Abstract
Circulating factors in the blood and lymph support critical functions of living tissues. Parabiosis refers to the condition where two entire living animals are conjoined and share a single circulatory system. This surgically created animal model is inspired by naturally occurring pairs of conjoined twins. Parabiosis experiments testing whether humoral factors from one animal affect the other animal have been performed for over 150 years and have led to advances in endocrinology, neurology, musculoskeletal biology, and dermatology. The development of high-throughput genomics and proteomics approaches permitted the identification of potential circulating factors and rekindled scientific interest in parabiosis studies. For example, this technique may be used to assess how circulating factors may affect skin homeostasis, skin differentiation, skin aging, wound healing, and potentially skin cancer.
Keywords: Methods, Models, Regenerative Medicine, Tissue Regeneration
Introduction
The techniques resulting in parabiosis began in the 1860’s when French biologist Bert tested the viability of skin allografts by joining two rats together and attaching flaps of skin from one animal to another (Bert 1864). He showed that a viable cross-circulation was established by injecting fluid into the tail vein of one animal and observing its appearance in the partner animal. In 1908, Sauerbruch and Heyde coined the term parabiosis and modified the technique by extending the length of the incision and adding an intestinal anastomosis (Sauerbruch and Heyde 1908). In 1933, Bunster and Meyer improved on the technique by joining the skin, muscle layers, and abdominal wall together (Bunster and Mayer 1933). The union became more stable, and this protocol remains the basis of most modern approaches. Interest in parabiosis peaked in the 1960–1970s as scientists used it to study a variety of topics, including: cancer, lifespan, blood pressure, and energy balance.
In 1959, Hervey used parabiosis to demonstrate that a circulating factor was involved in energy balance (Hervey 1959). He parabiosed young rats together and damaged the ventromedial hypothalamus of one parabiont to induce hyperphagia and obesity. Despite unlimited access to food, the non-lesioned partner stopped eating, lost weight, and appeared to be responding to a circulating “satiety” factor released by the other rat. These studies were later confirmed with ob/ob (obese) and db/db (diabetic) mice, which showed that obese mice lacked a circulating signal that regulated energy balance and diabetic mice appeared insensitive to the signal (see review, (Harris 2013)). The signal was identified to be leptin and subsequent parabiosis experiments confirmed leptin’s ability to circulate between parabionts.
In recent years, investigators resurrected parabiosis to study questions of aging in somatic stem cells. As muscle function declines with age, they asked if change was due to stem-cell intrinsic change or whether stem cell functionality may be influenced by their surroundings (Conboy et al. 2005). The results of these studies unequivocally demonstrated that the aged environment impairs regenerative potential of older individuals. When exposed to young blood, aged stem cells adopted a more youthful potential. When young stem cells are exposed to aged blood, they lost regenerative potential (Brack et al. 2007). Similar studies have now been extended for neurons and brain function (Villeda et al. 2011; Villeda et al. 2014).
Our group used parabiosis in studies of scar formation and skin regeneration. Physicians have observed that surgical wounds in the elderly heal with thinner scars than wounds in young patients. We showed that full-thickness skin wounds in aged, but not young, mice fully regenerate (Nishiguchi et al. 2018). We connected aged and young mice together, termed heterochronic parabiosis, to elucidate whether the observed phenotypes—scar formation and skin regeneration—are caused by circulating factors or local signaling (Figure 1). Our results demonstrated that exposure of aged animals to blood from young mice counteracts their regenerative capacity (Figure 2). We identified the factor by performing global transcriptomic analysis of wound-edge tissue from regenerating and non-regenerating mice. We distilled a list of 80 potential genes to 13 genes by looking for circulating proteins. We showed that stromal cell-derived factor-1 (SDF1), is expressed at higher levels in wounded skin of young mice, and genetic deletion of SDF1 in young skin enhanced tissue regeneration.
