Abstract
Objectives:
We aimed to study how frequently patient reported outcomes (PROs) are collected in registered clinical studies of atrial fibrillation (AF).
Background:
Improving symptom burden and quality of life are important goals in the treatment of AF, and are best measured with PROs.
Methods:
We analyzed data from clinicaltrials.gov to identify PROs in AF studies. All studies reporting AF as the disease condition were included, and PROs were identified by search terms within the Outcomes Measures. Generic and AF-specific PROs were identified, and assessed by study type and year. Clinicaltrials.gov reporting was compared with published reports of linked studies in PubMed.
Results:
From 1999-2018, 1709 studies including AF patients were posted; 238 (14%) included PROs. Collection of PROs was reported in 22% (n=83/386) of trials studying procedural interventions and 11% (n=18/168) of all Phase 3 studies. Among the 238 studies with PROs, most described ‘quality of life’ (n=194, 82%), and most (n=198, 83%) included only generic (not AF-specific) PROs. Only 17% (n=40) of studies reporting PROs specified a previously-published, AF-specific tool, most commonly the AFEQT (n=20, 8.4%). Among the available PubMed citations of 391 studies, 74 (19%) described collecting a specific PRO tool (n=29 [7.4%] for an AF-specific PRO).
Conclusions:
Despite increased emphasis on the importance of PROs in AF, a minority of registered clinical trials reported collecting PROs, with very few using validated, AF-specific PROs. Improving outcomes that are most important to patients will necessitate increased emphasis on these PROs in pivotal clinical studies.
Keywords: atrial fibrillation, patient reported outcomes, clinicaltrials.gov, quality of life, outcomes
Condensed Abstract
Improving symptom burden and quality of life are important goals in the treatment of AF, best measured with patient reported outcomes (PROs). We assessed PRO use in AF studies registered in clinicaltrials.gov. From 1999-2018, 1709 studies including AF patients were posted; 238 (14%) included PROs. Only 17% (n=40) of studies reporting PROs specified an AF-specific tool, most commonly the AFEQT (n=20, 8.4%). Among PubMed citations (n=391 studies), 29 (7.4%) described collection of an AF-specific PRO. Despite increased emphasis on PROs in AF, a minority of registered clinical trials reported collecting PROs, with very few using validated, AF-specific PROs.
Patient reported outcomes (PROs) have been defined as, “any report of the status of a patient’s health condition that comes directly from the patient (i.e., without interpretation of the patient’s response by a clinician or anyone else)”.(1) Understanding patients’ perspectives is important for atrial fibrillation (AF) because: (1) the subjective experience can vary dramatically between patients (some are asymptomatic whereas others can have debilitating symptoms); (2) AF occurs more commonly in older patients who often prioritize quality of life over longevity; and (3) substantial resources are frequently devoted to improving patients’ health status.(2,3) The burden of AF symptoms can be comparable to that of ischemic heart disease or congestive heart failure,(4) and strategies such as catheter ablation have been shown to improve arrhythmia-free survival and improve health-related quality of life(hrQoL).(5–7) Yet there is limited evidence that aggressive rhythm control (compared to symptomatic treatment with rate control) improves clinical endpoints of stroke and/or mortality, and thus the primary indication for pursuing a rhythm control strategy remains improvement of AF symptoms.(8,9) Despite this, it is unclear whether pivotal studies of AF are directly assessing hrQoL using PROs.(8)
We used data from the clinicaltrials.gov repository to assess the reported use of PROs in clinical studies of patients with AF. The objectives of the current analysis were to (1) measure the proportion of registered clinical studies of AF that included PROs as primary outcome measures; (2) assess trends in use of PROs over time; and (3) assess use of AF-specific PROs versus generic instruments in trials of AF. Results of the clinicaltrials.gov analysis were then validated in PubMed searches for the registered studies.
Methods
We analyzed publicly-available data from the clinicaltrials.gov database to identify registered AF studies that reported use of PROs. Data on all studies registered under the condition “atrial fibrillation” were downloaded, and up to date as of July 10, 2018. No other search filters were used. The studies were further restricted to only those that indicated “atrial fibrillation” in the Conditions field. Studies were further stratified by inclusion of PRO terms in the reported Outcome Measures (see Supplemental Material). Presence of any PRO term indicated reported use of PROs in the study. These included generic PRO measurement tools, generic terms for PROs (e.g., “quality of life”), as well as named, AF-specific PRO tools.(4,10–12) Clinician reported outcomes (CROs), such as European Heart Rhythm Association or Canadian Cardiovascular Society classifications of AF, were not included. See Supplemental Material for more detailed methods.
