohen’s d = 1.00; hour 6: t(21) = 2.27, p = 0.02, Cohen’s d = 0.95]; and (c) the first 4 hours following drug injections on day 14 [hour 1: t(21) = 4.46, p = 0.0001, Cohen’s d = 1.71; hour 2: t(21) = 5.71, p= 0.0001, Cohen’s d = 2.35; hour 3: t(21) = 3.79, p = 0.0005, Cohen’s d = 1.55; hour 4: t(21) = 3.67, p = 0.0007, Cohen’s d = 1.51].
Figure 5.
Mean (+ SEM) hourly HR of male prairie voles on days 5 (Panel A), 10 (Panel B), and 14 (Panel C) of the drug injection period in order to explore the unexpected observation of increased long-term HR in sertraline-treated animals. The data points represent multiple segments of ECG data collected at hourly intervals for 24 consecutive hours on each day. All ECG segments were manually inspected for accuracy of R-wave detection. Manual corrections were made for incorrect or missed detection of R-waves; and data with artifact due to animal movement or poor signal-to-noise ratio were excluded. The dashed line indicates injection time of either sertraline (16 mg/kg/day) or vehicle. * = Significant difference between sertraline- and vehicle-treated group at the same time point.
The mixed-design ANOVA for physical activity across the exploratory post-injection time period yielded no significant main effect of group [F(1,84) = 0.15, p = 0.699] or time [F(4,84) = 2.31, p = 0.065], and no group by time interaction [F(4,84) = 1.28, p = 0.286]. No follow-up tests were conducted (data not shown).
4. Conclusions
The present study investigated whether treatment with the antidepressant drug sertraline would buffer negative consequences in behavior, cardiac function, and long-term neural activity following prolonged social stress. The current experiment extends previous work demonstrating that isolation of a male prairie vole from its female partner for 5 days induced cardiac dysfunction, depression-relevant behavior, and exaggerated stress reactivity (Bosch et al., 2009; McNeal et al., 2014). Similarly, the current study employed a 5-day isolation period before the administration of sertraline for two primary reasons. First, previous evidence indicates that 5 days of social isolation in prairie voles is sufficient to produce changes in depression-relevant behaviors, autonomic regulation of the heart, and neuroendocrine activation (Bosch et al., 2009; McNeal et al., 2017; McNeal et al., 2014). Second, this study design allowed for the ability to investigate the potential protective effects of sertraline over a time course that mirrors events in human conditions – that is, seeking treatment after a problem has surfaced. The present results indicate that sertraline administration was partially, but not completely, effective at protecting against negative consequences of social stress. Contrary to the initial hypotheses, sertraline did not prevent depression-relevant behaviors in two operational measures of depression (FST and TST); and it was associated with an unexpected short- and long-term increase in HR (versus vehicle). However, sertraline-treated animals displayed changes in ΔFosB expression in brain regions that regulate behavior and cardiovascular function, consistent with improved neurobiological stress regulation. This alteration in long-term neural activity, combined with the observation of partially improved HRV recovery following the TST (including improved parasympathetic regulation of the heart), indicates that sertraline administration may yield limited improvements in neurobiological functions and cardiac recovery in prairie voles following the disruption of a social bond.
Consistent with previous findings (McNeal et al., 2014), the current results indicate that shortterm social isolation from an opposite-sex partner induces an increase in resting HR. But surprisingly, sertraline was associated with an increase, rather than a decrease, in HR in the present study. Relative to the vehicle-treated group, an increase in HR in the sertraline group was observed in several contexts: (a) in the short-term responses, immediately after the drug injections; (b) in the long-term responses, on days 5, 10, and 14 of the drug injection period; and (c) following a behavioral stressor, including for approximately the first 9 hours after the TST. Increased HR responses in the sertraline-treated group were not a function of changes in locomotor activity, nor were they a function of ECG artifact. The unexpected observation of increased HR is in contrast to similar time courses of sertraline administration in humans and other animal models. For instance, in healthy humans, 14 days of sertraline administration (50 mg) was associated with a decrease in HR and other measures of basal sympathetic nervous system activity (e.g., skin conductance) (Siepmann et al., 2003). Further, in rats, 14 days of sertraline administration (10 mg/kg) following an experimentally-induced myocardial infarction was associated with decreased limbic system apoptosis and increased active behavioral responses to stress (Wann et al., 2009). However, a variety of administration protocols using fluoxetine in rat models have yielded inconsistent reports, with some studies observing either minimal or no cardiovascular influences of various time courses of SRI administration (Grippo et al., 2006; Moffitt & Johnson, 2004), while others observed increases in HR and blood pressure (Almeida et al., 2015; Crestani et al., 2011).