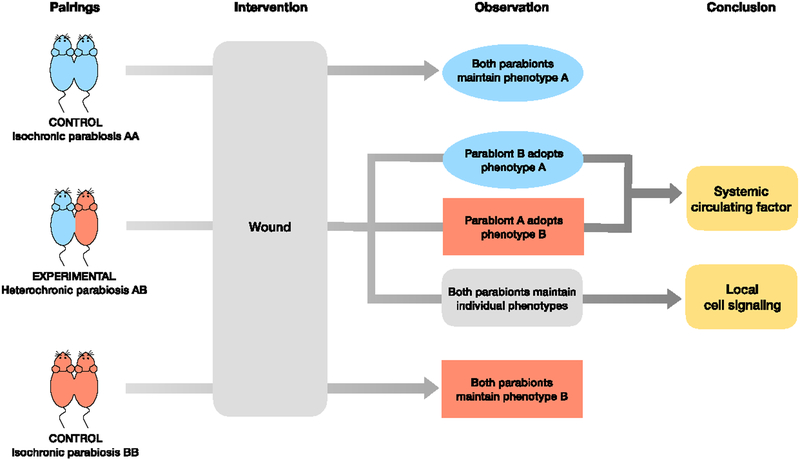
Figure 1. Example of parabiosis experiment to test age-defined phenotypes.
. Comparing heterochronic parabiosis pairs to isochronic parabiosis control pairs allows for assessment of age-mediated changes. An optional intervention, such as skin wounding or UV exposure, may be added. Isochronic control pairs should maintain their baseline individual phenotype. If so, we conclude that the parabiosis procedure itself does not alter the phenotype. If both mice in a heterochronic parabiosis pair maintain their original individual phenotypes, we conclude that no circulating factor is involved in this phenotype. If either parabiont adopts a new phenotype, we conclude that a systemic circulating factor may be responsible for the phenotypic change.
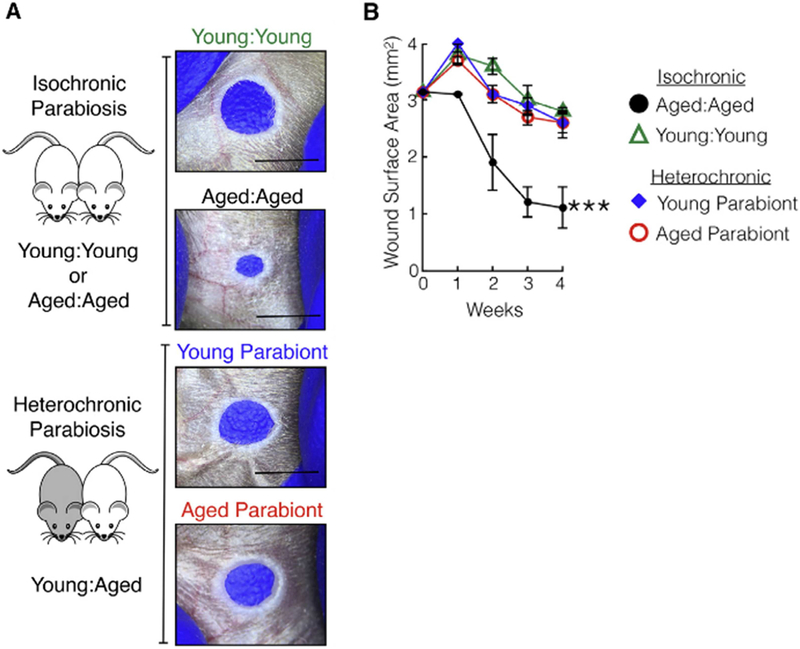
Figure 2. A circulating factor promotes scar formation in aged mice.
. (a) Isochronic aged:aged parabiosis led to significant wound closure, while isochronic young:young parabiosis did not, as photographed. Within the heterochronic parabiosis pairs, the aged parabiont did not close its ear hole and adopted the young parabiont phenotype. (b) Ear hole measurements of individual parabionts within each pair. n = 5. ***p < 0.001, comparing aged:aged with young:young or either parabiont of young:aged. Aged:aged parabionts had significant wound closure compared to young:young or young:aged parabionts.
