Abstract
While it was long held that T cells were the primary mediators of multiple sclerosis (MS) pathogenesis, the beneficial effects observed in response to treatment with Rituximab, a monoclonal antibody (mAb) targeting CD20, shed light on a key contributor to MS that had been previously underappreciated: B cells. This has been reaffirmed by results from clinical trials testing the efficacy of subsequently developed B cell-depleting mAbs targeting CD20 as well as studies revisiting the effects of previous disease-modifying therapies (DMTs) on B cell subsets thought to modulate disease severity. In this review, we summarize current knowledge regarding the complex roles of B cells in MS pathogenesis and current and potential future B cell-directed therapies.
Introduction
The contribution of B cells to autoimmune disease pathogenesis has historically been viewed primarily via the production of pathogenic autoantibodies, as exemplified in diseases such as myasthenia gravis (MG) and neuromyelitis optica (NMO). However, seminal observations in recent years have challenged this simplistic view1. It is now well-established that, in addition to the production of autoantibodies, B cells are able to drive autoimmunity through the presentation of autoantigen to autoreactive T cells, the secretion of proinflammatory cytokines, and the establishment of tertiary lymphoid organs (TLOs) in chronically inflamed tissues2. Paradoxically, B cells have also been shown to exert several regulatory functions critical for the prevention or resolution of inflammation accompanying several autoimmune diseases3–7. Collectively, these findings demonstrate a complex role for B cells as regulators of autoimmunity. B cells have become a focal point in recent years regarding their degree of involvement in the pathogenesis of multiple sclerosis (MS), which has canonically been considered a predominantly T cell-mediated autoimmune disease1,8–10. Corroborated by the success of clinical trials of B cell depleting therapies, this newfound role for B cells has warranted a shift in the approach to the treatment of MS.
B cells were first implicated in the pathogenesis of MS through the discovery of oligoclonal bands (OCBs) or abnormal production of clonally expanded IgG in the cerebral spinal fluid (CSF), but not plasma of patients with MS11. Since then, cells isolated from the CSF and peripheral blood of patients with MS have been found to produce these oligoclonal bands12–14. However, unlike what is observed in NMO and MG, there is significant heterogeneity in the antigen specificity of these oligoclonal antibodies, which may target pathogens as well as autoantigens8. Multiple studies have demonstrated the presence of clonally expanded B cells within lesions, as well as TLOs, and B cells can be found within the parenchyma, CSF, and meninges of patients with multiple sclerosis15–24. The clinical success of B cell depleting therapies such as anti-CD20 monoclonal antibodies (mAbs) corroborated these results, solidifying the contribution of B cells in the pathogenesis of multiple sclerosis25–27.
B cell tolerance: an overview
The adaptive immune response requires not only the ability of B and T cells to detect and respond to any encountered foreign antigen, but to do so in a highly specific way28,29. In order to accomplish this, each cell type expresses an antigen receptor with a particular specificity, conducive to their respective roles in this process. However, the B and T cell receptor (BCR and TCR, respectively) differ in important aspects: Firstly, the affinity of the BCR for antigen is several orders of magnitude higher than that of the TCR, allowing the BCR to recognize soluble antigens whereas antigen presentation to the TCR is determined by binding of peptides to major histocompatibility complex (MHC) molecules30. Secondly, the specificity and binding affinity of the BCR is not static, in contrast to the TCR, but can be edited through participation in a germinal center (GC) reaction31,32, the process responsible for the T cell-dependent generation of high affinity memory B cell and plasma cells.
Given that the specificities of the primary BCR repertoire are generated by random recombination of genes encoding the antigen binding region of the BCR, the generation of B cells possessing an autoreactive BCR seems inevitable31. Indeed, it has been established that a considerable majority of the initial BCR repertoire exhibits significant self-reactivity32. To prevent, or at least limit the emergence of autoreactive B cell responses, several mechanisms exist which effectively restrict the persistence of autoreactive B cells and thereby mitigate the risk of developing autoimmunity. The establishment of B cell tolerance can conceptually be divided into two separate “checkpoints”: Central tolerance, which occurs during the early stages of B cell development within the bone marrow, and peripheral tolerance, which occurs upon T cell-dependent activation and subsequent entry into the GC reaction33,34.
Central tolerance
In the bone marrow, the process of B cell development has two critical goals: The first is to generate a primary repertoire of BCRs that is maximally diverse, so as to facilitate recognition of foreign antigen. The second is to prevent the emigration of B cells expressing self-reactive BCRs into the periphery29,32,35.
The diversification of the primary BCR repertoire is accomplished through the rearrangement of the variable (V), diversity (D), and joining (J) gene segments encoding the variable regions of the BCR, in a process termed V(D)J recombination36. However, as a consequence of the stochastic and error-prone nature of this process, a majority of B cells (50–80%) initially express a self-reactive BCR33–35,37,38, at which point central tolerance mechanisms go into effect to eliminate these cells. The fate, i.e. death, survival, or anergy of these self-reactive B cells is determined by the strength of the signals derived from the bone marrow microenvironment and transduced through the BCR and its coreceptors32. However, B cells that strongly bind self-antigen can be rescued by reactivating V(D)J recombination in an attempt to generate a BCR that is either non-reactive or only slightly reactive to self-antigen, a process also termed receptor editing. B cells that still do not produce a BCR with lessened autoreactivity undergo apoptosis (clonal deletion)37. Lastly, any remaining B cells with BCRs that still weakly bind self-antigen are thought to become anergic. B cells that have undergone anergy are desensitized to BCR signaling and as a result typically will not undergo T-dependent activation and maturation into plasma cells or memory B cells in the periphery. Nevertheless, with sufficient stimulation, anergic B cells are still able to participate in GC responses39.
Last, it must be noted that some self-reactive B cell clones may not encounter their respective antigen in the bone marrow, and thereby evade central tolerance mechanisms by “clonal ignorance”. The control of these autoreactive B cells therefore relies on peripheral tolerance mechanisms, as discussed below. It may be these clones that fail both central and peripheral tolerance that are key in promoting autoimmune pathology in many conditions.
Peripheral tolerance
While central tolerance mechanisms are estimated to eliminate 90% of the autoreactive B cell clones produced, there are still some cells that escape into the immune periphery and which need to be controlled to prevent undue development of autoimmune pathology40. Interestingly, in contrast to a number of autoimmune diseases that are thought to be the result of defects in both central and peripheral tolerance mechanisms, the development of MS is thought to result from a deficiency primarily in peripheral tolerance mechanisms41,42, which will be discussed next. Peripheral B cell tolerance mechanisms rely on many of the signaling requirements for the activation of mature B cells prior to or upon participating in the GC reaction. GCs are specialized microenvironments that form in secondary lymphoid organs to facilitate the T cell-dependent activation and affinity maturation of mature B cells and subsequent generation of high affinity memory B cells and plasma cells. Briefly, B cells that have encountered their cognate antigen migrate from B cell-rich follicles to adjacent T cell-rich T cell zones43. These B cells internalize, process, and present a fragment of that antigen to CD4+ T cells in the form of a linear peptide complexed with MHC class II (MHC II)43. Upon receiving T cell help in the form of cytokines and stimulation of costimulatory receptors (described in more detail later), the presenting B cells begin proliferating, thus initiating the GC reaction.
The GC is comprised of the dark zone (DZ) and the light zone (LZ)44. In the DZ, activated B cells proliferate and undergo affinity maturation through somatic hypermutation (SHM) of the genes comprising the BCR. B cells that have completed this process enter the LZ where they reencounter antigen presented on the surface of follicular dendritic cells (FDCs) and compete for survival signals derived from follicular T helper (Tfh) cells. B cell clones are then selected based on affinity; those expressing higher affinity BCRs either reenter the DZ or differentiate directly into memory B cells or plasma cells, while lower affinity clones that do not receive Tfh cell help undergo apoptosis37.
Mature B cells, upon receiving the first activating signal through stimulation of the BCR, require costimulatory signals typically derived from helper T cells sharing the same antigenic specificity to become fully activated, and subsequently either directly differentiate into plasmablasts or memory B cells, or to be recruited into the GC reaction. Cells that receive one signal without the other undergo apoptosis or become anergic45. The principal B cell surface receptors involved in this process are the BCR, the CD40 receptor, and the Fas receptor (CD95). The ultimate fate of a given B cell is the sum of the relative activity of these signaling pathways, briefly summarized here.
BCR stimulation initiates signaling cascades that result in survival and proliferation as well as apoptosis. Apoptosis, mediated by the BH3-only protein Bim, functions as a default program. Therefore, survival and proliferation require sufficient “positive” costimulatory signals, such as stimulation of CD40 by T cell-derived CD40 ligand (CD40L), to avoid apoptosis. In addition to apoptosis, BCR signaling in the absence of costimulation can also result in anergy. BCR binding occupancy is the deciding variable in this circumstance, with high occupancy favoring apoptosis and intermediate (5–45%) occupancy favoring anergy46,47. Goodnow and colleagues elegantly demonstrated this concept using transgenic mice expressing a BCR with high affinity for hen egg lysozyme (HEL) as well as soluble HEL. As anergic B cells exhibit attenuated BCR signaling, this mechanism primarily serves to prevent the aberrant activation of ignorant B cells (autoreactive B cells that have not encountered their antigen yet). Ignorant B cells are able to undergo the initial steps of activation with proficiency comparable to non-autoreactive naïve mature B cells. However, since autoreactive CD4+ T cells are similarly regulated by self-tolerance mechanisms, the likelihood that corresponding autoreactive CD4+ T cells exist in the periphery is low; therefore, without T cell help, these previously ignorant B cells will be activated in the absence of costimulation, and either undergo apoptosis48 or become anergic45. CD40 stimulation is able to deter apoptosis temporarily, however it must be compounded with BCR signaling to fully activate B cells. Importantly, signaling via the CD40 molecule by CD40L expressed on T cells also induces the expression of CD95 on B cells, which is essential to the maintenance of peripheral tolerance49. CD40L, in turn, is upregulated upon cognate binding of a CD4+ TCR to the peptide:MHC complex on antigen-presenting cells (APCs). Upon proper activation, T cells will also upregulate Fas ligand (FasL), which will then bind to CD95 on activated B cells, inducing caspase-mediated apoptosis. This mechanism is relevant specifically for the control of anergic B cell responses.
Anergic B cells have a shortened lifespan (2–5 days)45 and exhibit impaired signaling through the BCR and repressed expression of the costimulatory molecule CD8649. Although these cells are capable of overcoming anergy in the absence of costimulation given sufficient BCR or toll-like receptor (TLR) stimulation49–51, anergic B cells are eliminated typically by follicular exclusion, in which these cells are outcompeted by non-autoreactive B cells for entry into the follicle46 as well as for the critical survival cytokine, B cell-activating factor (BAFF)45.
In the rare event that an anergic B cell enters the GC reaction and presents self-antigen to a T cell specific for the same autoantigen, it is signaled to undergo apoptosis via CD9537, a consequence of the attenuated BCR signaling characteristic of anergic B cells. Without sufficient BCR or toll-like receptor (TLR) signaling to counter signaling through CD95, these B cells will undergo apoptosis52. Taken together, the control of aberrant ignorant and anergic B cell activation and proliferation, and therefore the maintenance of tolerance, relies on both activating and death-inducing stimuli.
It is important to note that SHM is able to result in the generation of an autoreactive BCR, but in addition to being controlled by the tolerance mechanisms summarized here, these B cells are additionally controlled by follicular regulatory T cells (Tfrs), although the mechanism by which they exert these suppressive effects is poorly understood53. Indeed, MS patients exhibit a decrease in circulating Tfrs as well as reduced suppressive function of the Tfrs present compared to healthy control subjects54.
The role of B cells in the pathogenesis of MS
Initial evidence
Experimental autoimmune encephalomyelitis (EAE) induced in rodents or non-human primates has been widely studied as an animal model of human MS55. A number of rodent EAE models have been studied over the past decades, which differ in the nature of the neuroantigen used for disease induction, the use of different susceptible mouse strains, and whether disease is induced by immunization (“active EAE”), adoptive transfer of pathogenic lymphocytes (“passive EAE), or develops spontaneously in mouse lines transgenically expressing pathogenic T cell or B cell receptors. Many different neuroantigen preparations have been used for the induction of EAE, including whole spinal cord homogenate, purified or recombinant neuroantigen proteins, or immunodominant peptides identified from encephalitogenic neuroantigens. The choice of neuroantigen and mouse strain has important implications for the cellular autoimmune mechanisms driving EAE and the pathological phenotype. For example, induction of EAE in C57BL/6 mice with myelin oligodendrocyte (MOG) protein induces autoantibodies that are important for the effector mechanism driving disease in this model (i.e. B cells), whereas induction of disease with MOG peptide does not depend on autoantibody and B cells. Thus, the choice of EAE model may inform on the mechanism of B cell-depleting strategies and the role of autoantibodies in MS, which do not deplete plasma cells.