Characteristics of studies reporting PRO collection were compared to those that did not report PROs as outcome measures. Reporting of PRO collection was assessed over the duration of the reporting, and stratified by study type (interventional versus observational studies; expanded access was classified as observational) and intervention type (device or procedure only vs. drug only; studies with both types of intervention [or other interventions] were excluded from that stratification). Among studies that did report PROs, the use of generic PRO tools versus AF-specific PROs is described.
PubMed Review
In order to assess under- or over-reporting of PROs in the clinicaltrials.gov database, we searched PubMed for any related citation for each of the identified AF studies. Citations were matched based on clinicaltrials.gov identifier, and full-text manuscripts were reviewed (when accessible). Citations were examined for PRO collection, as part of the study methods, as well as the reporting of PROs results in any citation linked to the respective identifier. Because the objective was to assess study use and collection of PROs, all registered studies were included in the search (both completed and not yet completed), and ‘Rationale and Design’ manuscripts were reviewed for outcomes measures. As the description of possible PROs is variable, citations were examined for (a) any report of measuring quality of life outcomes, (b) the use of a specific tool to collect PROs, and (c) the use of a PRO tool specific to AF. Simply the collection of ‘symptomatic AF’ as an outcome (as interpreted by the clinician) was not considered as a collection of PROs or quality of life.
Statistical Methods
Categorical variables are summarized as number (percentage) and univariate comparisons were performed with Chi-squared. A two-sided p-value of <0.05 was considered significant. All analyses were performed using R (Version 3.5.2) and RStudio (Version 1.1.463),(13) with applicable packages.(14)
Results
We identified 1709 entries in clinicaltrials.gov that included patients with AF and were first posted from October 28, 1999, to June 29th, 2018. Overall, 43% (n=735) were reported as completed (n=260 [35.4%] of completed studies had a linked citation in PubMed; see below), and 90% (n=1534) did not have results available on clinicaltrials.gov. Among the 1709 registered AF studies, 238 (14%) reported PROs terms in their Outcome Measures (Table 1). Those reporting PROs as outcomes versus those not collecting PROs were more likely to be classified as interventional (82% vs. 64%, p<0.001), to be of smaller size (11% targeting >1000 participants, vs. 19%, p<0.001), and more likely to involve a procedural intervention (35% vs. 21%, p<0.001). However, among the 386 studies with specifically a procedural intervention, only 83 (22%) reported collecting PROs.
Table 1.
Characteristics of clinical trials that did and did not include patient reported outcomes.
| Overall (n=1709) | No PROs Reported (n=1471) | PROs Reported (n=238) | p | |
|---|---|---|---|---|
| Study Type (%) | <0.001 | |||
| Expanded Access | 1 (0.1) | 1 (0.1) | 0 (0.0) | |