Basic Principles of Parabiosis: Experimental Design, Methodology, and Interpretations
The technique of establishing the parabiotic state was recently reviewed, and an excellent video describes the surgical procedure (Kamran et al. 2013). Prior to parabiosis surgery, pairs should be co-housed for 1-week for proper acclimation. We remove fur 24 hours prior to surgery to shorten anesthesia time during the formal procedure. Standard aseptic surgical procedures are used, and the animals are kept warm with a heating pad. Briefly, mirror-image incisions at the left and right flanks are made through the skin, and skin is gently freed from superficial fascia. At this point, investigators may choose to join the peritoneal walls of the mice (Villeda et al., 2011; 2014). We and others omit the peritoneal joining to minimize invasiveness of the procedure, and we still establish an effective blood exchange. Elbow and knee joints from each parabiont are sutured together. After joints are stabilized, the skin flaps of the mice are sutured together—first dorsally, then ventrally. Kamran et al. (2013) recommends a continuous suture, while in our experience the use of single interrupted sutures minimizes wound dehiscence if the suture fails or is removed by the animal. Other techniques call for stapling the skin of each mouse together (Villeda et al., 2011; 2014). The time to complete the surgery ranges between 30–60 minutes depending on the experience of the surgeon. In particular, the decisions of whether to join the peritoneums and how extensively to join the limbs will influence the duration of the surgery and the stability of the pairings.
The post-procedure care is more critical that the procedure itself. For post-operative pain, each mouse is treated with antibiotics, subcutaneous normal saline, meloxicam, and buprenorphine hydrochloride. We have found that placing steristrips vertically over the skin sutures 2 to 3 days post-procedure provides a physical barrier against parabionts removing the suture. Several recovery characteristics are analyzed daily after surgery, including weight, grooming responses, urination, and defecation. Animals are excluded if they failed overall health inspections. If sutures are removed by the mice, they are replaced on a daily basis. The incisions are healed 2-weeks post-surgery, and sutures may be removed. For our skin wounding studies, we waited 4-weeks after surgical connection to perform additional skin injury to maximize the recovery from the procedure. This time period may be adjusted based on the specific experiment. We and others have kept parabiotic pairs connected for 8 months without significant problems (Kamran et al. 2013). Taken together, female, background-matched mice of similar size and weight offer the greatest chance of success for parabiosis experiments.
Technical challenges:
Perioperative mortality remains one of the major challenges in parabiotic experiments. However, survival of parabiotic pairs have improved significantly with better anesthesia and post-operative monitoring. In our experience, more than 90% of our pairs recover from the procedure, similar to results reported by others (Conboy et al. 2013).
Parabiosis involves the continuous exchange of fluids and cells between partners, and a second period of high mortality, called parabiotic disease (also known as parabiotic disharmony or parabiotic intoxication), occurs 1–2 weeks after surgery when the vascular anastomoses are maturing. The condition is independent of the procedure. Incidence can still be as high as 20–30% of pairs in highly inbred strains of mice and rats and 60–70% in outbred strains of mice (Conboy et al. 2013; Finerty and Panos 1951). One parabiont becomes pale, anemic, stops eating, and dies within a few days. The other member develops hyperemia, best noted by reddening and dilation of blood vessels of the feet, ear, and tail (Harris 2013). If the parabiosis pair is separated when these symptoms are first noted, it is possible that both of the individual animals will survive (Hall and Hall 1957). Parabiotic disease likely represents underlying graft-versus-host disease, where the rejected “organ” may be the vascular anastomoses. Lethal irradiation of one of the parabionts abrogates parabiotic disease, suggesting that the immune system is responsible. How the immune system of one mouse becomes dominant over the other mouse remains an open question. Finally, we noticed that incidence of parabiotic disease is much less frequent when members of a pair were littermates, which has been observed in other studies (Binhammer et al. 1963; Eichwald et al. 1959).