The results of the current study, coupled with the unknown antidepressant actions of sertraline in prairie voles, highlight the need to further explore the pharmacological actions of sertraline in this rodent model. For example, it is possible that the dose or time course of sertraline used here was not an ideal choice for this species. It is also possible that the a priori choice of administering sertraline after 5 days of social isolation did not allow for the drug to remediate behavioral and cardiac reactivity; however if the drug had been administered at the beginning of the isolation period it may have been able to prevent some of these consequences. Although sertraline has not been studied previously in the context of social stress in the prairie vole model, fluoxetine has been observed to influence prairie vole pup retrieval latencies, indicating that SRIs indeed result in behavioral modifications in this species (Villalba et al., 1997). Sertraline may influence prairie vole behavior and physiology differently than in other animal or human models; or behavioral and cardiovascular benefits may be observed following a higher dose, longer time course, or altered time course of administration.
Although behavioral stress responses and HR were not improved in the sertraline-treated group in the present study, longer-term HRV recovery was improved relative to vehicle treatment. Hourly assessments of cardiac variables following the TST indicate that the sertraline group displayed a delayed recovery of HR, yet subsequently displayed a higher SDNN index and RMSSD in the later stages of recovery, relative to the vehicle-treated group (e.g., Figure 3). The group differences in SDNN index and RMSSD during the TST recovery period were not a function of differences in locomotor activity or ECG artifact. Mechanistically, the improvement in HRV recovery could be achieved by an enhancement in parasympathetic cardiac control, a reduction in sympathetic tone, or both (Grippo et al., 2007b; Lewis et al., 2017; Roos et al., 2017). Supporting this theory, sertraline administration has been shown to stimulate gastric acid secretion via a vagal pathway in anesthetized rats (Abdel Salam, 2004). This theory could also explain findings from human studies indicating that sertraline use may improve mortality risk in patients experiencing negative mood states and cardiovascular disease (Berkman et al., 2003; Glassman et al., 2002; Roose et al., 1998; Shapiro et al., 1999). Some of these investigations have linked sertraline treatment with improvements in low and ultra-low frequency HRV power (Glassman et al., 2007; Glassman et al., 2002). The analysis of RMSSD in the current study indicates the possibility that parasympathetic mechanisms may underlie the recovery from a short-term stressor (e.g., Figure 3C). Importantly, decreased HRV is associated with increased mortality (Carney et al., 2001; Carney et al., 2002; Jiang et al., 2008; O'Connor et al., 2010; Shapiro et al., 1999). Therefore, elucidating the mechanisms through which pharmacological treatment can improve this biomarker has important implications for improving human health.
Coupled with the improvement in HRV recovery following a behavioral stressor, sertraline administration also altered neuronal activity in limbic regions involved in stress reactivity and cardiovascular regulation, including the PVN and CeA. The central autonomic network (CAN) is theorized to exert control over visceromotor, neuroendocrine, and behavioral responses needed for goal-directed behavior, adaptability, and health (Thayer & Brosschot, 2005). This network includes the anterior cingulate, insular, orbitofrontal/ventromedial cortices, CeA, PVN, other nuclei of the hypothalamus, periaqueductal gray, parabrachial nucleus, nucleus tractus solitarius (NTS), regions of the ventromedial medulla, and medullary tegmental field (Thayer & Brosschot, 2005). In general, the CAN receives top-down modulatory input from the prefrontal cortex (Thayer & Brosschot, 2005; Thayer & Lane, 2009) and then coordinates sympathetic and parasympathetic reflexes (McKlveen et al., 2015). Stress is hypothesized to bias this network towards autonomic imbalance which is characterized by increased sympathetic and/or decreased parasympathetic tone. One possible driving mechanism for unbalancing the system involves decreased medial prefrontal cortex GABAergic input, resulting in disinhibition of the CeA and brainstem communication (McKlveen et al., 2015; Thayer et al., 2012; Thayer & Lane, 2009). These changes may influence cardiac rate and rhythm disturbances.