To assess the role of B cells in MS, and its animal model experimental autoimmune encephalomyelitis (EAE), B cell depletion has been studied using a number of different experimental approaches. One of the most utilized models for studying the contribution of B cells is μMT mice in which the IgM heavy chain is mutated, and where the animals therefore lack mature B cells56. Another approach consists of using monoclonal antibodies, for example anti-CD19 or anti-CD20 mAbs, to deplete subsets of B cells57. CD19 is expressed on B cells from the pro-B cell stage on, but to a lower extent in plasma cells57. CD20 is expressed on pre-B cells and continues to be expressed on B cells until the plasmablast stage57. A subset of CD3+ T cells also expresses CD20 and are depleted with anti-CD20 mAb treatment; however the effect that this has on therapeutic efficacy is not known57. Studies indicate that the pathogenic nature of B cells depends greatly on autoantigen, subset of B cells depleted, and activation of the B cells. B cell-deficient μMT mice on the C57BL/6 background immunized with truncated recombinant human myelin oligodendrocyte glycoprotein1–120 (rhMOG1–120) protein do not develop EAE, but μMT mice immunized with MOG35–55 peptide are still susceptible to EAE58. Similarly, μMT mice on the B10.PL background and immunized with MBP1–11 peptide are still susceptible to EAE6. Interestingly, the μMT mice in this latter study showed impaired recovery from EAE, which provided seminal evidence for a regulatory function of B cells in neuroinflammatory disease. Regulatory B cells will be discussed later. Irrespective, mice immunized with recombinant mouse MOG1–117 protein (rmMOG1–117) and treated with anti-CD20 mAb weekly 21 days prior to immunization or after EAE was fully developed also showed less severe disease, while MOG35–55 peptide-immunized mice treated with anti-CD20 mAb 21 days prior to immunization or after EAE was fully developed showed exacerbated disease59. Similarly, human CD20 transgenic mice backcrossed on the C57BL/6 background that were immunized with rhMOG1–120 protein and treated with Rituximab for three days prior to immunization showed less severe disease than untreated mice60. μMT mice that were reconstituted with activated rhMOG1–120 protein-primed B cells restored the susceptibility to EAE induced with protein myelin antigen58. A caveat to these studies is that μMT mice still produce IgA+ B cells61 and have also been shown to produce IgG1, IgG2a, and IgE under certain conditions62. In another B cell depletion model, Weber, et al. demonstrated that, when mice are immunized with rmMOG1–117 protein, anti-CD20 mAb depletion reduced disease severity by decreasing the differentiation of rmMOG1–117 protein-specific Th1 and Th17 cells, but not in the MOG35–55 peptide-induced EAE model. They hypothesized that this was due to activation of B cells and subsequent antigen processing and presentation by B cells in the rmMOG1–117 protein model, but not the MOG35–55 peptide model59 In contrast, in a relapsing-remitting EAE model where Biozzi mice, which produce high titers of antibodies, were immunized with spinal cord homogenate, anti-CD20 mAb treatment did not rescue the mice from disease63. This could be due to the type of antigen utilized, depletion of regulatory B cells, or intrinsic differences of the Biozzi mouse EAE model compared with the recombinant MOG protein and MOG35–55 peptide models. When anti-CD20 mAb was administered 21 days post-sensitization in the marmoset model of active EAE using MOG34–56 peptide or rhMOG1–125 protein, clinical disease was lower or absent in the anti-CD20 mAb treated group64,65. Thus, the results in models inducing EAE with myelin protein antigen are consistent with the clinical data in MS patients showing reduced relapse rates after anti-CD20 mAb treatment in relapsing-remitting MS (RRMS) patients. However, anti-CD20 mAb treatment may not significantly modulate disease overall in patients with progressive MS25,26,66,67. Anti-CD19 mAb treatment for B cell depletion is also explored in EAE and MS. Treatment of active EAE induced with rhMOG1–125 protein with a single dose anti-CD19 mAb before disease induction as well as 7 days post-immunization ameliorated disease severity and has been reported to inhibit disease to a greater extent than anti-CD20 mAb treatment68,69. In MS, anti-CD19 mAb treatment reduced lesion size in newly forming lesions70. Taken together, current data support that B cells play a primarily pathogenic role in MS and EAE, and it seems that disease outcome depends greatly upon the autoantigen, the cooperation between T and B cells, the stage at which the depletion occurs, and the subset of B cells that are targeted. However, it is important to note that there is evidence for specific B cell effector functions that are protective in MS and EAE, which will be discussed. In the following sections we will provide an overview of the current understanding of pathogenic and protective mechanisms of B cell effector functions in MS and its murine model EAE.
Antibody-dependent mechanisms
I. Production of autoantibodies
As mentioned above, circulating B cells possessing a self-reactive BCR exist in the immune periphery of most healthy individuals, suggesting that they have escaped central tolerance mechanisms34,37,71,72. Most autoreactive B cells remain ignorant or anergic and are subsequently eliminated or are controlled through the peripheral tolerance mechanisms described earlier. While this should prevent the production of autoantibodies, it is important to note that autoantibodies, such as B1 cell-derived natural autoantibodies, typically of the IgM isotype, also exist in the serum of healthy individuals and are believed to carry out beneficial, rather than pathogenic functions, such as the clearance of apoptotic cells73,74.
Autoantibody-driven pathogenesis was first implicated by the detection of OCBs and the presence of class-switched autoantibodies in lesions and CSF of MS patients11,20,22,75,76. In contrast to autoimmune diseases classically considered to be autoantibody-mediated, such as MG or Grave’s disease77, where the nature of the autoantigens targeted by autoantibodies is often known, the molecular targets and pathogenic role of autoantibodies in MS patients are still not fully resolved, with some notable exceptions such as in neuromyelitis optica1 (NMO). Nevertheless, studies from CSF and blood of patients with MS and from EAE have provided some insights into ways that antibodies may contribute to MS.
In a small clinical study of 14 MS patients, IgG was isolated from lesions of post-mortem patients and was found to bind to rhMOG1–121 protein78. In corroboration of this observation, higher titers of autoantibodies specific for full-length human MOG protein were found in MS patients compared with controls and antibodies from these patients enhanced disease severity when transferred to mice with EAE when compared with diseased mice that were not treated with these antibodies79. Similarly, Lyons and colleagues reconstituted μMT mice with serum from rhMOG1–120 protein-immunized mice and found that this serum was capable of restoring EAE induced with MOG protein in μMT mice58. In further support of this notion, mice with guinea pig myelin basic protein (MBP)-induced active and passive EAE that have been treated with an anti-MOG mAb exhibit more demyelination than controls80, and when EAE is induced with MOG35–55 peptide, depletion of IgG antibodies leads to significantly reduced disease scores81. Another study assessed the proteolytic activity of MBP-specific antibodies from MS patients and found increased cleavage of MBP protein correlate with worse scores on the MS expanded disability status scale (EDSS)82.
Thus, some evidence suggests a pathogenic role for autoantibodies in MS. However, autoantibodies detected in the serum of patients with clinically isolated syndrome (CIS) do not correlate with progression to definite MS83, and myelin-binding autoantibodies can also be found in the serum of healthy controls84. This indicates that specific conditions may be necessary for myelin-binding autoantibodies to mediate pathogenic function in MS, and leaves room for investigation of specific effector mechanisms such as antibody-induced demyelination79,82,85–87, complement activation88–91, and opsonization and phagocytosis76.
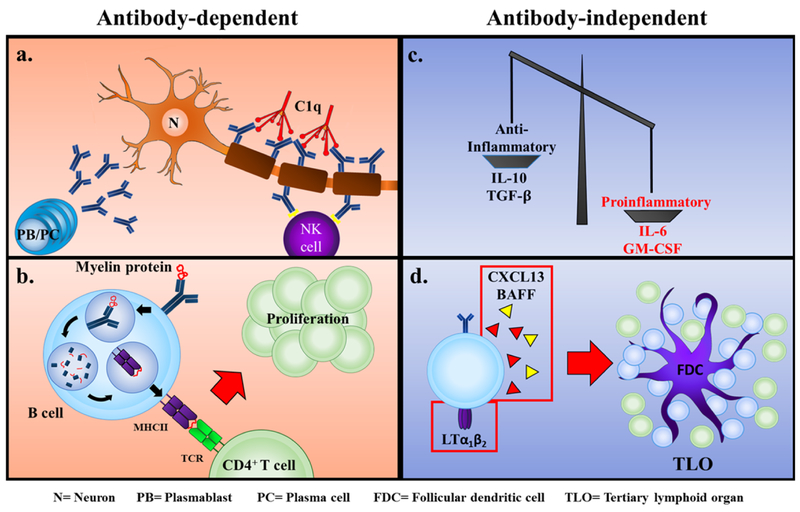
Antibodies have been associated with demyelinating lesions in both MS and EAE86,92,93. As mentioned before, IgG isolated from lesions of MS patients can proteolytically cleave MBP protein, and this correlated with EDSS scores82. This indicates that antibodies may cause damage to the myelin sheath. Along these lines, anti-MOG protein-specific antibodies have been associated with CNS demyelination86,92,94, which may be due to the ability of these antibodies to destabilize and alter the structure of the myelin sheaths95,96 (Fig. 1a).
Figure 1. B cells mediate MS pathology through antibody-dependent and antibody-independent mechanisms.
(a) Myelin-reactive antibodies secreted by autoreactive plasmablasts and plasma cells mediate destruction of the myelin sheath by binding C1q and activating the classical complement pathway or initiating FcγRIIIa-mediated antibody-dependent cell-mediated cytotoxicity by NK cells. b) B cells expressing a myelin-reactive BCR are capable of processing myelin protein and presenting myelin peptide to autoreactive CD4+ T cells, resulting in their activation and proliferation. (c, d) B cells are able to foster an environment conducive to autoimmunity by (c) secreting higher amounts of pro-inflammatory cytokines such as IL-6 and GM-CSF compared with regulatory cytokines such as IL-10 and TGF-b and (d) by secreting cytokines/chemokines including CXCL13 and BAFF which promote the differentiation of FDCs and establishment of TLOs, which facilitate the activation and expansion of autoreactive B and T cells. N= Neuron, PB= Plasmablast, PC= Plasma cell, FDC= Follicular dendritic cell, TLO= Tertiary lymphoid organ.
Autoantibodies have also been associated with pathogenesis of EAE by complement activation as indicated by the presence of active complement in MS lesions88. Complement activation was characteristic in recombinant rat MOG1–125 (rrMOG1–125) protein-induced EAE87, and mice that have been decomplemented show a delay of disease onset91. Studies have demonstrated autoantibody-driven, complement-dependent demyelination during MBP–PLP fusion protein-induced EAE and in brains of patients with MS90,97.
Autoantibodies can also act to increase opsonization and phagocytosis of autoantigens by macrophages and dendritic cells to present to autoreactive T cells98. Macrophages that bound myelin antigen as well as immunoglobulin and activated complement have been previously identified in a patient with MS88, and incubation with IgG antibodies isolated from the CSF of EAE-induced mice increases uptake of myelin by macrophages99. Kinzel, et al. demonstrated that when MOG protein-specific antibodies are deposited, APCs take up the antigen via the Fc receptor and proceed to process and present the antigen and activate T cells98,100. Autoantibodies specific for MOG protein opsonize to the greatest extent when compared with antibodies against other myelin antigens, and the anti-MOG mAb with the highest myelin uptake was shown to enhance EAE and demyelination101. MBP protein-specific antibodies were also able to increase myelin uptake, and increase inflammation during EAE101.
II. Autoantigen presentation to T cells
Dendritic cells (DCs) are considered the archetypal professional APCs. However, studies using transgenic mice lacking MHC II expression selectively on B cells59,102 have underscored the importance of B cell APC function in the pathogenesis of several autoimmune diseases1.
Specifically, memory B cells are particularly efficient APCs and myelin-specific memory B cells have been found in the peripheral blood of MS patients103,104. This is primarily attributed to the unique ability of B cells to present antigens that are present in very low concentrations105–107 through BCR-mediated uptake, which is of vastly superior efficiency compared with pinocytosis, the primary mechanism of antigen uptake employed by other APCs108–111.
B cells can activate T cells through MHC II-dependent antigen presentation and CD80 co-stimulation. Evidence from MS patients to support this notion include that there is an increase in CD80+ B cells in patients with active MS112, and that B cell-depleting therapies also cause a decrease in T cells in the CSF of patients with MS25. In fact, in certain EAE models it has been shown that mice that have MHC II-deficient B cells are resistant to EAE regardless of the presence of myelin-specific antibodies102. Moreover, T cells from B cell-deficient mice could infiltrate into the CNS when EAE was induced with rrMOG1–125 protein, but T cell reactivation did not occur113, and it has been shown that when B cells have the same antigen specificity as T cells there is more differentiation towards proinflammatory Th1 and Th17 cells59. Of particular interest, disease severity is increased when T and B cells share antigen specificity58–60,87,98,114,115. In summary, antigen presentation by B cells, particularly when T and B cells overlap in their specificity for myelin antigen, and B cell-derived costimulation are important pathogenic processes during EAE and MS (Fig. 1b).