Kinetic considerations:
The “on-rate” for any parabiotic effect is 1–2 weeks after surgery to permit sufficient vascular connections to develop and mature. This rate seems to be similar in animals of all ages. For our studies of skin wound healing, we waited for 1-month before further skin injury. An objective way to determine whether the parabiosis procedure established sufficient blood exchange is to connect a mouse carrying a reporter gene (e.g. mTmG mouse) and a non-reporter mouse. Three weeks after parabiosis, we drew venous blood from the mice and routinely obtained mixing rates between 35–40%, which is consistent with those reported by others (Conboy et al. 2005; Nishiguchi et al. 2018). Other groups have reported similar findings where mixing equilibrium is reached within 14 days but not 7 days after surgery (Gibney et al. 2012).
A second kinetic consideration is the rate of clearance of factors from the circulation. The rate of blood exchange in parabiosis models is relatively slow. Some proteins may be cleared from the circulation faster than they can exchange. Thus, some factors may not reach the partner in a parabiotic pair, and this result may lead to a false negative conclusion that a circulating factor is not involved. Harris et al. (2013) provide a more in-depth discussion on blood exchange and clearance rates in parabiosis. Acute cross circulation studies achieved by directly connecting large blood vessels between two animals results in complete mixing of blood in less than 10 minutes, and it is more likely that rapidly cleared factors will be present at high enough concentrations to be active in the other animal (Epstein et al. 1966; Laplace 1980; Stewart et al. 1963).
Applications of Parabiosis
There are three common applications where parabiosis experiments may be helpful in skin research.
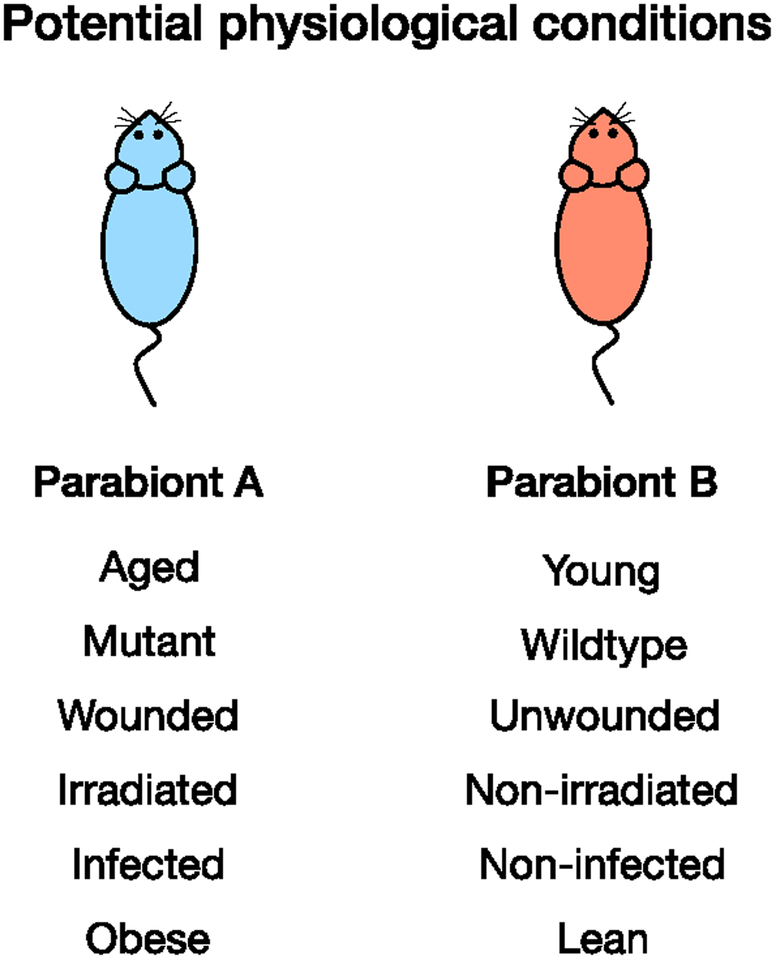
The most common use of parabiosis in dermatology studies is to test whether a circulating factor may be involved in a specific skin physiologic process, i.e. differentiation, neoplastic formation, or wound healing. The circulating factor is induced in one animal and the paired animal is assessed for a change in phenotype. Mice used for parabiosis can vary in physiological condition, making parabiosis an ideal technique for understanding a variety of biological processes (Figure 3). Parabiosis surgery is also reversible, which allows one to confirm that a circulating factor is responsible for a phenotypic change.