Thayer et al. (Thayer & Brosschot, 2005) postulate that activating the CeA (via reductions in prefrontal inhibition) may result in increased HR and decreased HRV. This may be accomplished through three different routes: (a) increasing net sympathetic activity by reducing the inhibition from the tonically active neurons in the caudal ventrolateral medulla (CVLM), which influence the disinhibition of tonically active sympathoexcitatory neurons of the rostral ventrolateral medulla (RVLM); (b) decreasing parasympathetic activity by inhibiting NTS neurons, such that the tonically active nucleus ambiguus and dorsal vagal motor nucleus are inhibited; and (c) increasing sympathetic activity by directly activating sympathoexcitatory RVLM neurons (McKlveen et al., 2015; Thayer et al., 2012; Thayer & Lane, 2009). The PVN is also implicated in the control of cardiac and renal sympathetic nerve activity (Ramchandra et al., 2013). Current evidence suggests the majority of this influence is mediated through the modulation of NTS output to other regions (Kawabe et al., 2008); however, the PVN also directly innervates the RVLM, dorsal vagal motor nucleus, and nucleus ambiguus (Badoer, 2001, 2010; Geerling et al., 2010). The PVN modulates neuronal functions through a variety of hormones and neurotransmitters, including for instance: (a) oxytocin input to the NTS to influence bradycardia (Michelini, 2007); (b) tonically active glutamatergic input to the medial NTS that restrains exaggerated pressor responses (Michelini, 2007); and (c) cardiopulmonary (i.e., increased blood volume) feedback suppression of renal sympathetic nerve activity (Badoer, 2001). Importantly, the CAN components are themselves targeted by serotoninergic projections that modulate their activity.
Therefore, in the context of serotonergic interactions with the CAN, the current findings inform our understanding of how sertraline may beneficially influence some aspects of cardiac neurobiological control in socially isolated animals. As described above, the PVN and CeA play pivotal roles in modulating cardiac activity. Sertraline administration was associated with decreased CeA and increased PVN activity, which may consequently reduce excitatory input to the sympathetic nervous system and/or enhance tonic parasympathetic cardioregulatory control. These functional changes may enable improved long-term recovery from acute stressors, such as the improvement in HRV recovery observed in the sertraline-treated group following the TST. In support of this hypothesis, sertraline treatment was associated with significant improvements in cardiovascular indicators (e.g., improved flow-mediated dilation and indicators of atherosclerosis), versus placebo, in patients with depression (Sherwood et al., 2016).
In conclusion, contrary to our initial hypotheses, sertraline was partially (but not completely) effective in protecting against some consequences of social stress in male prairie voles. Although sertraline did not have behavioral antidepressant properties and was associated with unexpected increases in HR, it showed some efficacy in improving longer-term HRV recovery after a stressor, including improved parasympathetic regulation of the heart. Also, measures of long-term neural activity indicate that CAN regions involved in the modulation of autonomic outflow displayed altered function to a state consistent with improved HRV control in sertraline-treated prairie voles. However, the mechanism(s) through which sertraline administration produced increased short- and long-term HR responses following social isolation remain to be determined. It is possible that alterations in the experimental procedures will yield observable improvements in behavioral and cardiovascular measures, such as investigation of an altered time course or higher dose of sertraline, or exploration of different SRIs. Given the complexity of serotonergic innervation to many brain structures – including communication with central sympathetic and parasympathetic structures (Dergacheva et al., 2014; Deuchars & Lall, 2015) – evaluating additional central mechanisms such as hindbrain, brainstem, and spinal structures responsible for autonomic efferent control, will also improve our understanding of the potential benefits of SRI therapy in the context of social experiences. The current study therefore provides a foundation for improving healthcare practices for individuals who experience social stress.
Highlights.
Social stressors contribute to emotional and autonomic dysfunction
The prairie vole is useful model to study the effects of social stressors
Sertraline improved heart rate variability recovery after stress in isolated voles
Sertraline’s benefits may involve changes in central autonomic network regions
5. Acknowledgements
The authors would like to thank Sarah Ciosek, William Colburn, Miranda Cox, Nicole Holzapfel, Melissa-Ann Scotti, Sarah Sujet, and Matthew Woodbury for valuable assistance.
6. Funding
This research was supported in part by the National Institutes of Health (HL112350); and the generous donation of sertraline hydrochloride from Pfizer, Inc. through the Compound Transfer Program (WI184247).