Antibody-independent mechanisms
I. Secretion of proinflammatory cytokines
Another form of stimulation B cells provide is via proinflammatory cytokine and chemokine secretion. B cells can secrete lymphotoxin (LT), tumor necrosis factor (TNF)-α, interleukin (IL)-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), BAFF, a proliferation-inducing ligand (APRIL), and IL-15 (Fig. 1c). Patients with MS show an increased LT to IL-10 ratio, and increased levels of LT and TNF-α upon reactivation with TLR-9 stimulation or interferon (IFN)-γ stimulation116. Culture of oligodendrocytes with LT and TNF-α demonstrates the ability of these two molecules to induce oligodendrocyte apoptosis117. Somewhat in disagreement with this observation was a study investigating the effect of co-culturing B cells and B cell secretory products from healthy controls and patients with MS with oligodendrocytes, which found no correlation between the levels of LT or TNF-α and oligodendrocyte death. However, secretory products from MS patient B cells induced more oligodendrocyte death than those from healthy controls118. In a MOG35–55 peptide-induced EAE model, LT-deficient mice show lower EAE disease severity than WT controls119. Moreover, it has been demonstrated that LT-β receptor is important for the establishment of TLOs (which will be discussed later) during EAE120. However, LT-deficient mice lack lymph nodes, which impairs T and B cell priming, and therefore could account for the observation that these animals do not develop EAE121,122; thus, the contribution of B cell-derived LT to EAE may not have been conclusively addressed yet. TNF-α confers both protective and pathogenic effects. TNF-α is important for induction of EAE123, but also for mediating CNS remyelination in vivo124 and regulating the induction of regulatory T cells125. These effects are likely due to different downstream effects of signals through TNF receptor 1 vs TNF receptor 2. However, B cell-specific TNF-signaling deficiency has not been studied. B cells from patients with MS produce more IL-6 than B cells from healthy controls, and mice had reduced EAE scores when B cells were deficient for IL-6126. This indicates a pathogenic role for B cell derived IL-6.
Recently, evidence from EAE models has suggested an important role of GM-CSF in MS. GM-CSF deficient C57BL/6 mice are resistant to development of EAE127. Moreover, GM-CSF promotes chronic EAE, where mice do not remit from disease128. Furthermore, lack of the GM-CSF receptor on T cells altered the extent of chronic EAE disease severity128, mice with GM-CSF retrovirally transduced antigen-specific T cells show severe and chronic disease compared with controls129, and patients with MS show significantly more GM-CSF+ T cells than controls130. Additionally, T cell derived GM-CSF is important for the effector phase of EAE131, and is required to activate microglia during EAE132. Interestingly, GM-CSF-producing B cells have been identified in patients with MS and these cells could activate myeloid cells in vitro133. However, the effect of B cell-specific GM-CSF (e.g. in mice with B cell-specific knockout of GM-CSF or GM-CSF receptor) has so far not been directly tested in EAE models.
BAFF and APRIL are regulators of B cell survival, maturation, and activation that are dysregulated in patients with MS134–138. APRIL has a soluble or membrane bound form and binds the transmembrane activator and cyclophilin ligand interactor (TACI), and the B cell maturation antigen (BCMA) receptors. Marmosets induced with EAE and treated with anti-APRIL antibodies showed decreased disease severity139, but the exact mechanism by which this cytokine acts in this context is unknown. Like APRIL, BAFF can be membrane-bound or soluble and can signal through the receptors BCMA, TACI, and, unlike APRIL, BAFF receptor (BAFF-R)137. BAFF-overexpressing mice show increased disease severity, ligand KO mice show decreased disease severity when compared with WT mice140, and marmosets induced with EAE and treated with anti-BAFF antibodies show a decrease in disease severity139. BAFF has also been implicated in the induction of TLOs134. Overall, current evidence indicates that BAFF signaling is pathogenic in EAE. Studies on the receptors of BAFF reveal a more pleotropic role for BAFF signaling. BAFF-R-deficient mice show exacerbated EAE141, but treatment of mice with a ligand antagonist that binds to BCMA suppresses disease142, and anti-TACI mAb treatment has been shown to reduce the number of pathogenic Th1 and Th17 T cell subsets, but not memory T cell subsets, but disease severity was not reported in this study143. Taken together, this indicates that there are either specific roles unique to each BAFF receptor, or specific roles for subsets of B cells that express these receptors. Specifically, while BAFF-R stimulation seems to be protective, BCMA and TACI signaling seems to be pathogenic during EAE. In addition to potentially signaling to induce pathogenic T cells140,143, BAFF is known to inhibit the production of a B cell-produced cytokine, IL-15144, potentially through the TACI receptor143. While the main function of IL-15 is to drive proliferation of CD8+ T cells and NK cells145, IL-15 KO mice exhibit exacerbated disease146. This could be because IL-15 is known to induce proliferation of a subset of CD8+ CD122+ T cells that have the capacity to suppress EAE by modulating Th17 cells147. While inhibiting BAFF-R signaling is protective in mice, the translational potential of targeting this pathway in MS patients was cast into question by adverse outcomes observed in a clinical trial with the BAFF- and APRIL-depleting TACI-Fc fusion protein, atacicept148, which will be elaborated on later.
II. Induction/establishment of tertiary lymphoid organs
Finally, B cells can contribute to the establishment of structures known as TLOs, a feature of diseases characterized by chronic inflammation, and as such a feature of disease such as rheumatoid arthritis (RA), Sjögren’s disease, and MS149. At the induction of TLO architecture, B cells along with T cells express LTα1/β2, promoting the differentiation of resident stromal cells into FDCs150–152. Additionally, B cells will express CXCL13103 and BAFF134, ultimately facilitating the formation of an architecture similar to GCs in secondary lymphoid organs1,149 (Fig. 1d). As these GC-like structures persist, they can potentially become a continual reservoir of autoreactive plasmablasts, plasma cells, and memory B cells1,153–155, perpetuating or even exacerbating autoimmune disease149. These structures have been identified in patients with MS and are thought to be important in relapses of MS, and in the trafficking and re-priming of other leukocytes relevant to the pathogenesis of MS23,24,153,154,156–160.
Protective B cell functions
The protective functions of B cells in MS are well-documented and include the production of anti-inflammatory cytokines and the use of inhibitory surface receptors to regulate other immune cells. Some studies have also shown that antibodies can exert beneficial functions in MS, such as inducing remyelination161,162 or clearing apoptotic cells73,74.
Regulatory B cells (Bregs), particularly the B10 subset in humans, have the capacity to secrete anti-inflammatory cytokines such as IL-10, TGF-β, and IL-35163–166. B cell-derived TGF-β has protective effects during EAE by inhibiting APC function167. IL-10 production is a common feature of several Breg subsets that are found in both humans and mice165,168 and have been shown to be necessary for recovery in EAE7. Interestingly, plasma cells can also secrete IL-10 in humans7,9. Finally, EAE studies showed that Breg binding of glucocorticoid-induced tumor necrosis factor-related receptor (GITR) by its ligand, (GITR-L), which is expressed on B cells, resulted in the expansion of regulatory T cell (Treg) populations independent of IL-101,169. Moreover, B cells can protect against EAE by suppressing T cell responses through the inhibitory receptor PD-1170. Collectively, these protective functions are thought to be a major contributor to the beneficial effects that accompany existing therapies, which we describe later in this review.
B cell-depleting therapies
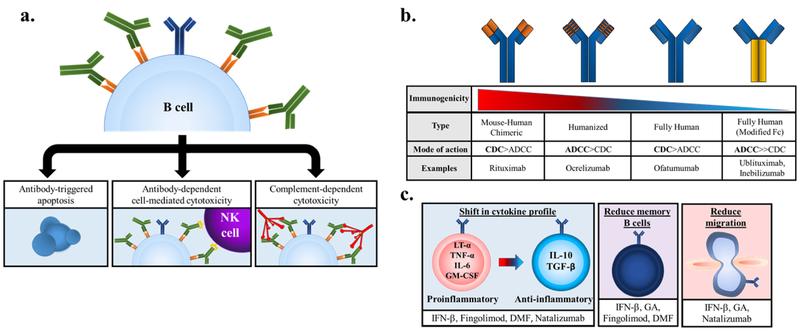
Several B cell-targeted therapies have been developed based on the growing body of evidence, from both mouse and human studies, of significant B cell involvement in the pathogenesis of MS. The most effective therapies employ mAbs to deplete B cells through NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-triggered apoptosis1,3,171 (Fig. 2a). Of note, these therapies do not typically reduce the levels of serum IgG, calling into question the role of autoantibodies in MS25,66,172. In clinical trials, B cell depletion therapies using mAbs directed against CD20 or CD19 have yielded promising results for the treatment of MS111.
Figure 2. The mechanisms of action of B cell-depleting msAbs and earlier MS DMTs.
(a) Binding of anti-CD19 or anti-CD20 mAbs mediates B cell depletion via different mechanisms: directly catalyzing antibody-triggered apoptosis (left), initiating antibody-dependent cell-mediated cytotoxicity (ADCC) by binding of the mAb’s Fc region by NK cell-expressed FcγRIIIa (middle), or initiating complement-dependent cytotoxicity (CDC) by binding C1q and activating the classical complement pathway (right). (b) B cell-depleting mAbs display differences in immunogenicity and preferential mechanism of action. (c) DMTs can shift B cell cytokine profiles from pro- to anti-inflammatory (left), reduce circulating memory B cells (middle), or impair migration through down-regulation or inhibition of surface receptors (right).
Anti-CD20 mAbs
CD20 is a surface receptor present primarily on B cells, functioning as an ion channel which serves to amplify calcium signals transduced by the BCR57,173. Importantly, CD20 is not expressed on pre-B cells or plasma cells, enabling reconstitution of the B cell compartment following depletion as well as preservation of pre-existing humoral immunity57,174. Following depletion, reconstitution primarily consists of naïve and immature B cells, while the memory B cells population remains low10,173,175. Considering that memory B cells exhibit an enhanced ability to stimulate myelin-reactive T cells, the sustained reduction in memory B cells suggests that targeting the APC function of B cells in MS might significantly contribute to the efficacy of anti-CD20 antibody therapies173.
Additionally, the reconstituted B cells exhibit reduced proinflammatory (GM-CSF, LT-α, TNF-α) and increased anti-inflammatory (IL-10) cytokine production173. Of note, a decrease in CD3+ T cells in the CSF of treated MS patients is also observed57. This could be a result of the depletion of CD20+ T cells, which are known to produce more inflammatory cytokines176,177 compared with CD20− T cells, although this may be somewhat speculative; more likely this decrease may be the result of the increased regulatory B cell activity173.
Taken together, these observations suggest that the depletion and subsequent reconstitution of the peripheral B cell compartment effectively shifts the B cell response from proinflammatory to anti-inflammatory111.
Presently there are 4 different anti-CD20 mAbs that have moved to phase III clinical trials for the treatment of RRMS: rituximab (RTX), ocrelizumab (OCR), ofatumumab (OFT), and ublituximab (UTX). In clinical trials, all four mAbs displayed similar efficacy and had favorable safety profiles, differing slightly in structure, target epitope, and predominant mechanism of B cell depletion3,111,178 (Fig. 2b).
RTX
Rituximab is a mouse-human chimeric IgG1 mAb to CD20, depleting B cells primarily through CDC3,178. RTX was the first anti-CD20 mAb tested as a treatment for MS and, after yielding surprisingly promising results in its initial phase II clinical trial, provided the rationale for investigating anti-CD20 as an effective treatment for MS, RRMS specifically111,173,178. In spite of the striking reduction in the number of new contrast-enhancing lesions as well as relapse rates, RTX has not entered subsequent phase III clinical trials for various reasons and therefore is used as an off-label therapy for MS patients173.
Ocrelizumab
The next mAb developed was ocrelizumab. Importantly, OCR differed from RTX in that it was a humanized IgG1 mAb. The reasoning for this was two-fold: firstly, since the chimeric nature of RTX resulted in 24.6% of patients developing anti-idiotypic antibodies in response to RTX treatment, a humanized mAb would be less immunogenic111,178; secondly, the humanized Fc region displayed a higher affinity for the FcγRIIIa receptors present on NK cells173, thereby enhancing the ADCC activity of OCR171,173,179,180. The efficacy of OCR was tested in three phase III trials, the results of which established OCR as the first anti-CD20 mAb to be approved by the FDA to treat both RRMS as well as primary progressive MS (PPMS)57.
Ofatumumab
Ofatumumab is a fully human mAb and, consequently, is less immunogenic than OCR181. A second unique feature is that OFT not only binds an epitope that is completely distinct from that of RTX or OCR178, but it does so with slower rate of dissociation from CD20 compared with RTX, resulting in enhanced CDC activity57,182–186. A phase III clinical trial (ASCLEPIOS I) testing the efficacy and safety of OFT compared to teriflunomide in the treatment of RRMS is currently underway (Clinicaltrials.gov number: NCT02792218).
Ublituximab
Ublituximab is, like OFT, a fully human mAb and binds a unique epitope on CD20. However, the Fc region has been glycoengineered to increase the affinity for FcγRIIIa, resulting in increased ADCC activity187. The results from a recently concluded phase II clinical trial are promising, and recruiting is underway for a phase III trial (ULTIMATE 1) comparing the efficacy of UTX to that of teriflunomide (Clinicaltrials.gov number: NCT03277261).
Anti-CD19 mAb
While both CD19 and CD20 are involved in B cell activation and differentiation, specifically through the modulation of BCR signaling57, they differ in the timing of their expression during the course of B cell development and maturation. Importantly, CD19 is expressed on plasmablasts and some plasma cells after CD20 expression is lost57. This differential expression allows for anti-CD19 mAb therapies to have a longer-lasting depletion effect188,189 and to eliminate pathogenic short-lived antibody-secreting cells, while preserving long-lived plasma cell populations.
MEDI-551 (inebilizumab), in addition to being humanized, which confers reduced immunogenicity, also lacks a fucose residue in the Fc region, which enables tighter binding to FcγRIIIa. MEDI-551, therefore, exhibits superior NK cell- and macrophage-mediated ADCC57,189.