| Interventional | 1132 (66.2) | 937 (63.7) | 195 (81.9) | |
| Observational | 576 (33.7) | 533 (36.2) | 43 (18.1) | |
| Year First Posted | 0.045 | |||
| 1999 -2009 | 382 (22.4) | 315 (21.4) | 67 (28.2) | |
| 2010-2014 | 622 (36.4) | 536 (36.4) | 86 (36.1) | |
| >=2015 | 705 (41.3) | 620 (42.1) | 85 (35.7) | |
| Study Size | <0.001 | |||
| <100 | 551 (32.7) | 487 (33.7) | 64 (26.9) | |
| 100-499 | 705 (41.8) | 587 (40.6) | 118 (49.6) | |
| 500-999 | 128 (7.6) | 99 (6.8) | 29 (12.2) | |
| >=1000 | 301 (17.9) | 274 (18.9) | 27 (11.3) | |
| AF Type Included | ||||
| Only Paroxysmal | 111 (6.5) | 91 (6.2) | 20 (8.4) | 0.252 |
| Paroxysmal | 121 (7.1) | 101 (6.9) | 20 (8.4) | 0.471 |
| Persistent | 81 (4.7) | 70 (4.8) | 11 (4.6) | 1.000 |
| Permanent | 10 (0.6) | 8 (0.5) | 2 (0.8) | 0.922 |
| Not specified | 1510 (88.4) | 1305 (88.7) | 205 (86.1) | 0.297 |
| Study Funding | ||||
| Industry | 683 (40.0) | 592 (40.2) | 91 (38.2) | 0.606 |
| NIH | 39 (2.3) | 34 (2.3) | 5 (2.1) | 1.000 |
| Other | 1241 (72.6) | 1060 (72.1) | 181 (76.1) | 0.229 |
| Federal Government (US) | 15 (0.9) | 14 (1.0) | 1 (0.4) | 0.659 |
| Intervention | ||||
| Drug | 527 (30.8) | 464 (31.5) | 63 (26.5) | 0.135 |
| Procedure | 386 (22.6) | 303 (20.6) | 83 (34.9) | <0.001 |
| Device | 415 (24.3) | 363 (24.7) | 52 (21.8) | 0.388 |
| Behavior | 52 (3.0) | 32 (2.2) | 20 (8.4) | <0.001 |
| Other Intervention | 196 (11.5) | 153 (10.4) | 43 (18.1) | 0.001 |
| Ablation | 329 (19.3) | 253 (17.2) | 76 (31.9) | <0.001 |
| Status (%) | 0.163 | |||
| Active, not recruiting | 118 (6.9) | 98 (6.7) | 20 (8.4) | |
| Completed | 735 (43.0) | 633 (43.0) | 102 (42.9) | |
| Enrolling by invitation | 18 (1.1) | 17 (1.2) | 1 (0.4) | |
| No longer available | 1 (0.1) | 1 (0.1) | 0 (0.0) | |
| Not yet recruiting | 85 (5.0) | 76 (5.2) | 9 (3.8) | |
| Recruiting | 377 (22.1) | 321 (21.8) | 56 (23.5) | |
| Suspended | 7 (0.4) | 4 (0.3) | 3 (1.3) | |
| Terminated | 107 (6.3) | 88 (6.0) | 19 (8.0) | |
| Unknown status | 220 (12.9) | 194 (13.2) | 26 (10.9) | |
| Withdrawn | 41 (2.4) | 39 (2.7) | 2 (0.8) | |
| No Results Available (%) | 1534 (89.8) | 1312 (89.2) | 222 (93.3) | 0.070 |
| Phases (%) | <0.001 | |||
| Early Phase 1 | 4 (0.4) | 4 (0.4) | 0 (0.0) | |
| Not Applicable | 590 (52.1) | 467 (49.8) | 123 (63.1) | |
| Phase 1 | 24 (2.1) | 22 (2.3) | 2 (1.0) | |
| Phase 1|Phase 2 | 12 (1.1) | 12 (1.3) | 0 (0.0) | |
| Phase 2 | 96 (8.5) | 93 (9.9) | 3 (1.5) | |
| Phase 2|Phase 3 | 11 (1.0) | 10 (1.1) | 1 (0.5) | |
| Phase 3 | 168 (14.8) | 150 (16.0) | 18 (9.2) | |
| Phase 4 | 227 (20.1) | 179 (19.1) | 48 (24.6) |
PRO: patient reported outcomes
Characteristics of included studies of atrial fibrillation, as reported by most recent posting.
Values are presented as n (%).
Proportions of studies reporting PRO collection, by year, are shown in Figure 1 (stratified by study type) and in the Central Illustration (stratified by intervention type). During the period where clinicaltrials.gov data are available, the number of posted studies including patients with AF increased from 382 for the first 10 years of reporting (1999-2009) up to 705 in the most recent 3 years (2015-2018).
Figure 1.