This work was supported in part by the National Institutes of Health (HL112350). Sertraline was generously donated by Pfizer Inc. via the Compound Transfer Program (WI184247). The funding agencies had no further role in the design of the experiments, data collection, analyses, interpretation, decision to publish, or preparation of the manuscript.
7. Role of the Funding Sources
The funding agencies had no further role in the design of the experiments, data collection, analyses, interpretation, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors declare no conflicts of interest.
9. Literature Cited
- Abdel Salam OM. (2004). Fluoxetine and sertraline stimulate gastric acid secretion via a vagal pathway in anaesthetised rats. Pharmacological Research 50(3):309–316. [DOI] [PubMed] [Google Scholar]
- Almeida J, Duarte JO, Oliveira LA, Crestani CC. (2015). Effects of nitric oxide synthesis inhibitor or fluoxetine treatment on depression-like state and cardiovascular changes induced by chronic variable stress in rats. Stress 18(4):462–474. [DOI] [PubMed] [Google Scholar]
- Andrews MH, Matthews SG. (2004). Programming of the hypothalamo-pituitary-adrenal axis: serotonergic involvement. Stress 7(1): 15–27. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. (2003). A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. Journal of Neuroscience 23(8):3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoer E (2001). Hypothalamic paraventricular nucleus and cardiovascular regulation. Clinical and Experimental Pharmacology and Physiology 28:95–99. [DOI] [PubMed] [Google Scholar]
- Badoer E (2010). Role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure. American Journal of Physiology - Renal Physiology 298(4):F839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. (2006). Effects of stress on parental care are sexually dimorphic in prairie voles. Physiology and Behavior 87(2):424–429. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N, Enhancing Recovery in Coronary Heart Disease Patients I. (2003). Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. Journal of the American Medical Association 289(23):3106–3116. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. (2004). Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience 124(2):439–448. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. (2009). The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Glasper ER, Neigh GN. (2014). SSRI or CRF antagonism partially ameliorate depressive-like behavior after adolescent social defeat. Behavioural Brain Research 270:295–299. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Thisted RA. (2010). Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago health, aging, and social relations study. Psychology and Aging 25(2):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O'Connor C, Stone PH, Freedland KE. (2001). Depression, heart rate variability, and acute myocardial infarction. Circulation 104:2024–2028. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Miller GE, Jaffe AS. (2002). Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. Journal of Psychosomatic Research 53(4):897–902. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. (1993). Monogamy and the prairie vole. Scientific American 268:100–106. [DOI] [PubMed] [Google Scholar]
- Chiou RJ, Kuo CC, Liang KC, Yen CT. (2009). State-dependent amygdala stimulation-induced cardiovascular effects in rats. Chinese Journal of Physiology 52(6):432–440. [DOI] [PubMed] [Google Scholar]
- Clinard CT, Bader LR, Sullivan MA, Cooper MA. (2015). Activation of 5-HT2a receptors in the basolateral amygdala promotes defeat-induced anxiety and the acquisition of conditioned defeat in Syrian hamsters. Neuropharmacology 90:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Tavares RF, Guimaraes FS, Correa FM, Joca SR, Resstel LB. (2011). Chronic fluoxetine treatment alters cardiovascular functions in unanesthetized rats. European Journal of Pharmacology 670(2-3) :527–533. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. (2005a). The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neuroscience and Biobehavioral Reviews 29:571–625. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. (2005b). Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology 182:335–344. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. (2005c). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and Biobehavioral Reviews 29:547–569. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le W, Hoffman GE, Carter CS. (2003). Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Research 965(1-2):203–211. [DOI] [PubMed] [Google Scholar]