In the EAE model, MEDI-551 has been shown to reduce leukocyte infiltration into the spinal cord and reduce the numbers of short- and long-lived autoreactive plasma cells in the spleen and bone marrow, while maintaining and expanding protective Breg and myelin-specific Treg populations, respectively57.
In human clinical trials, MEDI-551 has also been shown to reduce the mean number of cumulative new or enlarging MRI lesions over 24 weeks57.
Revisiting the effects of earlier DMTs on B cells
As mentioned previously, B cells can contribute by different effector mechanisms to the pathogenesis of MS, namely via autoantibody production, antigen presentation to and stimulation of autoreactive T cells, establishment of ectopic lymphoid structures, and secretion of proinflammatory cytokines such as IL-6 and LT-α9. While several therapies have been developed which directly target B cells, it has also been shown that earlier disease-modifying therapies (DMTs) have appreciable effects on the B cell contribution to MS9,112,173,190–199 (Fig. 2c), although the exact mechanism responsible for the beneficial effects observed remain incompletely understood3. These DMTs are thought to exert their effects on multiple cell types, further underscoring the complex inter-cellular dynamics that collectively result in the pathogenesis of MS. Here we will summarize the effects of earlier DMTs on the B cell component of MS pathogenesis.
Interferon-β
The mechanism underlying the therapeutic efficacy of IFN-β is poorly understood, but is generally attributed to the combination of various anti-inflammatory effects on several immune cell types. While IFN-β treatment results in a shift from Th1 to Th2 responses, it reduces the percentages of naïve B cells expressing the costimulatory molecule CD86196 and the chemokine receptor CCR5200, as well as reducing the memory B cell population201. Further adding to its cumulative anti-inflammatory effects, IFN-β also increases the frequency of IL-10-producing regulatory transitional B cells202,203. This was coupled with an increase in BAFF levels in the serum and plasma, the source of which was suggested to be monocytes and granulocytes8.
Glatiramer acetate
Glatiramer acetate (GA) is a synthetic amino acid copolymer comprised of L-alanine, L-lysine, L-glutamic acid, and L-tyrosine. GA, along with IFN-β, is a first-line medication used to treat MS. Although GA is known to act on B and T cells, the exact protective mechanisms remain incompletely understood204. Similar to IFN-β, GA treatment results in an increase in B cell-derived IL-10205 with a concomitant decrease in the production of IL-6, LT-α,3 and TNF-α173 in humans, corroborated by results from EAE studies showing a shift towards a regulatory B cell phenotype3. In RRMS patients, GA treatment causes a decrease in the expression of CXCR5 and intracellular adhesion molecule (ICAM)-3 in B cells, reducing their migratory potential182,206, as well as a decrease in the total circulating memory B cells and plasmablasts8, likely due to the reduced levels of BAFF in the CNS173.
Daclizumab
Daclizumab is a humanized IgG1 mAb to CD25, the alpha subunit of the IL-2 receptor. This treatment is primarily utilized to deplete the autoreactive CD4 T cell pool, however, it also reduces the memory B cell population, of which 60% express CD25171,207, and in addition a specific Breg subset208. However, daclizumab has been withdrawn from the market in light of 12 recent reports of serious inflammatory brain disorders, three of them being fatal209.
Fingolimod
Fingolimod, an antagonist to the sphingosine-1-phosphate (S1P) receptor, results in a reduction of circulating lymphocytes as well as decreasing the infiltration of potentially pathogenic or autoreactive lymphocytes into CNS8. In the periphery, the relative frequency of naïve and memory B cells is reduced198,210–213. Although the total number of B cells within the CNS is not significantly changed, there is an increase in the frequency of IL-10-producing Breg subsets in CSF3,208,213–220 as well as the peripheral blood173. Overall, treatment with fingolimod favors an increase in the frequency of regulatory B cells210,212,213,221.
Dimethyl fumarate
Although the exact mechanism of action is not well understood, the overall effect on B cells is similar to fingolimod. DMF treatment results in a decrease in total circulating B cells, a decrease in the frequency of naïve and memory B cells199,222–225 with a concurrent increase in IL-10-producing B cells in peripheral blood226, and a shift from a proinflammatory to an anti-inflammatory cytokine profile.
Natalizumab
Natalizumab prevents leukocyte migration into the CNS through the blood brain barrier (BBB)227 by targeting VLA-4, an adhesion molecule expressed on T and B cells, as well as on monocytes, basophils, neutrophils, and eosinophils228. The beneficial effects were initially attributed to the effects on T cell migration, however it was later shown in vitro that human B cell migration across endothelial cells was reduced to a greater extent than T cells229. Treatment causes an increase in memory B cells and a decrease in naïve B cells in the periphery230, but leads to B cell reduction in the CSF231,232. Natalizumab has also been reported to reduce the amount of IgG, IgM, and OCBs in the CSF3,173,231,233–236. An increase in peripheral memory B cells is also seen following treatment due to their high level of VLA-4 expression229,237,238, which could be potentially problematic upon discontinuation207. Natalizumab treatment is also associated with an increase in regulatory B cells171.
Alemtuzumab
Alemtuzumab is a humanized mAb to CD52, which is expressed on over 95% of B and T cells, but is also expressed on multiple other leukocyte populations239. Though its definitive function is unknown, evidence suggests that it serves as a costimulatory molecule for T cell activation and contributes to T cell migration240. Treatment with anti-CD52 mAb results in the ADCC- and CDC-dependent depletion of B and T cells173,182. Notably, immature transitional B cells and naïve mature B cells are increased upon reconstitution of the B cell compartment, while memory B cells remain reduced3.
Positive clinical outcomes from phase III clinical trials showing reduced relapse rates compared with IFN-β1α, are tempered by concerns over increasing occurrence of secondary autoimmune disease241, specifically antibody-mediated autoimmunity such as Graves’ disease and hypothyroidism among others182. It is thought that this effect could possibly be due to accelerated repopulation of immature B cells3 in the absence of regulation by T cells242, as B cells repopulate much faster than T cells3,173. Increases in serum IL-21, a cytokine implicated in the induction of Th17 cells, B cell differentiation and antibody production, and inhibition of Treg cells, correlates with the incidence of secondary autoimmunity following alemtuzumab treatment243 and is therefore a second contributor to this adverse effect.
Cladribine
Cladribine (NCT03364036/Mavenclad) is a chlorinated analogue of deoxyadenosine that eliminates B and T cells by manipulating the purine salvage pathway to ultimately impair DNA synthesis and repair244. The phosphorylation of cladribine is catalyzed by DCK but can be abrogated by the enzyme 5’-neucleotidase (5’-NTase)245. B and T cells express low levels of 5’-NTase relative to DCK245. Previous reports emphasized the primary target population as T cells, however the depletion of B cells is comparatively greater245,246. This selectivity is likely due to the finding that most B cell subsets express higher concentrations of DCK compared with T cells245. Importantly, it was recently shown that cladribine exerts its protective effects through the long-term depletion of memory B cells245 and that it has the ability to penetrate the BBB, a shortcoming of B cell-targeting mAbs246–248. Cladribine was shown to be efficacious in the treatment of RRMS in a phase III clinical trial. A 2-year phase IV clinical trial is currently recruiting to determine the onset of action in subjects with highly active RRMS.
Plasmapheresis
One of the earliest clinical observations pointing to the involvement of B cells in MS was the detection of OCBs in the CSF of MS patients57. In response to this, plasmapheresis, which is the removal of soluble products from the plasma, namely proinflammatory Ig and cytokines178,249, was applied to the treatment for MS. Although intrathecal OCBs are one of the hallmarks of MS, detected in the CSF of >95% of patients250–253, only patients exhibiting the most common demyelination pattern, pattern II, respond favorably to plasmapheresis3,254, suggesting that the role of B cells in MS is not primarily antibody-mediated. This is further supported by the maintenance of CSF immunoglobulin levels in patients treated with RTX or OCR3,178. However, plasmapheresis, plasma absorption, and therapeutic plasma exchange (TPE) are still used as suitable treatments for patients with severe and refractory MS relapses, particularly those unresponsive to steroids8, though whether the beneficial outcome is due to the removal of autoreactive immunoglobulin remains unknown3.
B cell-directed treatment strategies currently unexplored in MS
Anti-CD22 mAb (epratuzumab)
Epratuzumab is a humanized mAb targeting CD22, which is thought to be a negative regulator of BCR-derived activation signals and primarily expressed on mature B cells178. Epratuzumab induces only partial B cell depletion and was well tolerated in a phase III clinical trial for systemic lupus erythematosus (SLE), but has not yet been explored as a treatment for MS255.
Targeting plasmablasts and plasma cells
Treatments are also being explored which would target plasmablasts and plasma cells specifically. Daratumumab, a mAb to CD38, which is thought to selectively deplete plasmablasts and some plasma cells, is currently used to treat multiple myeloma and plasma cell malignancies, but is also being considered for MS treatment111,173. Anti-CD38 mAb treatment also depletes memory B cells, the reduction of which is thought to significantly contribute to the efficacy of several of the aforementioned DMTs256. However, the potential effectiveness of this treatment is called into question by studies showing that plasmablasts in humans are a source of IL-10, along with immature/transitional B cells, which also highly express CD3810,214,215. Regulatory myeloid-derived suppressor cells (MDSCs) and a novel subpopulation of Tregs also highly express CD38 and are therefore susceptible to depletion by daratumumab, although the degree to which this affects clinical outcome requires further investigation256.
Bortezomib, a small molecule proteasome inhibitor, has been approved for treatment of multiple myeloma. Additional studies using animal models of SLE, RA, autoimmune hemolytic anemia, and MG have shown promise in effectively depleting plasma cells10,178, though it is noteworthy that there is a lack of definitive evidence implicating plasmablasts and plasma cells as significant contributors to MS pathology.
Targeting B cell survival factors
BAFF and APRIL are soluble factors essential for the maturation, activation, and survival of B cells8,178,257. There are three separate receptors capable of binding both molecules, albeit with different affinities: BAFF-R, TACI, and BCMA. Adding to the complexity of this system is the observation that expression of these receptors varies greatly depending on the context. Evidence of increased serum levels of BAFF in MS patients suggested it as a potential target. However, in a phase II clinical trial for the TACI-Fc fusion protein atacicept, which depletes soluble BAFF and APRIL, treatment unexpectedly worsened MS in the participants and the trial was halted8,148,258. Additionally, in a phase II clinical trial to control optic neuritis, atacicept precipitated CNS inflammation and augmented the conversion of people with optic neuritis to clinically definite MS. Proposed reasons for its failure include the possible depletion of Breg populations, the evidence that APRIL has been suggested as a negative regulator of autoimmunity, and the relative increase in memory B cells173, although the exact reason is unknown8.
Since the atacicept trial, four additional antibodies targeting components of this system have been developed: belimumab and tabalumab, which are humanized mAbs to BAFF; VAY736, a fully human IgG1 anti-BAFF-R mAb; and human BCMA fused to IgG1 Fc (hBCMA-Fc)259. However, none of these have progressed past phase II clinical trials8,178.
Targeting B cell-produced GM-CSF
GM-CSF is thought to be involved in multiple aspects of MS pathogenesis and it is secreted by multiple cell types260. The frequency of GM-CSF-producing B cells is increased in MS patients, which is thought to enhance the proinflammatory myeloid cell responses133,261. Results from EAE studies showed that using antibodies against GM-CSF or its receptor ameliorates disease or prevented its onset260. Otilimab (MOR103/GSK3196165), a human recombinant antibody with high specificity for GM-CSF, has been developed, initially to treat RA. It has been tested on RRMS and secondary progressive MS (SPMS) patients in a small phase Ib clinical trial, the results showing that it was well-tolerated. Thus, selective suppression of GM-CSF production by pathogenic B cells may be beneficial because these cells are significant producers of this cytokine in MS260.
Targeting B cell signaling pathways
Signals received within inflamed tissues are known to skew B cells towards more pro-inflammatory and pathogenic subsets9. Therefore, interfering with intracellular signaling cascades in B cells using small molecule inhibitors has generated interest as a potential treatment for MS173. Particularly attractive is the potential of small molecular compounds to cross the BBB, a current deficiency of mAb therapies. One example is tofacitinib, which interferes with the JAK/STAT signaling pathway through the selective inhibition of Jak1 and Jak3. Tofacitinib treatment was shown to impair plasmablast development and thereby reduce Ig secretion in mouse and human B cells262. Memory B cell formation was also impaired, however, class switching was unaffected262. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, has been studied in the treatment of SLE, providing the basis for exploring its efficacy in the treatment of MS9.
Inducing IL-10-producing regulatory B cells
Given that the enhanced frequency and activity of IL-10-producing regulatory B cells is a key feature of several approved therapies, as detailed above, induction of regulatory B cell subsets would represent a promising treatment of MS3,10,263. Unfortunately, numerous obstacles have hindered the development of this therapeutic approach, namely the disparities between animal and human models regarding the phenotype of regulatory B cells5,168 and lack of suitable methods for inducing them10. One proposed method to facilitate the generation of regulatory B cells involves the expansion of IL-10+ B cells from patient PBMCs in vivo and subsequent transfer back into the patient10. However, further advances in characterizing the function and phenotype of B cell subsets in humans will be essential before this particular treatment becomes clinically feasible.