Parabiosis has been instrumental in answering questions about systemic regulation of cell and tissue aging in multiple organs. Heterochronic parabiosis allows researchers to test whether constant exposure to young or old blood changes physiologic skin processes (Figure 1). Here, we used heterochronic parabiosis to assess changes in scar formation and wound healing with age. A similar approach may be used to study skin aging and response to UV damage. Isolation of aged cells exposed, in vivo, to young blood has revealed clear molecular changes that may persist for some period of time (Goodell and Rando 2015). Aged skin and young skin have well-characterized phenotypic differences (thickness, rate of cell proliferation) (Adler et al. 2007; Leung et al. 2013). Parabiosis experiments will lead to a better understanding of cellular plasticity or the epigenetic regulation of the cellular state that defines a cell as being “young” or “old.” This reprogramming is clearly different than induced pluripotent stem cell (iPS) cell reprogramming, because these cells do not lose their differentiated state. The cells continue to be of the same lineages; the only change is that their regenerative tendencies become rejuvenated by the young blood milieu. These experiments would separate the differences between dedifferentiation and rejuvenation and allow for a more precise molecular definition of skin aging.
Application of parabiosis using genetically altered mouse strains allows for direct testing of signaling pathways/networks involved in regulating an identified process. To definitely show that circulating SDF1 from young blood was responsible for promoting scar formation in aged mice, we performed parabiosis between young skin-deficient SDF1 mice and aged WT mice (Figure 2) (Nishiguchi et al. 2018). In this instance, young blood deficient in SDF1 was not sufficient to promote scar formation in aged mice.
Figure 3. Potential physiological conditions to be studied using parabiosis.
. Parabiosis may be used to study a variety of phenotypes. Potential physiological conditions are not limited to the provided list.
As a powerful experimental system to identify molecular and cellular mechanisms, parabiosis has a distinguished history in dissecting fundamental biological processes across multiple fields. The combination of parabiosis with high-throughput genomics and proteomics approaches will continue to answer important unanswered questions in skin biology, particularly related to the epigenetics of skin aging and skin rejuvenation.
Supplementary Material
Summary Points.
Parabiosis experiments connect two living animals together, where they share a single circulatory system.
Parabiosis experiments assess whether a circulating factor in blood or lymph from one animal may affect the other animal.
Heterochronic parabiosis (connecting young and aged animals together) asks whether physiologic skin processes are affected by young blood milieu.
Limitations.
Animals should be background-matched to have the best chance of survival.
Circulatory system anastomosis requires 2-weeks to mature before steady-state is reached.
Multiple Choice Questions.
- For best results, mice should be:
- Female
- Background-matched
- Of similar size
- All of the above
- What protein variable can give a false negative in parabiosis experiments?
- Size
- Molecular weight
- Synthetic rate
- Clearance rate
- Parabiotic disease is the most common cause of death for parabiosis pairs 1–2 weeks after surgery. What is the incidence in outbred strains?
- 20%
- 40%
- 60%
- 100%
- Parabiosis experiments were instrumental in identifying the first circulating factor in satiety. What is this factor?
- Insulin
- Resistin
- Leptin
- Limastatin
- Perioperative mortality in parabiosis experiments is:
- 10%
- 20%
- 30%
- 40%
- 50%
Correct Answers:
d. Mice should be sex-matched and ideally female, background matched and size matched (within reason) for greatest success rate and minimal stress on the animals.
d. A false negative can occur as a result of the factor of interest having a high clearance rate from the blood and never reaching the other parabionts blood stream.
c. Incidence of parabiotic disease (also known as intoxication and disharmony) is 60–70% in outbred strains and 20–30% in inbred strains.
c. In 1959, Hervey used parabiosis in rats to determine that there is a circulating satiety factor, which was later identified as leptin.
a. Perioperative mortality is less prevalent in modern day parabiosis procedures as anesthesia has improved, and it stands at approximately 10%.