- Dale E, Bang-Andersen B, Sanchez C. (2015). Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochemical Pharmacology 95(2):81–97. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Wiegand LA, Dyavanapalli J, Mares J, Wang X, Mendelowitz D (2014). Function and modulation of premotor brainstem parasympathetic cardiac neurons that control heart rate by hypoxia-, sleep-, and sleep-related diseases including obstructive sleep apnea. Progress in Brain Research 212:39–58. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Lall VK. (2015). Sympathetic preganglionic neurons: properties and inputs. Comprehensive Physiology 5:829–869. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS. (1995). Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proceedings of the National Academy of Sciences of the United States of America 92(17):7744–7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval F, Mokrani MC, Monreal J, Weiss T, Fattah S, Hamel B, Macher JP. (2002). Interaction between the serotonergic system and HPA and HPT axes in patients with major depression: implications for pathogenesis of suicidal behavior. Dialogues in Clinical Neuroscience 4(4):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JE, Wood SK. (2016). Neuroinflammation at the interface of depression and cardiovascular disease: Evidence from rodent models of social stress. Neurobiology of Stress 4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament MF, Lane RM, Zhu R, Ying Z. (1999). Predictors of an acute antidepressant response to fluoxetine and sertraline. International Clinical Psychopharmacology 14(5):259–275. [PubMed] [Google Scholar]
- Flandreau EI, Bourke CH, Ressler KJ, Vale WW, Nemeroff CB, Owens MJ. (2013). Escitalopram alters gene expression and HPA axis reactivity in rats following chronic overexpression of corticotropin-releasing factor from the central amygdala. Psychoneuroendocrinology 38(8): 1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez D, Laorden ML, Milanes MV, Nunez C. (2012). Glucocorticoids regulation of FosB/DeltaFosB expression induced by chronic opiate exposure in the brain stress system. PLoS ONE 7(11):e50264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. (2010). Paraventricular hypothalamic nucleus: axonal projections to the brainstem. Journal of Comparative Neurology 518(9): 1460–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. (2007). The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132(1):397–414. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT, Gaffney M, Van Zyl LT. (2007). Heart rate variability in acute coronary syndrome patients with major depression: influence of sertraline and mood improvement. Archives of General Psychiatry 64(9): 1025–1031. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr., Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, McLvor M (2002). Sertraline treatment of major depression in patients with acute MI or unstable angina. Journal of the American Medical Association 288(6):701–709. [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ. (2012). Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychological Medicine 42(12):2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore S (1978). The effect of social support in moderating the health consequences of unemployment. Journal of Health and Social Behavior 19(2): 157–165. [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. (2006). The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biological Psychiatry 59:309–316. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. (2007a). Cardiac regulation in the socially monogamous prairie vole. Physiology and Behavior 90:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. (2007b). Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biological Psychiatry 62:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. (2008). Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and Anxiety 25:E17–E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L, Kitzman DW, Register TC, Shively CA. (2014). Effect of depression and sertraline treatment on cardiac function in female nonhuman primates. Psychosomatic Medicine 76(2): 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology 6(2):603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestermann D, Temel Y, Blokland A, Lim LW. (2014). Acute serotonergic treatment changes the relation between anxiety and HPA-axis functioning and periaqueductal gray activation. Behavioural Brain Research 273:155–165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. (1991). Activity of brain serotonergic neurons in the behaving animal. Pharmacological Reviews 43(4):563–578. [PubMed] [Google Scholar]
- Jensen JB, Jessop DS, Harbuz MS, Mork A, Sanchez C, Mikkelsen JD. (1999). Acute and long-term treatments with the selective serotonin reuptake inhibitor citalopram modulate the HPA axis activity at different levels in male rats. Journal of Neuroendocrinology 11(6):465–471. [DOI] [PubMed] [Google Scholar]
- Jiang W, O'Connor C, Silva SG, Kuchibhatla M, Cuffe MS, Callwood DD, Zakhary B, Henke E, Arias RM, Krishnan R. (2008). Safety and efficacy of sertraline for depression in patients with CHF (SADHART-CHF): a randomized, double-blind, placebo-controlled trial of sertraline for major depression with congestive heart failure. American Heart Journal 156(3):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Salonen JT, Cohen RD, Brand RJ, Syme SL, Puska P. (1988). Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. American Journal of Epidemiology 128(2):370–380. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. (2008). Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius in the rat. Neuroscience 153(3):605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. (2006). HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation 13(5-6):268–276. [DOI] [PubMed] [Google Scholar]
- Lesch KP. (2007). Linking emotion to the social brain. EMBO reports 8(1S):S24–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R, Wilkins B, Benjamin B, Curtis JT. (2017). Cardiovascular control is associated with pair-bond success in male prairie voles. Autonomic Neuroscience 208:93–102. [DOI] [PubMed] [Google Scholar]