Future challenges/Concluding remarks
The benefit of targeting the B cell component in MS pathogenesis has been firmly established in light of the striking efficacy seen in clinical trials for B cell-depleting mAbs. However, variations in disease outcome represent a significant shortcoming of this type of therapy10. Several possible explanations for this have been postulated, including the inability to reach pathogenic B cell populations within the CNS, the depletion of beneficial regulatory B cell populations, and genetic polymorphisms affecting ADCC. Last, one might have to consider the likelihood that MS is the common clinical outcome of several differing pathogenic mechanisms, some which may not depend on B cells.
As depletion occurs only in the peripheral blood, perivascular space, and, less effectively, within CSF259, B cell-depleting mAbs may not sufficiently reach areas within the CNS in which pathogenic B cell populations are thought to reside, such as meningeal TLOs247,248,264. This is further supported by evidence showing that current anti-CD20 mAbs do not efficiently cross the BBB, primarily as a result of their size29,173. However, advances in antibody engineering are seeking to overcome this by exploiting receptor-mediated transcytosis (RMT) as a way for mAbs to cross the BBB without its disruption265. For example, engineered bispecific mAbs (bsAbs) where the antigen-binding fragment (Fab) of one antibody arm is directed against a physiological receptor on the BBB (e.g. transferrin receptor) and the other Fab is comprised of a “therapeutic arm” directed against a therapeutic target, show enhanced passage across the BBB265,266. Results using bsAbs in mouse models of Alzheimer’s showed that they could be a promising approach for improving B cell-targeted mAb treatments265.
The risk of eliminating beneficial anti-inflammatory regulatory B cells with global B cell-depleting therapies in addition to the intended pathogenic B cell populations may be ameliorated by developing novel therapies that more selectively target specific cell populations3,9. Additional hurdles stem from difficulties in definitively identifying certain B cell subsets in humans due to phenotypic differences between murine versus human B cells, namely memory B cell and regulatory B cell subsets168,267. Future advances in phenotypic and functional analysis of different B cell subsets will allow us to move toward developing therapies that are able to deplete only the pathogenic B cell populations while sparing those that are beneficial.
Another possible explanation for patients experiencing no significant improvement on B cell-depleting therapies is the existence of polymorphisms between patients in the Fc receptor region149. Since B cell-depleting mAbs utilize ADCC as a mechanism of action, these polymorphisms could potentially reduce the binding efficiency of the receptor to the depleting antibody, thereby limiting the effectiveness of treatment149.
Last but not least, a possible reason for inconsistent treatment outcomes seen in B cell-directed therapies may rest with the fact that the exact role B cells in MS is still somewhat enigmatic. Great strides have been made towards a better understanding of which B cell subsets are pathogenic, where they reside, and how they function, but there is still much left to be discovered. Thus, development of more effective B cell-directed therapies will be contingent on an enhanced understanding of B cell biology in health and disease, as well as a better understanding of the pathogenesis of MS. The mounting body of work deconvoluting the involvement of B cells in MS pathogenesis provides key insights aiding in the development of more effective B cell-directed therapeutics going forward.
Acknowledgments
This work was supported by grants NS42809, NS84201, and G12MD007591 from the National Institute of Health, and grants RG3701, RG5501, and RG1602 from the National Multiple Sclerosis Society (T.G.F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NMO was considered a subtype of MS until the discovery of aquaporin-4 (AQP4) autoantibodies.
REFERENCES CITED
- 1.Hampe CS B Cell in Autoimmune Diseases. Scientifica (Cairo) 2012, doi: 10.6064/2012/215308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manz RA, Hauser AE, Hiepe F & Radbruch A Maintenance of serum antibody levels. Annu Rev Immunol 23, 367–386, doi: 10.1146/annurev.immunol.23.021704.115723 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Lehmann-Horn K, Kinzel S & Weber MS Deciphering the Role of B Cells in Multiple Sclerosis-Towards Specific Targeting of Pathogenic Function. Int J Mol Sci 18, doi: 10.3390/ijms18102048 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lampropoulou V et al. Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll-like receptors in immunity. Immunological reviews 233, 146–161, doi: 10.1111/j.0105-2896.2009.00855.x (2010). [DOI] [PubMed] [Google Scholar]
- 5.Rosser EC & Mauri C Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612, doi: 10.1016/j.immuni.2015.04.005 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Wolf SD, Dittel BN, Hardardottir F & Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med 184, 2271–2278 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D & Anderton SM B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3, 944–950, doi: 10.1038/ni833 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Staun-Ram E & Miller A Effector and regulatory B cells in Multiple Sclerosis. Clinical immunology 184, 11–25, doi: 10.1016/j.clim.2017.04.014 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Li R, Patterson KR & Bar-Or A Reassessing B cell contributions in multiple sclerosis. Nat Immunol, doi: 10.1038/s41590-018-0135-x (2018). [DOI] [PubMed] [Google Scholar]
- 10.Hofmann K, Clauder AK & Manz RA Targeting B Cells and Plasma Cells in Autoimmune Diseases. Frontiers in immunology 9, 835, doi: 10.3389/fimmu.2018.00835 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabat EA, Moore DH & Landow H An Electrophoretic Study of the Protein Components in Cerebrospinal Fluid and Their Relationship to the Serum Proteins. J Clin Invest 21, 571–577, doi: 10.1172/JCI101335 (1942). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Budingen HC, Harrer MD, Kuenzle S, Meier M & Goebels N Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur J Immunol 38, 2014–2023, doi: 10.1002/eji.200737784 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Bankoti J et al. In multiple sclerosis, oligoclonal bands connect to peripheral B-cell responses. Annals of neurology 75, 266–276, doi: 10.1002/ana.24088 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermeier B et al. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. Journal of neuroimmunology 233, 245–248, doi: 10.1016/j.jneuroim.2011.01.010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baranzini SE et al. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. Journal of immunology 163, 5133–5144 (1999). [PubMed] [Google Scholar]
- 16.Palanichamy A et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Science translational medicine 6, 248ra106, doi: 10.1126/scitranslmed.3008930 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern JN et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Science translational medicine 6, 248ra107, doi: 10.1126/scitranslmed.3008879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo M et al. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. Journal of immunology 164, 2782–2789 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Qin Y et al. Intrathecal B-cell clonal expansion, an early sign of humoral immunity, in the cerebrospinal fluid of patients with clinically isolated syndrome suggestive of multiple sclerosis. Lab Invest 83, 1081–1088 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Beltran E et al. Intrathecal somatic hypermutation of IgM in multiple sclerosis and neuroinflammation. Brain 137, 2703–2714, doi: 10.1093/brain/awu205 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Eggers EL et al. Clonal relationships of CSF B cells in treatment-naive multiple sclerosis patients. JCI Insight 2, doi: 10.1172/jci.insight.92724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomakin YA et al. Polyreactive monoclonal autoantibodies in multiple sclerosis: functional selection from phage display library and characterization by deep sequencing analysis. Acta Naturae 5, 94–104 (2013). [PMC free article] [PubMed] [Google Scholar]
- 23.Michel L et al. B Cells in the Multiple Sclerosis Central Nervous System: Trafficking and Contribution to CNS-Compartmentalized Inflammation. Frontiers in immunology 6, 636, doi: 10.3389/fimmu.2015.00636 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovato L et al. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain 134, 534–541, doi: 10.1093/brain/awq350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cross AH, Stark JL, Lauber J, Ramsbottom MJ & Lyons JA Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. Journal of neuroimmunology 180, 63–70, doi: 10.1016/j.jneuroim.2006.06.029 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monson NL, Cravens PD, Frohman EM, Hawker K & Racke MK Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol 62, 258–264, doi: 10.1001/archneur.62.2.258 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Cross AH, Klein RS & Piccio L Rituximab combination therapy in relapsing multiple sclerosis. Therapeutic advances in neurological disorders 5, 311–319, doi: 10.1177/1756285612461165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlissel M Allelic exclusion of immunoglobulin gene rearrangement and expression: why and how? Seminars in immunology 14, 207–212; discussion 225–206 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Krumbholz M & Meinl E B cells in MS and NMO: pathogenesis and therapy. Semin Immunopathol 36, 339–350, doi: 10.1007/s00281-014-0424-x (2014). [DOI] [PubMed] [Google Scholar]
- 30.Hennecke J & Wiley DC T Cell Receptor-MHC Interactions up Close. Cell 104, 1–4, doi: 10.1016/S0092-8674(01)00185-4 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Brink R The imperfect control of self-reactive germinal center B cells. Current opinion in immunology 28, 97–101, doi: 10.1016/j.coi.2014.03.001 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Nemazee D Mechanisms of central tolerance for B cells. Nature reviews. Immunology 17, 281–294, doi: 10.1038/nri.2017.19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meffre E The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci 1246, 1–10, doi: 10.1111/j.1749-6632.2011.06347.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wardemann H et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377, doi: 10.1126/science.1086907 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Theofilopoulos AN, Kono DH & Baccala R The multiple pathways to autoimmunity. Nat Immunol 18, 716–724, doi: 10.1038/ni.3731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonegawa S Somatic generation of antibody diversity. Nature 302, 575–581 (1983). [DOI] [PubMed] [Google Scholar]
- 37.Brink R & Phan TG Self-Reactive B Cells in the Germinal Center Reaction. Annu Rev Immunol 36, 339–357, doi: 10.1146/annurev-immunol-051116-052510 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Wardemann H & Nussenzweig MC B-cell self-tolerance in humans. Adv Immunol 95, 83–110, doi: 10.1016/S0065-2776(07)95003-8 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Sabouri Z et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proceedings of the National Academy of Sciences of the United States of America 111, E2567–2575, doi: 10.1073/pnas.1406974111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gururajan M, Sindhava V & Bondada S B Cell Tolerance in Health and Disease. Antibodies 3, 116 (2014). [Google Scholar]
- 41.Kinnunen T et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood 121, 1595–1603, doi: 10.1182/blood-2012-09-457465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinnunen T et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest 123, 2737–2741, doi: 10.1172/JCI68775 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamel KM, Liarski VM & Clark MR Germinal center B-cells. Autoimmunity 45, 333–347, doi: 10.3109/08916934.2012.665524 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Gatto D & Brink R The germinal center reaction. The Journal of allergy and clinical immunology 126, 898–907; quiz 908–899, doi: 10.1016/j.jaci.2010.09.007 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Yarkoni Y, Getahun A & Cambier JC Molecular underpinning of B-cell anergy. Immunological reviews 237, 249–263, doi: 10.1111/j.1600-065X.2010.00936.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cyster JG & Goodnow CC Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity 3, 691–701, doi: 10.1016/1074-7613(95)90059-4 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Gauld SB, Benschop RJ, Merrell KT & Cambier JC Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nature Immunology 6, 1160, doi: 10.1038/ni1256 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Enders A et al. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med 198, 1119–1126, doi: 10.1084/jem.20030411 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koncz G & Hueber AO The Fas/CD95 Receptor Regulates the Death of Autoreactive B Cells and the Selection of Antigen-Specific B Cells. Frontiers in immunology 3, 207, doi: 10.3389/fimmu.2012.00207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews SF & Wilson PC The anergic B cell. Blood 115, 4976–4978, doi: 10.1182/blood-2010-03-276352 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Cappione A 3rd et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest 115, 3205–3216, doi: 10.1172/JCI24179 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathmell JC, Townsend SE, Xu JC, Flavell RA & Goodnow CC Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell 87, 319–329 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Vanderleyden I, Linterman MA & Smith KG Regulatory T cells and control of the germinal centre response. Arthritis research & therapy 16, 471 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhaeze T et al. Circulating Follicular Regulatory T Cells Are Defective in Multiple Sclerosis. Journal of immunology 195, 832–840, doi: 10.4049/jimmunol.1500759 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Procaccini C, De Rosa V, Pucino V, Formisano L & Matarese G Animal models of Multiple Sclerosis. Eur J Pharmacol 759, 182–191, doi: 10.1016/j.ejphar.2015.03.042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitamura D, Roes J, Kuhn R & Rajewsky K A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350, 423–426, doi: 10.1038/350423a0 (1991). [DOI] [PubMed] [Google Scholar]