Acknowledgements:
T.H.L. receives support from the NIH (K08-AR066661 and P30-AR069589), VA (I01RX002701), the Moseley Foundation, and the H.T. Leung Foundation. We thank C. Natale for careful reading of the manuscript.
Abbreviations used:
- iPS,
induced pluripotent stem cell
- SDF-1
stromal cell-derived factor 1
- UV,
ultraviolet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors state no conflict of interest.
References
- Adler AS, Sinha S, Kawahara TLA, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. Cold Spring Harbor Lab; 2007;21(24):3244–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert P Experiences et Considerations Sur la Greffe Animale. J Anatomie Physiologie. 1864;1:69–87 [Google Scholar]
- Binhammer RT, Epstein S, Whitehouse A. Development of parabiosis intoxication in rat parabionts. Anat. Rec 1963;145:503–11 [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–10 [DOI] [PubMed] [Google Scholar]
- Bunster E, Mayer RK. An improved method of parabiosis. Anat. Rec 1933;57:339–43 [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4 [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12(3):525–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichwald EJ, Lustgraaf EC, Strainer M. Genetic factors in parabiosis. J. Natl. Cancer Inst 1959;23:1193–213 [PubMed] [Google Scholar]
- Epstein RB, Graham TC, Buckner CD, Bryant J, Thomas ED. Allogeneic marrow engraftment by cross circulation in lethally irradiated dogs. Blood. 1966;28(5):692–707 [PubMed] [Google Scholar]
- Finerty JC, Panos TC. Parabiosis intoxication. Proc. Soc. Exp. Biol. Med 1951;76(4):833–5 [DOI] [PubMed] [Google Scholar]
- Gibney BC, Chamoto K, Lee GS, Simpson DC, Miele LF, Tsuda A, et al. Cross-circulation and cell distribution kinetics in parabiotic mice. J. Cell. Physiol 2012;227(2):821–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Rando TA. Stem cells and healthy aging. Science. American Association for the Advancement of Science; 2015;350(6265):1199–204 [DOI] [PubMed] [Google Scholar]
- Hall CE, Hall O. Postseparation recovery from parabiosis intoxication in the rat. Am. J. Physiol 1957;189(3):495–7 [DOI] [PubMed] [Google Scholar]
- Harris RBS. Contribution made by parabiosis to the understanding of energy balance regulation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832(9):1449–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J. Physiol. (Lond.) 1959;145(2):336–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran P, Sereti K-I, Zhao P, Ali SR, Weissman IL, Ardehali R. Parabiosis in mice: a detailed protocol. JoVE. 2013;(80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplace JP. Compensatory hypertrophy of the residual small intestine after partial enterectomy. A neurohumoral feedback? Ann. Rech. Vet 1980;11(2):165–77 [PubMed] [Google Scholar]
- Leung TH, Zhang LF, Wang J, Ning S, Knox SJ, Kim SK. Topical hypochlorite ameliorates NF-κB–mediated skin diseases in mice. J. Clin. Invest. American Society for Clinical Investigation; 2013;123(12):5361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi MA, Spencer CA, Leung DH, Leung TH. Aging Suppresses Skin-Derived Circulating SDF1 to Promote Full-Thickness Tissue Regeneration. CellReports. 2018;24(13):3383–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbruch R, Heyde M. Ueber Parabiose kunstlich vereinigter Warmbliiter. Munch Med Wchnschr. 1908;55:153–6 [Google Scholar]
- Stewart JD, Williams JS, Kluge DN, Drapanas T. Effect of Cross Circulation on Metabolism Following Hepatectomy. Annals of Surgery. Lippincott, Williams, and Wilkins; 1963;158(5):812–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med 2014;20(6):659–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.