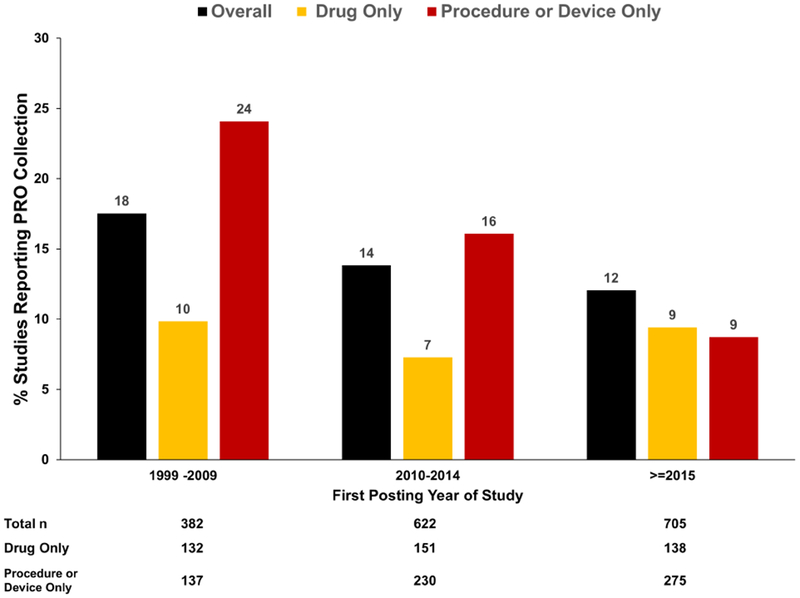
Trends in reported collection of PROs over time, stratified by reported study type in clinicaltrials.gov.
ROs: patient reported outcomes
Central Illustration.
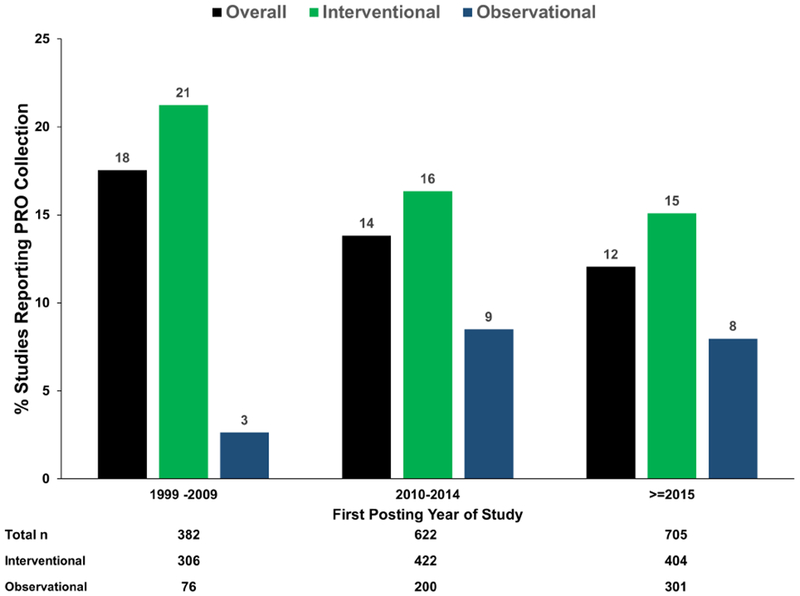
Trends in reported collection of PROs over time, stratified by reported study intervention type in clinicaltrials.gov. Drug studies include studies that reported only a drug as an intervention; procedure or device studies included procedure, device, or ablation as the intervention (without drug). Studies with other interventions or with both drug and procedure/device interventions were not included in the stratified data (thus the sum does not equal total).
PROs: patient reported outcomes
Reporting of generic PRO tools and terms, versus AF-specific instruments, are shown in Table 2, stratified by study type. Of the 238 entries that reported PROs, 40 (17%) referenced an AF-specific instrument, and this was not significantly different between interventional or observational studies (p=0.90). Among AF-specific PRO measures, the AFEQT was most commonly cited (n=20, 8.4% of studies with PRO terms). Thirty-five studies reported collecting both an AF-specific and a generic PRO measure, none of which were Phase 3 studies (other either drugs or devices).
Table 2.
Generic and AF-specific PROs identified as outcomes, among all studies reporting PROs, by study type.