- 57.Forsthuber TG, Cimbora DM, Ratchford JN, Katz E & Stuve O B cell-based therapies in CNS autoimmunity: differentiating CD19 and CD20 as therapeutic targets. Therapeutic advances in neurological disorders 11, 1756286418761697, doi: 10.1177/1756286418761697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyons JA, San M, Happ MP & Cross AH B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol 29, 3432–3439, doi: (1999). [DOI] [PubMed] [Google Scholar]
- 59.Weber MS et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Annals of neurology 68, 369–383, doi: 10.1002/ana.22081 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monson NL et al. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PloS one 6, e17103, doi: 10.1371/journal.pone.0017103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macpherson AJ et al. IgA production without mu or delta chain expression in developing B cells. Nat Immunol 2, 625–631, doi: 10.1038/89775 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Ghosh S, Hoselton SA & Schuh JM mu-chain-deficient mice possess B-1 cells and produce IgG and IgE, but not IgA, following systemic sensitization and inhalational challenge in a fungal asthma model. Journal of immunology 189, 1322–1329, doi: 10.4049/jimmunol.1200138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sefia E, Pryce G, Meier UC, Giovannoni G & Baker D Depletion of CD20 B cells fails to inhibit relapsing mouse experimental autoimmune encephalomyelitis. Mult Scler Relat Disord 14, 46–50, doi: 10.1016/j.msard.2017.03.013 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Jagessar SA et al. B-cell depletion abrogates T cell-mediated demyelination in an antibody-nondependent common marmoset experimental autoimmune encephalomyelitis model. J Neuropathol Exp Neurol 71, 716–728, doi: 10.1097/NEN.0b013e3182622691 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Kap YS et al. Late B cell depletion with a human anti-human CD20 IgG1kappa monoclonal antibody halts the development of experimental autoimmune encephalomyelitis in marmosets. Journal of immunology 185, 3990–4003, doi: 10.4049/jimmunol.1001393 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Hauser SL et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. The New England journal of medicine 358, 676–688, doi: 10.1056/NEJMoa0706383 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Hawker K et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Annals of neurology 66, 460–471, doi: 10.1002/ana.21867 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Chen D et al. Autoreactive CD19+CD20- Plasma Cells Contribute to Disease Severity of Experimental Autoimmune Encephalomyelitis. Journal of immunology 196, 1541–1549, doi: 10.4049/jimmunol.1501376 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Chen D et al. Single dose of glycoengineered anti-CD19 antibody (MEDI551) disrupts experimental autoimmune encephalomyelitis by inhibiting pathogenic adaptive immune responses in the bone marrow and spinal cord while preserving peripheral regulatory mechanisms. Journal of immunology 193, 4823–4832, doi: 10.4049/jimmunol.1401478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agius MA et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: Results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler, 1352458517740641, doi: 10.1177/1352458517740641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McHeyzer-Williams MG & Nossal GJ Clonal analysis of autoantibody-producing cell precursors in the preimmune B cell repertoire. Journal of immunology 141, 4118–4123 (1988). [PubMed] [Google Scholar]
- 72.Shlomchik MJ Sites and stages of autoreactive B cell activation and regulation. Immunity 28, 18–28, doi: 10.1016/j.immuni.2007.12.004 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Chou MY et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 119, 1335–1349, doi: 10.1172/JCI36800 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardy RR & Hayakawa K Development of B cells producing natural autoantibodies to thymocytes and senescent erythrocytes. Springer Semin Immunopathol 26, 363–375, doi: 10.1007/s00281-004-0183-1 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Correale J & de los Milagros Bassani Molinas, M. Oligoclonal bands and antibody responses in multiple sclerosis. J Neurol 249, 375–389, doi: 10.1007/s004150200026 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Genain CP, Cannella B, Hauser SL & Raine CS Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 5, 170–175, doi: 10.1038/5532 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Lopomo A & Berrih-Aknin S Autoimmune Thyroiditis and Myasthenia Gravis. Front Endocrinol (Lausanne) 8, 169, doi: 10.3389/fendo.2017.00169 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Connor KC et al. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. Journal of immunology 175, 1974–1982 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou D et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America 103, 19057–19062, doi: 10.1073/pnas.0607242103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schluesener HJ, Sobel RA, Linington C & Weiner HL A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol 139, 4016–4021 (1987). [PubMed] [Google Scholar]
- 81.Challa DK et al. Autoantibody depletion ameliorates disease in murine experimental autoimmune encephalomyelitis. mAbs 5, 655–659, doi: 10.4161/mabs.25439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ponomarenko NA et al. Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunology letters 103, 45–50, doi: 10.1016/j.imlet.2005.10.006 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Kuhle J et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. The New England journal of medicine 356, 371–378, doi: 10.1056/NEJMoa063602 (2007). [DOI] [PubMed] [Google Scholar]
- 84.O’Connor KC et al. Anti-myelin antibodies modulate clinical expression of childhood multiple sclerosis. Journal of neuroimmunology 223, 92–99, doi: 10.1016/j.jneuroim.2010.02.019 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Marta CB et al. Signaling cascades activated upon antibody cross-linking of myelin oligodendrocyte glycoprotein: potential implications for multiple sclerosis. The Journal of biological chemistry 280, 8985–8993, doi: 10.1074/jbc.M413174200 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Ohtani S, Kohyama K & Matsumoto Y Autoantibodies recognizing native MOG are closely associated with active demyelination but not with neuroinflammation in chronic EAE. Neuropathology 31, 101–111, doi: 10.1111/j.1440-1789.2010.01131.x (2011). [DOI] [PubMed] [Google Scholar]
- 87.von Budingen HC et al. Frontline: Epitope recognition on the myelin/oligodendrocyte glycoprotein differentially influences disease phenotype and antibody effector functions in autoimmune demyelination. Eur J Immunol 34, 2072–2083, doi: 10.1002/eji.200425050 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Storch MK et al. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Annals of neurology 43, 465–471, doi: 10.1002/ana.410430409 (1998). [DOI] [PubMed] [Google Scholar]
- 89.Breij EC et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Annals of neurology 63, 16–25, doi: 10.1002/ana.21311 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Hundgeburth LC et al. The complement system contributes to the pathology of experimental autoimmune encephalomyelitis by triggering demyelination and modifying the antigen-specific T and B cell response. Clinical immunology 146, 155–164, doi: 10.1016/j.clim.2012.12.007 (2013). [DOI] [PubMed] [Google Scholar]
- 91.Terenyi N et al. Transient decomplementation of mice delays onset of experimental autoimmune encephalomyelitis and impairs MOG-specific T cell response and autoantibody production. Mol Immunol 47, 57–63, doi: 10.1016/j.molimm.2008.12.026 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Genain CP et al. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. J Clin Invest 96, 2966–2974, doi: 10.1172/JCI118368 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lucchinetti C et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47, 707–717 (2000). [DOI] [PubMed] [Google Scholar]
- 94.Dale RC et al. Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurology(R) neuroimmunology & neuroinflammation 1, e12, doi: 10.1212/NXI.0000000000000012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marta CB, Oliver AR, Sweet RA, Pfeiffer SE & Ruddle NH Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology. Proceedings of the National Academy of Sciences of the United States of America 102, 13992–13997, doi: 10.1073/pnas.0504979102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Menon KK, Piddlesden SJ & Bernard CC Demyelinating antibodies to myelin oligodendrocyte glycoprotein and galactocerebroside induce degradation of myelin basic protein in isolated human myelin. J Neurochem 69, 214–222 (1997). [DOI] [PubMed] [Google Scholar]
- 97.Liu Y et al. Myelin-specific multiple sclerosis antibodies cause complement-dependent oligodendrocyte loss and demyelination. Acta Neuropathol Commun 5, 25, doi: 10.1186/s40478-017-0428-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flach AC et al. Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proceedings of the National Academy of Sciences of the United States of America 113, 3323–3328, doi: 10.1073/pnas.1519608113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sommer MA, Forno LS & Smith ME EAE cerebrospinal fluid augments in vitro phagocytosis and metabolism of CNS myelin by macrophages. Journal of neuroscience research 32, 384–394, doi: 10.1002/jnr.490320310 (1992). [DOI] [PubMed] [Google Scholar]
- 100.Kinzel S et al. Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta neuropathologica 132, 43–58, doi: 10.1007/s00401-016-1559-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van der Goes A, Kortekaas M, Hoekstra K, Dijkstra CD & Amor S The role of anti-myelin (auto)-antibodies in the phagocytosis of myelin by macrophages. Journal of neuroimmunology 101, 61–67 (1999). [DOI] [PubMed] [Google Scholar]
- 102.Molnarfi N et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med 210, 2921–2937, doi: 10.1084/jem.20130699 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.von Budingen HC, Palanichamy A, Lehmann-Horn K, Michel BA & Zamvil SS Update on the autoimmune pathology of multiple sclerosis: B-cells as disease-drivers and therapeutic targets. Eur Neurol 73, 238–246, doi: 10.1159/000377675 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harp CT et al. Memory B cells from a subset of treatment-naive relapsing-remitting multiple sclerosis patients elicit CD4(+) T-cell proliferation and IFN-gamma production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. Eur J Immunol 40, 2942–2956, doi: 10.1002/eji.201040516 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pierce SK et al. Antigen-presenting function of B lymphocytes. Immunological reviews 106, 149–180 (1988). [DOI] [PubMed] [Google Scholar]
- 106.Rivera A, Chen CC, Ron N, Dougherty JP & Ron Y Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol 13, 1583–1593 (2001). [DOI] [PubMed] [Google Scholar]
- 107.Chesnut RW & Grey HM Antigen presenting cells and mechanisms of antigen presentation. Crit Rev Immunol 5, 263–316 (1985). [PubMed] [Google Scholar]
- 108.Lanzavecchia A Antigen uptake and accumulation in antigen-specific B cells. Immunological reviews 99, 39–51 (1987). [DOI] [PubMed] [Google Scholar]
- 109.Watts C & Davidson HW Endocytosis and recycling of specific antigen by human B cell lines. EMBO J 7, 1937–1945 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bennett JL et al. B lymphocytes in neuromyelitis optica. Neurology(R) neuroimmunology & neuroinflammation 2, e104, doi: 10.1212/NXI.0000000000000104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greenfield AL & Hauser SL B-cell Therapy for Multiple Sclerosis: Entering an era. Annals of neurology 83, 13–26, doi: 10.1002/ana.25119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Genc K, Dona DL & Reder AT Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest 99, 2664–2671, doi: 10.1172/JCI119455 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pierson ER, Stromnes IM & Goverman JM B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. Journal of immunology 192, 929–939, doi: 10.4049/jimmunol.1302171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang LY & Fujinami RS Enhancement of EAE and induction of autoantibodies to T-cell epitopes in mice infected with a recombinant vaccinia virus encoding myelin proteolipid protein. Journal of neuroimmunology 75, 75–83 (1997). [DOI] [PubMed] [Google Scholar]
- 115.Matsumoto Y, Park IK, Hiraki K, Ohtani S & Kohyama K Role of pathogenic T cells and autoantibodies in relapse and progression of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis in LEW.1AV1 rats. Immunology 128, e250–261, doi: 10.1111/j.1365-2567.2008.02955.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bar-Or A et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Annals of neurology 67, 452–461, doi: 10.1002/ana.21939 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Selmaj K, Raine CS, Farooq M, Norton WT & Brosnan CF Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. Journal of immunology 147, 1522–1529 (1991). [PubMed] [Google Scholar]
- 118.Lisak RP et al. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. Journal of neuroimmunology 246, 85–95, doi: 10.1016/j.jneuroim.2012.02.015 (2012). [DOI] [PubMed] [Google Scholar]
- 119.Suen WE, Bergman CM, Hjelmstrom P & Ruddle NH A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med 186, 1233–1240 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Columba-Cabezas S et al. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin beta receptor-Ig fusion protein. Journal of neuroimmunology 179, 76–86, doi: 10.1016/j.jneuroim.2006.06.015 (2006). [DOI] [PubMed] [Google Scholar]
- 121.Banks TA et al. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. Journal of immunology 155, 1685–1693 (1995). [PubMed] [Google Scholar]
- 122.Chiang EY et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nature medicine 15, 766–773, doi: 10.1038/nm.1984 (2009). [DOI] [PubMed] [Google Scholar]
- 123.Nishibori T, Tanabe Y, Su L & David M Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med 199, 25–34, doi: 10.1084/jem.20020509 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arnett HA et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4, 1116–1122, doi: 10.1038/nn738 (2001). [DOI] [PubMed] [Google Scholar]