- Liu R, Dang W, Jianting M, Su C, Wang H, Chen Y, Tan Q. (2012). Citalopram alleviates chronic stress induced depression-like behaviors in rats by activating GSK3beta signaling in dorsal hippocampus. Brain Research 1467:10–17. [DOI] [PubMed] [Google Scholar]
- Liu X, Tang X, Sanford LD. (2009). Stressor controllability and Fos expression in stress regulatory regions in mice. Physiology and Behavior 97(3-4):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Burke AR, Zelin NS, Hale MW, Lowry CA. (2012). Post-weaning social isolation attenuates c-Fos expression in GABAergic interneurons in the basolateral amygdala of adult female rats. Physiology and Behavior 107(5):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neuroscience and Biobehavioral Reviews 38:173–192. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Herman JP. (2015). The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. Journal of Neuroendocrinology 27(6):446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Appleton KM, Johnson AK, Scotti ML, Wardwell J, Murphy R, Bishop C, Knecht A, Grippo AJ. (2017). The protective effects of social bonding on behavioral and pituitary-adrenal axis reactivity to chronic mild stress in prairie voles. Stress 20:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti ML, Wardwell J, Chandler DL, Bates SL, LaRocca MA, Trahanas DM, Grippo AJ. (2014). Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonomic Neuroscience: Basic and Clinical 180:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini LC. (2007). Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: reflex mechanisms and changes during exercise. Clinical and Experimental Pharmacology and Physiology 34:369–376. [DOI] [PubMed] [Google Scholar]
- Moffitt JA, Johnson AK. (2004). Short-term fluoxetine treatment enhances baroreflex control of sympathetic nervous system activity after hindlimb unloading. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 286:R584–R590. [DOI] [PubMed] [Google Scholar]
- Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. (2008). Serotonin: a review. Journal of Veterinary Pharmacology and Therapeutics 31(3): 187–199. [DOI] [PubMed] [Google Scholar]
- Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. (2017). Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neuroscience and Biobehavioral Reviews 74(Pt B):366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaiko E, Blessing WW. (2001). Raphe region mediates changes in cutaneous vascular tone elicited by stimulation of amygdala and hypothalamus in rabbits. Brain Research 891(1-2): 130–137. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. (2015). FosB: a transcriptional regulator of stress and antidepressant responses. European Journal of Pharmacology 753:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Chapin DS, Johnson JL Jr., Torgersen LK (1992). Sertraline, a serotonin-uptake inhibitor,reduces food intake and body weight in lean rats and genetically obese mice. American Journal of Clinical Nutrition 55(1 Suppl):185s–189s. [DOI] [PubMed] [Google Scholar]
- Nishijima T, Kawakami M, Kita I. (2013). Long-term exercise is a potent trigger for DeltaFosB induction in the hippocampus along the dorso-ventral axis. PLoS ONE 8(11):e81245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, Krishnan R. (2010). Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. Journal of the American College of Cardiology 56(9):692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Fava M. (2008). Predictors, moderators, and mediators (correlates) of treatment outcome in major depressive disorder. Dialogues in Clinical Neuroscience 10(4):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2005). The rat brain in stereotaxic coordinates (5th ed.). New York: Elsevier. [DOI] [PubMed] [Google Scholar]
- Ramanathan M, Kumar SN, Suresh B. (2003). Evaluation of cognitive function of fluoxetine, sertraline and tianeptine in isolation and chronic unpredictable mild stress-induced depressive Wistar rats. Indian Journal of Experimental Biology 41:1269–1272. [PubMed] [Google Scholar]
- Ramchandra R, Hood SG, Frithiof R, McKinley MJ, May CN. (2013). The role of the paraventricular nucleus of the hypothalamus in the regulation of cardiac and renal sympathetic nerve activity in conscious normal and heart failure sheep. Journal of Physiology 591(1):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, Wannamethee SG. (2008). Social engagement and the risk of cardiovascular disease mortality: results of a prospective population-based study of older men. Annals of Epidemiology 18(6):476–483. [DOI] [PubMed] [Google Scholar]
- Roos LE, Knight EL, Beauchamp KG, Berkman ET, Faraday K, Hyslop K, Fisher PA. (2017). Acute stress impairs inhibitory control based on individual differences in parasympathetic nervous system activity. Biological Psychology 125:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT Jr., Pollock BG, Gaffney A, Narayan M, Finkel MS, McCafferty J, Gergel I (1998). Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. Journal of the American Medical Association 279(4):287–291. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Khoenkhoen SJ, Ottenhof KW, Koeter MW, Mocking RJ, Schene AH. (2015). Longitudinal effects of the SSRI paroxetine on salivary cortisol in major depressive disorder. Psychoneuroendocrinology 52:261–271. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Breeding M, Knoth K, Tsutsumi M. (1989). Sertraline-induced desensitization of the serotonin 5HT-2 receptor transmembrane signaling system. Psychopharmacology 99(1):64–69. [DOI] [PubMed] [Google Scholar]