| All Studies Reporting PROs (n=238) | Interventional (n=195) | Observational (n=43) | p | |
|---|---|---|---|---|
| EQ-5D | 24 (10.1) | 22 (11.3) | 2 (4.7) | 0.304 |
| “Short-form” | 2 (0.8) | 2 (0.2) | 0 (0.0) | 0.793 |
| SF-36 | 24 (10.1) | 22 (11.3) | 2 (4.7) | 0.304 |
| SF-12 | 8 (3.4) | 6 (3.1) | 2 (4.7) | 0.959 |
| Quality of Life | 194 (81.5) | 170 (87.2) | 24 (55.8) | <0.001 |
| “Questionnaire” | 65 (27.3) | 46 (23.6) | 19 (44.2) | 0.011 |
| “Inventory” | 2 (0.8) | 0 (0.0) | 2 (4.7) | 0.036 |
| “Depression” | 8 (3.4) | 6 (0.5) | 2 (0.3) | 0.880 |
| PHQ-9 | 3 (1.3) | 3 (1.5) | 0 (0.0) | 0.949 |
| MLWHF | 3 (1.3) | 3 (1.5) | 0 (0.0) | 0.949 |
| DASS | 1 (0.4) | 1 (0.5) | 0 (0.0) | 1.000 |
| AF-Specific PROs | 40 (16.8) | 32 (16.4) | 8 (18.6) | 0.902 |
| Symptom checklist (SCL) | 9 (3.8) | 8 (4.1) | 1 (2.3) | 0.911 |
| AFEQT | 20 (8.4) | 19 (9.7) | 1 (2.3) | 0.199 |
| AFSS | 7 (2.9) | 6 (3.1) | 1 (2.3) | 1.000 |
| MAFSI | 1 (0.4) | 1 (0.5) | 0 (0.0) | 1.000 |
| PACT | 5 (2.1) | 0 (0.0) | 5 (11.6) | <0.001 |
| ASTA | 1 (0.4) | 1 (0.5) | 0 (0.0) | 1.000 |
| AF QoL | 3 (1.3) | 3 (1.5) | 0 (0.0) | 0.949 |
Terms reported in the search methods but not reported here did not yield any results.
PROs: patient reported outcomes; AF: atrial fibrillation; SF: Short-Form; PHQ-9: Patient Health Questionnaire; MLWHF: Minnesota Living with Heart Failure questionnaire; AFEQT: Atrial Fibrillation Effect on QualiTy-of-life; AFSS: Atrial fibrillation Symptom Score (University of Toronto); MAFSI: Mayo Atrial Fibrillation Symptom Inventory; PACT: Perception of Anticoagulant Treatment questionnaire; ASTA: Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia.
PubMed Review
Among the 1709 studies of AF in clinicaltrials.gov, 391 (23%) had at least one citation in PubMed associated with the clinicaltrials.gov registration number (Table 3), including either ‘Rationale and Design’ manuscripts and/or publication of results; the majority of these had >1 full-text article that was available and accessed (n=371, 95% of studies with citations).
Table 3.
Description of patient reported outcomes among registered clinical trials of atrial fibrillation that have at least 1 associated PubMed citation.
| Studies With ≥1 Associated PubMed Citation (n=391) | |
|---|---|
| Study Marked ‘Completed’ in clinicaltrials.org | 260 (66.5) |
| PROs identified in clinicaltrials.org entry | 66 (16.9) |
| AF-specific PROs | 6 (1.5) |
| Generic PROs | 65 (16.6) |
| ≥1 full-text manuscript available and accessed from PubMed | 375 (95.9) |
| Rational & Design Citation Available | 91 (23.3) |
| Results Citation Available | 333 (85.2) |
| Collection of PROs Described in Methods | 86 (22.0) |
| Results of PROs Reported | 60 (15.3) |
| Specific PRO Tool Identified | 74 (18.9) |
| AF-specific PROs Tool Identified | 29 (7.4) |
| Any PROs Collected (according to either clinicaltrials.gov or PubMed) | 112 (28.6) |
| AF-Specific PROs Collected (according to either clinicaltrials.gov or PubMed) | 31 (7.9) |
PROs: patient reported outcomes; AF: atrial fibrillation.
Of the 1471 studies without PROs described as outcomes in clinicaltrials.gov, 46 (3%) had PubMed entries that described PRO collection. Among these 46, 36 described a specific PRO tool (e.g., EQ-5D), and 16 described an AF-specific PRO tool. Overall, among all of the 1709 registered studies, 65 (3.8%) describe use of an AF-specific PRO in either the registered outcomes and/or a linked PubMed citation.
Discussion
Over the past few decades, there has been increasing recognition of the importance of PROs in the assessment of important outcomes of clinical research studies, especially in chronic, non-fatal conditions. Our understanding of best methods to create and validate PRO tools has evolved, and new disease-specific measurement tools for AF have been introduced. However, in contrast to our expectations, this analysis of AF studies in the clinicaltrials.gov registry found a low proportion of studies reporting PRO collection and this proportion has not improved over time. Thus, while there has been substantial growth in the number of studies of AF patients, only 1 in 7 include PRO terms as Outcomes Measures. Pivotal phase III and intervention-based studies do not show any substantially higher use of PROs. Lastly, among studies that appeared to report PROs, only 17% included AF-specific PRO tools (versus more generic tools or terms).