- 125.Tsakiri N, Papadopoulos D, Denis MC, Mitsikostas DD & Kollias G TNFR2 on non-haematopoietic cells is required for Foxp3+ Treg-cell function and disease suppression in EAE. Eur J Immunol 42, 403–412, doi: 10.1002/eji.201141659 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Barr TA et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209, 1001–1010, doi: 10.1084/jem.20111675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McQualter JL et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med 194, 873–882 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duncker PC, Stoolman JS, Huber AK & Segal BM GM-CSF Promotes Chronic Disability in Experimental Autoimmune Encephalomyelitis by Altering the Composition of Central Nervous System-Infiltrating Cells, but Is Dispensable for Disease Induction. Journal of immunology 200, 966–973, doi: 10.4049/jimmunol.1701484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marusic S et al. Local delivery of granulocyte macrophage colony-stimulating factor by retrovirally transduced antigen-specific T cells leads to severe, chronic experimental autoimmune encephalomyelitis in mice. Neurosci Lett 332, 185–189 (2002). [DOI] [PubMed] [Google Scholar]
- 130.Rasouli J et al. Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-beta Therapy. Journal of immunology 194, 5085–5093, doi: 10.4049/jimmunol.1403243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Codarri L et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12, 560–567, doi: 10.1038/ni.2027 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Ponomarev ED et al. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol 178, 39–48 (2007). [DOI] [PubMed] [Google Scholar]
- 133.Li R et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Science translational medicine 7, 310ra166, doi: 10.1126/scitranslmed.aab4176 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Magliozzi R, Columba-Cabezas S, Serafini B & Aloisi F Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. Journal of neuroimmunology 148, 11–23, doi: 10.1016/j.jneuroim.2003.10.056 (2004). [DOI] [PubMed] [Google Scholar]
- 135.Krumbholz M et al. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201, 195–200, doi: 10.1084/jem.20041674 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haugen M, Frederiksen JL & Degn M B cell follicle-like structures in multiple sclerosis-with focus on the role of B cell activating factor. Journal of neuroimmunology 273, 1–7, doi: 10.1016/j.jneuroim.2014.05.010 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA & Mackay F The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev 24, 203–215, doi: 10.1016/j.cytogfr.2013.04.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Piazza F et al. Cerebrospinal fluid levels of BAFF and APRIL in untreated multiple sclerosis. Journal of neuroimmunology 220, 104–107, doi: 10.1016/j.jneuroim.2010.01.011 (2010). [DOI] [PubMed] [Google Scholar]
- 139.Jagessar SA et al. Antibodies against human BLyS and APRIL attenuate EAE development in marmoset monkeys. J Neuroimmune Pharmacol 7, 557–570, doi: 10.1007/s11481-012-9384-x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou X et al. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. PloS one 6, e23629, doi: 10.1371/journal.pone.0023629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim SS, Richman DP, Zamvil SS & Agius MA Accelerated central nervous system autoimmunity in BAFF-receptor-deficient mice. J Neurol Sci 306, 9–15, doi: 10.1016/j.jns.2011.04.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huntington ND et al. A BAFF antagonist suppresses experimental autoimmune encephalomyelitis by targeting cell-mediated and humoral immune responses. Int Immunol 18, 1473–1485, doi: 10.1093/intimm/dxl080 (2006). [DOI] [PubMed] [Google Scholar]
- 143.Wang X et al. Blockade of B-cell activating factor with TACI-IgG effectively reduced Th1 and Th17 cells but not memory T cells in experimental allergic encephalomyelitis mice. Cent Eur J Immunol 40, 142–148, doi: 10.5114/ceji.2015.52826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma N et al. BAFF suppresses IL-15 expression in B cells. Journal of immunology 192, 4192–4201, doi: 10.4049/jimmunol.1302132 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Schneider R et al. B cell-derived IL-15 enhances CD8 T cell cytotoxicity and is increased in multiple sclerosis patients. Journal of immunology 187, 4119–4128, doi: 10.4049/jimmunol.1100885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gomez-Nicola D, Spagnolo A, Guaza C & Nieto-Sampedro M Aggravated experimental autoimmune encephalomyelitis in IL-15 knockout mice. Exp Neurol 222, 235–242, doi: 10.1016/j.expneurol.2009.12.034 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Yu P, Bamford RN & Waldmann TA IL-15-dependent CD8+ CD122+ T cells ameliorate experimental autoimmune encephalomyelitis by modulating IL-17 production by CD4+ T cells. Eur J Immunol 44, 3330–3341, doi: 10.1002/eji.201444675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kappos L et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol 13, 353–363, doi: 10.1016/S1474-4422(14)70028-6 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Browning JL B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nature reviews. Drug discovery 5, 564–576, doi: 10.1038/nrd2085 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Ware CF Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 23, 787–819, doi: 10.1146/annurev.immunol.23.021704.115719 (2005). [DOI] [PubMed] [Google Scholar]
- 151.Kratz A, Campos-Neto A, Hanson MS & Ruddle NH Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med 183, 1461–1472 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drayton DL, Ying X, Lee J, Lesslauer W & Ruddle NH Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med 197, 1153–1163, doi: 10.1084/jem.20021761 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E & Aloisi F Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14, 164–174 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Magliozzi R et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007). [DOI] [PubMed] [Google Scholar]
- 155.Lehmann-Horn K, Wang SZ, Sagan SA, Zamvil SS & von Budingen HC B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue. JCI Insight 1, e87234, doi: 10.1172/jci.insight.87234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Howell OW et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–2771, doi: 10.1093/brain/awr182 (2011). [DOI] [PubMed] [Google Scholar]
- 157.Louveau A et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341, doi: 10.1038/nature14432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Magliozzi R et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Annals of neurology 68, 477–493, doi: 10.1002/ana.22230 (2010). [DOI] [PubMed] [Google Scholar]
- 159.Prineas JW & Wright RG Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab Invest 38, 409–421 (1978). [PubMed] [Google Scholar]
- 160.von Budingen HC et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest 122, 4533–4543, doi: 10.1172/JCI63842 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ciric B et al. Human monoclonal IgM antibody promotes CNS myelin repair independent of Fc function. Brain Pathol 13, 608–616 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wootla B, Denic A, Watzlawik JO, Warrington AE & Rodriguez M Antibody-Mediated Oligodendrocyte Remyelination Promotes Axon Health in Progressive Demyelinating Disease. Mol Neurobiol 53, 5217–5228, doi: 10.1007/s12035-015-9436-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Duddy ME, Alter A & Bar-Or A Distinct profiles of human B cell effector cytokines: a role in immune regulation? Journal of immunology 172, 3422–3427 (2004). [DOI] [PubMed] [Google Scholar]
- 164.Matsushita T, Horikawa M, Iwata Y & Tedder TF Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol 185, 2240–2252, doi: 10.4049/jimmunol.1001307 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M & Tedder TF Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 118, 3420–3430, doi: 10.1172/JCI36030 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shen P et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370, doi: 10.1038/nature12979 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bjarnadottir K et al. B cell-derived transforming growth factor-beta1 expression limits the induction phase of autoimmune neuroinflammation. Scientific reports 6, 34594, doi: 10.1038/srep34594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mauri C & Menon M The expanding family of regulatory B cells. Int Immunol 27, 479–486, doi: 10.1093/intimm/dxv038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ray A, Basu S, Williams CB, Salzman NH & Dittel BN A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. Journal of immunology 188, 3188–3198, doi: 10.4049/jimmunol.1103354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bodhankar S, Galipeau D, Vandenbark AA & Offner H PD-1 Interaction with PD-L1 but not PD-L2 on B-cells Mediates Protective Effects of Estrogen against EAE. J Clin Cell Immunol 4, 143, doi: 10.4172/2155-9899.1000143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dubey D et al. Clinical management of multiple sclerosis and neuromyelitis optica with therapeutic monoclonal antibodies: approved therapies and emerging candidates. Expert review of clinical immunology 11, 93–108, doi: 10.1586/1744666X.2015.992881 (2015). [DOI] [PubMed] [Google Scholar]
- 172.von Budingen HC et al. Onset of secondary progressive MS after long-term rituximab therapy - a case report. Ann Clin Transl Neurol 4, 46–52, doi: 10.1002/acn3.377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sabatino JJ Jr., Zamvil SS & Hauser SL B-Cell Therapies in Multiple Sclerosis. Cold Spring Harb Perspect Med, doi: 10.1101/cshperspect.a032037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Du FH, Mills EA & Mao-Draayer Y Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights 8, 12, doi: 10.1007/s13317-017-0100-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roll P, Palanichamy A, Kneitz C, Dorner T & Tony HP Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum 54, 2377–2386, doi: 10.1002/art.22019 (2006). [DOI] [PubMed] [Google Scholar]
- 176.Palanichamy A et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. Journal of immunology 193, 580–586, doi: 10.4049/jimmunol.1400118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schuh E et al. Features of Human CD3+CD20+ T Cells. Journal of immunology 197, 1111–1117, doi: 10.4049/jimmunol.1600089 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Dubey D, Forsthuber T, Flanagan EP, Pittock SJ & Stuve O B-cell-targeted therapies in relapsing forms of MS. Neurology(R) neuroimmunology & neuroinflammation 4, e405, doi: 10.1212/NXI.0000000000000405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kappos L et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 378, 1779–1787 (2011). [DOI] [PubMed] [Google Scholar]
- 180.Kausar F et al. Ocrelizumab: a step forward in the evolution of B-cell therapy. Expert opinion on biological therapy 9, 889–895, doi: 10.1517/14712590903018837 (2009). [DOI] [PubMed] [Google Scholar]
- 181.Sorensen PS New management algorithms in multiple sclerosis. Curr Opin Neurol 27, 246–259, doi: 10.1097/WCO.0000000000000096 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Milo R Therapeutic strategies targeting B-cells in multiple sclerosis. Autoimmun Rev 15, 714–718, doi: 10.1016/j.autrev.2016.03.006 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Li B et al. Development of novel tetravalent anti-CD20 antibodies with potent antitumor activity. Cancer Res 68, 2400–2408, doi: 10.1158/0008-5472.CAN-07-6663 (2008). [DOI] [PubMed] [Google Scholar]
- 184.Teeling JL et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104, 1793–1800, doi: 10.1182/blood-2004-01-0039 (2004). [DOI] [PubMed] [Google Scholar]
- 185.Teeling JL et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. Journal of immunology 177, 362–371 (2006). [DOI] [PubMed] [Google Scholar]
- 186.Sorensen PS et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 82, 573–581, doi: 10.1212/WNL.0000000000000125 (2014). [DOI] [PubMed] [Google Scholar]
- 187.Sharman JP et al. Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial. Br J Haematol 176, 412–420, doi: 10.1111/bjh.14447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gasperi C, Stuve O & Hemmer B B cell-directed therapies in multiple sclerosis. Neurodegener Dis Manag 6, 37–47, doi: 10.2217/nmt.15.67 (2016). [DOI] [PubMed] [Google Scholar]
- 189.Chen D, Gallagher S, Monson NL, Herbst R & Wang Y Inebilizumab, a B Cell-Depleting Anti-CD19 Antibody for the Treatment of Autoimmune Neurological Diseases: Insights from Preclinical Studies. J Clin Med 5, doi: 10.3390/jcm5120107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cupps TR, Gerrard TL, Falkoff RJ, Whalen G & Fauci AS Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation. J Clin Invest 75, 754–761, doi: 10.1172/JCI111757 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Duda PW, Schmied MC, Cook SL, Krieger JI & Hafler DA Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J Clin Invest 105, 967–976, doi: 10.1172/JCI8970 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Salama HH, Hong J, Zang YC, El-Mongui A & Zhang J Blocking effects of serum reactive antibodies induced by glatiramer acetate treatment in multiple sclerosis. Brain 126, 2638–2647, doi: 10.1093/brain/awg269 (2003). [DOI] [PubMed] [Google Scholar]
- 193.Duddy M et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. Journal of immunology 178, 6092–6099 (2007). [DOI] [PubMed] [Google Scholar]
- 194.Begum-Haque S et al. Increased expression of B cell-associated regulatory cytokines by glatiramer acetate in mice with experimental autoimmune encephalomyelitis. Journal of neuroimmunology 219, 47–53, doi: 10.1016/j.jneuroim.2009.11.016 (2010). [DOI] [PubMed] [Google Scholar]
- 195.Kala M et al. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp Neurol 221, 136–145, doi: 10.1016/j.expneurol.2009.10.015 (2010). [DOI] [PubMed] [Google Scholar]
- 196.Ramgolam VS et al. B cells as a therapeutic target for IFN-beta in relapsing-remitting multiple sclerosis. Journal of immunology 186, 4518–4526, doi: 10.4049/jimmunol.1000271 (2011). [DOI] [PubMed] [Google Scholar]
- 197.Miyazaki Y et al. A novel microRNA-132-sirtuin-1 axis underlies aberrant B-cell cytokine regulation in patients with relapsing-remitting multiple sclerosis [corrected]. PloS one 9, e105421, doi: 10.1371/journal.pone.0105421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakamura M et al. Differential effects of fingolimod on B-cell populations in multiple sclerosis. Mult Scler 20, 1371–1380, doi: 10.1177/1352458514523496 (2014). [DOI] [PubMed] [Google Scholar]