- Sasagawa T, Horii-Hayashi N, Okuda A, Hashimoto T, Azuma C, Nishi M. (2017). Long-term effects of maternal separation coupled with social isolation on reward seeking and changes in dopamine D1 receptor expression in the nucleus accumbens via DNA methylation in mice. Neuroscience Letters 641:33–39. [DOI] [PubMed] [Google Scholar]
- Shaffer F, Ginsberg JP. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health 5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F, McCraty R, Zerr CL. (2014). A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Frontiers in Psychology 5:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, McMunn A, Banks J, Steptoe A. (2011). Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychology 30(4):377–385. [DOI] [PubMed] [Google Scholar]
- Shapiro PA, Lesperance F, Frasure-Smith N, O'Connor CM, Baker B, Jiang JW, Dorian P, Harrison W, Glassman AH. (1999). An open-label preliminary trial of sertraline for treatment of major depression after acute myocardial infarction (the SADHAT Trial). Sertraline Anti-Depressant Heart Attack Trial. American Heart Journal 137(6): 1100–1106. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Blumenthal JA, Smith PJ, Watkins LL, Hoffman BM, Hinderliter AL. (2016). Effects of exercise and sertraline on measures of coronary heart disease risk in patients with major depression: results from the SMILE-II randomized clinical trial. Psychosomatic Medicine 78(5):602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. (2009). Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neuroscience and Biobehavioral Reviews 33:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. (2003). Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology 168(3):293–298. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. (2013). Corticotropin releasing factor and catecholamines enhance glutamatergic neurotransmission in the lateral subdivision of the central amygdala. Neuropharmacology 70:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitges M, Aldana BI, Gomez CD, Nekrassov V. (2012). The antidepressant sertraline prevents the behavioral and EEG changes induced in two animal models of seizures. Epilepsy & Behavior 25(4):511–516. [DOI] [PubMed] [Google Scholar]
- Sladek CD, Michelini LC, Stachenfeld NS, Stern JE, Urban JH. (2015). Endocrine-autonomic linkages. Comprehensive Physiology 5(3): 1281–1323. [DOI] [PubMed] [Google Scholar]
- Spasojevic N, Jovanovic P, Dronjak S. (2012). Chronic fluoxetine treatment affects gene expression of catecholamine enzymes in the heart of depression model rats. Indian Journal of Experimental Biology 50(11):771–775. [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. (1985). The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370. [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z. (2014). Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behavioural Brain Research 265:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. (1980). Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31(6):410–417. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology, North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93(5):1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews 36(2):747–756. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. (2005). Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 30(10): 1050–1058. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. (2009). Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews 33(2):81–88. [DOI] [PubMed] [Google Scholar]
- Villalba C, Boyle PA, Caliguri EJ, De Vries GJ. (1997). Effects of the selective serotonin reuptake inhibitor fluoxetine on social behaviors in male and female prairie voles (Microtus ochrogaster). Hormones and Behavior 32(3): 184–191. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. (2012). Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct 217(2) :337–351. [DOI] [PubMed] [Google Scholar]
- Wann BP, Bah TM, Kaloustian S, Boucher M, Dufort AM, Le Marec N, Godbout R, Rousseau G. (2009). Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. Journal of Psychopharmacology 23(4):451–459. [DOI] [PubMed] [Google Scholar]
- Watanasriyakul WT, Wardwell J, McNeal N, Schultz R, Woodbury M, Dagner A, Cox M, Grippo AJ. (2018). Voluntary physical exercise protects against behavioral and endocrine reactivity to social and environmental stressors in the prairie vole. Social Neuroscience 13:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and Behavior 26:339–349. [DOI] [PubMed] [Google Scholar]
- Wood SK. (2014). Individual differences in the neurobiology of social stress: implications for depression-cardiovascular disease comorbidity. Current Neuropharmacology 12(2) :205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E, Erol K, Ulupinar E. (2012). Effects of sertraline on behavioral alterations caused by environmental enrichment and social isolation. Pharmacology, Biochemistry and Behavior 101:278–287. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. (2004). The neurobiology of pair bonding. Nature Neuroscience 7:1048–1054. [DOI] [PubMed] [Google Scholar]