While controlling symptoms in patients with AF can be challenging, it is often the most important outcome to patients. Interventions to improve symptoms, particularly those geared towards rhythm control (e.g., catheter ablation) can be costly and are not without risk of harm. Furthermore, while emerging data suggest improved thromboembolic and mortality outcomes in subgroups of AF patients undergoing ablation,(15) there remains uncertainty as to whether these findings are applicable to the broader AF population.
Therefore, the current objective of rhythm control strategies in most patients remains symptom control. Yet, measurement of these vital outcomes remains challenging and are not being routinely collected or reported in the largest repository of clinical trials. Furthermore, “treatment success”, as defined by subsequent AF burden and freedom from recurrence, is poorly correlated to quality of life. Thus, “arrhythmia burden” is not an appropriate surrogate for PROs, and this has limited the interpretation of many AF trials to date – it is not clear that the endpoints most important to patients are measured, and therefore it is difficult to know which interventions are of highest value to them. The use of arrhythmia burden in existing trials is a valuable endpoint for clinicians and researchers, but of little value in understanding hrQoL implications of our interventions, which requires PROs.
These data may also reflect that state of AF clinical research in general. A recent analysis of only interventional AF studies in the clinicaltrials.gov dataset demonstrated some variability in the quality of trials – many only modest in size, and no consistent use of randomization or blinding.(16) Our analysis focuses on an even broader cohort of registered AF studies (all phases, all designs, any status), and these shortcomings appear to extend to a lack of PROs, particularly a lack of AF-specific PROs. This is despite the development and validation of several AF-specific PRO measurement tools (17) that have been studied extensively. Furthermore, there are robust data in support of using AF-specific PRO tools over general hrQoL assessments.(18) Lastly, the latest consensus on AF ablation “… recommends that all clinical trials incorporate some measure of patient reported outcomes and preferably measure them using both a general and an AF specific measurement scale.”(9) More detailed guidance on PRO incorporation in clinical trials has recently been provided by the SPIRIT-PRO group.(19) Nevertheless, our data demonstrate suboptimal implementation of PROs in AF research, according to the clinicaltrials.gov registry.
While the clinicaltrials.gov dataset may be limited and exclude smaller or observational studies that are not required to be registered, it remains the de facto registration repository for landmark and regulatory-pathway studies and registration is often required by journals before considering publication of study results. Therefore, the absence of PROs among studies included in this dataset represents an informative, if potentially incomplete, assessment of their use in AF clinical research. As there has been increasing scrutiny on the use of brief duration AF as an endpoint in clinical trials,(20) and since such events are often clinically inconsequential to patients, PROs offer a much better opportunity to demonstrate value of an intervention to patients. Furthermore, optimal and efficient implementation of PROs in studies, both generic and disease-specific, could be greatly enhanced with their routine incorporation into clinical care and the electronic health record.(21,22) This would also facilitate additional, much needed research, such as: (1) identifying minimum clinically important changes in PROs for AF; (2) understanding the correlation between PROs and more ‘traditional’ outcomes of arrhythmia recurrence, arrhythmia burden, and clinical events (e.g., thromboembolism, heart failure); and (3) using PROs to guide treatment decisions.
Limitations
The data analyzed here are derived from a publicly available registry, where data are entered manually, and often voluntarily, by study personnel. Not all data elements are required (requirements change over time) and many studies are not completed or are completed without results reported; only primary (and not secondary) outcomes are downloaded.(23) Additionally, changes over time in the patterns of trial registration (e.g., study types, etc.) and availability of PROs could influence the assessment of chronological trends. Lastly, responsible persons may not have included PROs in the clinicaltrials.gov registration, even if they were collected. However, this implies that such outcomes were neither (a) the primary outcome, nor (b) felt to be of sufficient import to include in this registry (though may have been identified during our PubMed review). This, in and of itself, reflects an under-emphasis of outcomes that are of primary concern to patients with AF – as technologies rapidly develop to more effectively detect and treat AF, it is incumbent on the field to ensure we are improving outcomes that matter to patients.