- 199.Li R et al. Dimethyl Fumarate Treatment Mediates an Anti-Inflammatory Shift in B Cell Subsets of Patients with Multiple Sclerosis. Journal of immunology 198, 691–698, doi: 10.4049/jimmunol.1601649 (2017). [DOI] [PubMed] [Google Scholar]
- 200.Niino M, Hirotani M, Miyazaki Y & Sasaki H Memory and naive B-cell subsets in patients with multiple sclerosis. Neurosci Lett 464, 74–78, doi: 10.1016/j.neulet.2009.08.010 (2009). [DOI] [PubMed] [Google Scholar]
- 201.Rizzo F et al. Interferon-beta therapy specifically reduces pathogenic memory B cells in multiple sclerosis patients by inducing a FAS-mediated apoptosis. Immunol Cell Biol 94, 886–894, doi: 10.1038/icb.2016.55 (2016). [DOI] [PubMed] [Google Scholar]
- 202.Stuve O, Chabot S, Jung SS, Williams G & Yong VW Chemokine-enhanced migration of human peripheral blood mononuclear cells is antagonized by interferon beta-1b through an effect on matrix metalloproteinase-9. Journal of neuroimmunology 80, 38–46 (1997). [DOI] [PubMed] [Google Scholar]
- 203.Schubert RD et al. IFN-beta treatment requires B cells for efficacy in neuroautoimmunity. Journal of immunology 194, 2110–2116, doi: 10.4049/jimmunol.1402029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ahn YH et al. Glatiramer acetate attenuates the activation of CD4(+) T cells by modulating STAT1 and −3 signaling in glia. Scientific reports 7, 40484, doi: 10.1038/srep40484 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ireland SJ et al. The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA neurology 71, 1421–1428, doi: 10.1001/jamaneurol.2014.1472 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sellner J et al. Glatiramer acetate attenuates the pro-migratory profile of adhesion molecules on various immune cell subsets in multiple sclerosis. Clin Exp Immunol 173, 381–389, doi: 10.1111/cei.12125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Baker D, Marta M, Pryce G, Giovannoni G & Schmierer K Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis. EBioMedicine 16, 41–50, doi: 10.1016/j.ebiom.2017.01.042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.de Andres C et al. New regulatory CD19(+)CD25(+) B-cell subset in clinically isolated syndrome and multiple sclerosis relapse. Changes after glucocorticoids. Journal of neuroimmunology 270, 37–44, doi: 10.1016/j.jneuroim.2014.02.003 (2014). [DOI] [PubMed] [Google Scholar]
- 209.Hawkes N MS drug is suspended after reports of inflammatory brain disorders. BMJ 360, k1114, doi: 10.1136/bmj.k1114 (2018). [DOI] [PubMed] [Google Scholar]
- 210.Blumenfeld S, Staun-Ram E & Miller A Fingolimod therapy modulates circulating B cell composition, increases B regulatory subsets and production of IL-10 and TGFbeta in patients with Multiple Sclerosis. J Autoimmun 70, 40–51, doi: 10.1016/j.jaut.2016.03.012 (2016). [DOI] [PubMed] [Google Scholar]
- 211.Claes N et al. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PloS one 9, e111115, doi: 10.1371/journal.pone.0111115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Grutzke B et al. Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann Clin Transl Neurol 2, 119–130, doi: 10.1002/acn3.155 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miyazaki Y et al. Suppressed pro-inflammatory properties of circulating B cells in patients with multiple sclerosis treated with fingolimod, based on altered proportions of B-cell subpopulations. Clinical immunology 151, 127–135, doi: 10.1016/j.clim.2014.02.001 (2014). [DOI] [PubMed] [Google Scholar]
- 214.Flores-Borja F et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Science translational medicine 5, 173ra123, doi: 10.1126/scitranslmed.3005407 (2013). [DOI] [PubMed] [Google Scholar]
- 215.Blair PA et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32, 129–140, doi: 10.1016/j.immuni.2009.11.009 (2010). [DOI] [PubMed] [Google Scholar]
- 216.Iwata Y et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117, 530–541, doi: 10.1182/blood-2010-07-294249 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Amu S, Tarkowski A, Dorner T, Bokarewa M & Brisslert M The human immunomodulatory CD25+ B cell population belongs to the memory B cell pool. Scand J Immunol 66, 77–86, doi: 10.1111/j.1365-3083.2007.01946.x (2007). [DOI] [PubMed] [Google Scholar]
- 218.Kessel A et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev 11, 670–677, doi: 10.1016/j.autrev.2011.11.018 (2012). [DOI] [PubMed] [Google Scholar]
- 219.Lemoine S, Morva A, Youinou P & Jamin C Regulatory B cells in autoimmune diseases: how do they work? Ann N Y Acad Sci 1173, 260–267, doi: 10.1111/j.1749-6632.2009.04651.x (2009). [DOI] [PubMed] [Google Scholar]
- 220.Matsumoto M et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41, 1040–1051, doi: 10.1016/j.immuni.2014.10.016 (2014). [DOI] [PubMed] [Google Scholar]
- 221.Piancone F et al. B Lymphocytes in Multiple Sclerosis: Bregs and BTLA/CD272 Expressing-CD19+ Lymphocytes Modulate Disease Severity. Scientific reports 6, 29699, doi: 10.1038/srep29699 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chi Y et al. Novel role of aquaporin-4 in CD4+ CD25+ T regulatory cell development and severity of Parkinson’s disease. Aging Cell 10, 368–382, doi: 10.1111/j.1474-9726.2011.00677.x (2011). [DOI] [PubMed] [Google Scholar]
- 223.Smith MD, Martin KA, Calabresi PA & Bhargava P Dimethyl fumarate alters B-cell memory and cytokine production in MS patients. Ann Clin Transl Neurol 4, 351–355, doi: 10.1002/acn3.411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Longbrake EE et al. Dimethyl fumarate induces changes in B- and T-lymphocyte function independent of the effects on absolute lymphocyte count. Mult Scler 24, 728–738, doi: 10.1177/1352458517707069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lundy SK et al. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurology(R) neuroimmunology & neuroinflammation 3, e211, doi: 10.1212/NXI.0000000000000211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dubey D et al. Dimethyl fumarate in relapsing-remitting multiple sclerosis: rationale, mechanisms of action, pharmacokinetics, efficacy and safety. Expert Rev Neurother 15, 339–346, doi: 10.1586/14737175.2015.1025755 (2015). [DOI] [PubMed] [Google Scholar]
- 227.Takeshita Y & Ransohoff RM Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunological reviews 248, 228–239, doi: 10.1111/j.1600-065X.2012.01127.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Goligorsky MS et al. in Comprehensive Toxicology (Second Edition) (ed Charlene McQueen A.) 213–244 (Elsevier, 2010). [Google Scholar]
- 229.Alter A et al. Determinants of human B cell migration across brain endothelial cells. Journal of immunology 170, 4497–4505 (2003). [DOI] [PubMed] [Google Scholar]
- 230.Planas R, Jelcic I, Schippling S, Martin R & Sospedra M Natalizumab treatment perturbs memory- and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur J Immunol 42, 790–798, doi: 10.1002/eji.201142108 (2012). [DOI] [PubMed] [Google Scholar]
- 231.Warnke C et al. Natalizumab exerts a suppressive effect on surrogates of B cell function in blood and CSF. Mult Scler 21, 1036–1044, doi: 10.1177/1352458514556296 (2015). [DOI] [PubMed] [Google Scholar]
- 232.Stüve O et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Annals of neurology 59, 743–747, doi:doi: 10.1002/ana.20858 (2006). [DOI] [PubMed] [Google Scholar]
- 233.Villar LM et al. Immunological markers of optimal response to natalizumab in multiple sclerosis. Arch Neurol 69, 191–197, doi: 10.1001/archneurol.2011.971 (2012). [DOI] [PubMed] [Google Scholar]
- 234.Mancuso R et al. Effects of natalizumab on oligoclonal bands in the cerebrospinal fluid of multiple sclerosis patients: a longitudinal study. Mult Scler 20, 1900–1903, doi: 10.1177/1352458514538111 (2014). [DOI] [PubMed] [Google Scholar]
- 235.Harrer A et al. Cerebrospinal fluid parameters of B cell-related activity in patients with active disease during natalizumab therapy. Mult Scler 19, 1209–1212, doi: 10.1177/1352458512463483 (2013). [DOI] [PubMed] [Google Scholar]
- 236.Amor S et al. Neurofilament light antibodies in serum reflect response to natalizumab treatment in multiple sclerosis. Mult Scler 20, 1355–1362, doi: 10.1177/1352458514521887 (2014). [DOI] [PubMed] [Google Scholar]
- 237.Niino M et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol 59, 748–754, doi: 10.1002/ana.20859 (2006). [DOI] [PubMed] [Google Scholar]
- 238.Putzki N, Baranwal MK, Tettenborn B, Limmroth V & Kreuzfelder E Effects of natalizumab on circulating B cells, T regulatory cells and natural killer cells. Eur Neurol 63, 311–317, doi: 10.1159/000302687 (2010). [DOI] [PubMed] [Google Scholar]
- 239.Rao SP et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PloS one 7, e39416, doi: 10.1371/journal.pone.0039416 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kasarello K, Cudnoch-Jedrzejewska A, Czlonkowski A & Mirowska-Guzel D Mechanism of action of three newly registered drugs for multiple sclerosis treatment. Pharmacol Rep 69, 702–708, doi: 10.1016/j.pharep.2017.02.017 (2017). [DOI] [PubMed] [Google Scholar]
- 241.Coles AJ et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380, 1829–1839, doi: 10.1016/S0140-6736(12)61768-1 (2012). [DOI] [PubMed] [Google Scholar]
- 242.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G & Schmierer K Interpreting Lymphocyte Reconstitution Data From the Pivotal Phase 3 Trials of Alemtuzumab. JAMA neurology 74, 961–969, doi: 10.1001/jamaneurol.2017.0676 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ruck T, Bittner S, Wiendl H & Meuth SG Alemtuzumab in Multiple Sclerosis: Mechanism of Action and Beyond. Int J Mol Sci 16, 16414–16439, doi: 10.3390/ijms160716414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Leist TP & Weissert R Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol 34, 28–35, doi: 10.1097/WNF.0b013e318204cd90 (2011). [DOI] [PubMed] [Google Scholar]
- 245.Ceronie B et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol 265, 1199–1209, doi: 10.1007/s00415-018-8830-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mao Z et al. Cladribine: Off-label disease modification for people with multiple sclerosis in resource-poor settings? Mult Scler J Exp Transl Clin 4, 2055217318783767, doi: 10.1177/2055217318783767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.esfandi S, Salimian S, Corboy J & Alvarez E Persistent B lymphocytes in multiple sclerosis plaques after rituximab treatment (P5.341). Neurology 88 (2017). [Google Scholar]
- 248.Liang A, Finney-Stable A, Lin J & Sadiq S Cerebrospinal Fluid (CSF) Cellular Analysis of Patients with Multiple Sclerosis (MS) Treated with Anti B-cell Therapy – Correlated with Treatment Response (P5.331). Neurology 88 (2017). [Google Scholar]
- 249.Li R & Bar-Or A The Multiple Roles of B Cells in Multiple Sclerosis and Their Implications in Multiple Sclerosis Therapies. Cold Spring Harb Perspect Med, doi: 10.1101/cshperspect.a029108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kabat EA, Freedman DA & et al. A study of the crystalline albumin, gamma globulin and total protein in the cerebrospinal fluid of 100 cases of multiple sclerosis and in other diseases. Am J Med Sci 219, 55–64 (1950). [DOI] [PubMed] [Google Scholar]
- 251.Siritho S & Freedman MS The prognostic significance of cerebrospinal fluid in multiple sclerosis. J Neurol Sci 279, 21–25, doi: 10.1016/j.jns.2008.12.029 (2009). [DOI] [PubMed] [Google Scholar]
- 252.Link H & Huang YM Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. Journal of neuroimmunology 180, 17–28, doi: 10.1016/j.jneuroim.2006.07.006 (2006). [DOI] [PubMed] [Google Scholar]
- 253.Keegan M et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet 366, 579–582, doi: 10.1016/S0140-6736(05)67102-4 (2005). [DOI] [PubMed] [Google Scholar]
- 254.Heigl F et al. Immunoadsorption in steroid-refractory multiple sclerosis: clinical experience in 60 patients. Atheroscler Suppl 14, 167–173, doi: 10.1016/j.atherosclerosissup.2012.10.025 (2013). [DOI] [PubMed] [Google Scholar]
- 255.Musette P & Bouaziz JD B Cell Modulation Strategies in Autoimmune Diseases: New Concepts. Frontiers in immunology 9, 622, doi: 10.3389/fimmu.2018.00622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cole S et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis research & therapy 20, 85, doi: 10.1186/s13075-018-1578-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mackay F & Browning JL BAFF: a fundamental survival factor for B cells. Nature reviews. Immunology 2, 465–475, doi: 10.1038/nri844 (2002). [DOI] [PubMed] [Google Scholar]
- 258.Sergott RC et al. ATON: results from a Phase II randomized trial of the B-cell-targeting agent atacicept in patients with optic neuritis. J Neurol Sci 351, 174–178, doi: 10.1016/j.jns.2015.02.019 (2015). [DOI] [PubMed] [Google Scholar]
- 259.Rahmanzadeh R, Weber MS, Bruck W, Navardi S & Sahraian MA B cells in multiple sclerosis therapy-A comprehensive review. Acta Neurol Scand 137, 544–556, doi: 10.1111/ane.12915 (2018). [DOI] [PubMed] [Google Scholar]
- 260.Kostic M, Zivkovic N, Cvetanovic A & Stojanovic I Granulocyte-macrophage colony-stimulating factor as a mediator of autoimmunity in multiple sclerosis. Journal of neuroimmunology 323, 1–9, doi: 10.1016/j.jneuroim.2018.07.002 (2018). [DOI] [PubMed] [Google Scholar]
- 261.Bracarda S et al. Comparing comparators: a look at control arms in kidney cancer studies over the years. Br J Cancer 112, 14–19, doi: 10.1038/bjc.2014.240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rizzi M et al. Impact of tofacitinib treatment on human B-cells in vitro and in vivo. J Autoimmun 77, 55–66, doi: 10.1016/j.jaut.2016.10.005 (2017). [DOI] [PubMed] [Google Scholar]
- 263.Mauri C & Menon M Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest 127, 772–779, doi: 10.1172/JCI85113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Avasarala J It’s Time For Combination Therapies: in Multiple Sclerosis. Innovations in Clinical Neuroscience 14, 28–30 (2017). [PMC free article] [PubMed] [Google Scholar]
- 265.Neves V, Aires-da-Silva F, Corte-Real S & Castanho MARB Antibody Approaches To Treat Brain Diseases. Trends in Biotechnology 34, 36–48, doi: 10.1016/j.tibtech.2015.10.005 (2016). [DOI] [Google Scholar]
- 266.Greenwood J et al. Current research into brain barriers and the delivery of therapeutics for neurological diseases: a report on CNS barrier congress London, UK, 2017. Fluids Barriers CNS 14, 31, doi: 10.1186/s12987-017-0079-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Weisel F & Shlomchik M Memory B Cells of Mice and Humans. Annu Rev Immunol 35, 255–284, doi: 10.1146/annurev-immunol-041015-055531 (2017). [DOI] [PubMed] [Google Scholar]