Conclusions
Despite increased emphasis on research for AF interventions, a minority of registered AF clinical trials reported the use of PROs as outcomes measures, and very few describe collection of validated, AF-specific PROs. These data reflect an under-emphasis of outcome measures most relevant to patients, and among the most important studies in AF. Improving the care of patients with AF will necessitate increased emphasis on these PROs in the pivotal clinical trials of contemporary interventions.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Despite recommendations from international governing bodies that patient reported outcomes (PROs) be included in all clinical studies of atrial fibrillation (AF), a minority of trials cite them as primary outcomes in clinicaltrials.gov registrations.
Translational Outlook
While there is contemporary evidence suggesting improvement clinical events and mortality among patients undergoing rhythm control for AF, the primary objective of this approach remains symptom control. However, validated, AF PROs are cited as primary outcomes in a minority of these registered trials. Understanding the impact of interventions on these outcomes that frequently matter most to patients will require greater emphasis and commitment to PROs in clinical studies of AF.
Acknowledgments
Disclosure Information
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156 (BAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
BAS, PD, KA, RH, DBM, PAN, and JS did not report any relevant disclosures. JPP receives grants for clinical research from Abbott, Boston Scientific, and Gilead, and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Johnson & Johnson, LivaNova, Medtronic, Sanofi, and Phillips.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsevat J, Dawson NV, Wu AW et al. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. Jama 1998;279:371–5. [DOI] [PubMed] [Google Scholar]
- 3.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–20. [DOI] [PubMed] [Google Scholar]
- 4.Dorian P, Jung W, Newman D et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303–9. [DOI] [PubMed] [Google Scholar]
- 5.Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson K, Al-Khatib SM. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol 2009;2:626–33. [DOI] [PubMed] [Google Scholar]
- 6.Morillo CA, Verma A, Connolly SJ et al. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-Line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2). Jama 2014;311:692. [DOI] [PubMed] [Google Scholar]
- 7.Al Halabi S, Qintar M, Hussein A et al. Catheter Ablation for Atrial Fibrillation in Heart Failure Patients: A Meta-Analysis of Randomized Controlled Trials. JACC Clinical electrophysiology 2015; 1: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spertus J, Dorian P, Bubien R et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 11.Aliot E, Botto GL, Crijns HJ, Kirchhof P. Quality of life in patients with atrial fibrillation: how to assess it and how to improve it. Europace 2014;16:787–96. [DOI] [PubMed] [Google Scholar]
- 12.Wokhlu A, Monahan KH, Hodge DO et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol 2010;55:2308–16. [DOI] [PubMed] [Google Scholar]
- 13.R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 14.Yoshida K, Bohn J. tableone: Create ‘Table 1’ to Describe Baseline Characteristics (R package). 0.9.2 ed, 2018.
- 15.Marrouche NF, Brachmann J, Andresen D et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 16.Patel RB, Venkateswaran RV, Singh A et al. The Current Landscape of Atrial Fibrillation and Atrial Flutter Clinical Trials: A Report of 348 Studies Registered With ClinicalTrials.gov. JACC Clinical electrophysiology 2018;4:944–954. [DOI] [PubMed] [Google Scholar]
- 17.Mark DB. Assessing quality-of-life outcomes in cardiovascular clinical research. Nat Rev Cardiol 2016;13:286–308. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner S, Deisenhofer I, Kindsmuller S et al. Prospective assessment of short- and long-term quality of life after ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:121–7. [DOI] [PubMed] [Google Scholar]
- 19.Calvert M, Kyte D, Mercieca-Bebber R et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension. Jama 2018;319:483–494. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg JS, O’Connell H, Li S, Ziegler PD. Thirty-Second Gold Standard Definition of Atrial Fibrillation and Its Relationship With Subsequent Arrhythmia Patterns: Analysis of a Large Prospective Device Database. Circ Arrhythm Electrophysiol 2018;11:e006274. [DOI] [PubMed] [Google Scholar]
- 21.Stehlik J, Rodriguez-Correa C, Spertus JA et al. Implementation of Real-Time Assessment of Patient-Reported Outcomes in a Heart Failure Clinic: A Feasibility Study. J Card Fail 2017;23:813–816. [DOI] [PubMed] [Google Scholar]
- 22.Biber J, Ose D, Reese J et al. Patient reported outcomes - experiences with implementation in a University Health Care setting. Journal of patient-reported outcomes 2017;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tse T, Fain KM, Zarin DA. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. Bmj 2018;361:k1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


