Abstract
Transcranial focused ultrasound (FUS) combined with intravenously circulating microbubbles can transiently and selectively increase blood–brain barrier permeability to enable targeted drug delivery to the central nervous system, and is a technique that has the potential to revolutionize the way neurological diseases are managed in medical practice. Clinical testing of this approach is currently underway in patients with brain tumors, early Alzheimer’s disease, and amyotrophic lateral sclerosis. A major challenge that needs to be addressed in order for widespread clinical adoption of FUS-mediated blood–brain barrier permeabilization to occur is the development of systems and methods for real-time treatment monitoring and control, to ensure that safe and effective acoustic exposure levels are maintained throughout the procedures. This review gives a basic overview of the oscillation dynamics, acoustic emissions, and biological effects associated with ultrasound-stimulated microbubbles in vivo, and provides a summary of recent advances in acoustic-based strategies for detecting, controlling, and mapping microbubble activity in the brain. Further development of next-generation clinical FUS brain devices tailored towards microbubble-mediated applications is warranted and required for translation of this potentially disruptive technology into routine clinical practice.
Introduction
Nearly two decades ago, it was discovered that low-intensity pulsed focused ultrasound (FUS) applied in the presence of intravenously circulating contrast agent microbubbles can temporarily and selectively increase blood–brain barrier (BBB) permeability in rabbits,1 providing a window for targeted delivery of therapeutics into the brain. Extensive pre-clinical work has demonstrated that a wide range of agents can be delivered to brain parenchyma using this approach (e.g. rodent models),2–8 and that various biological effects can result from FUS exposures with microbubbles but without additional agent delivery, including increased hippocampal neurogenesis,9,10 the clearance of amyloid-beta plaque pathology,11–13 as well as the stimulation of angiogenesis.14 Multiple laboratories have obtained positive therapeutic outcomes in animal models of brain tumors (e.g. rat models),7,15,16 Alzheimer’s disease (AD; e.g. transgenic mouse models,11–13,17 natural canine model of aging18) and Parkinson’s disease (e.g. transgenic rodent models).19–21 Based on the favorable safety profile of transcranial FUS-mediated BBB permeabilization observed in large animal experiments performed using a commercial brain system (e.g. non-human primate22 and trans-human skull porcine23 models), the first clinical trials in human subjects began at Sunnybrook Research Institute (Toronto, ON) in late 2015. Two separate safety trials have recently completed with this extracorporeal FUS device (30 cm diameter hemispherical phased array, 1024 elements, 220–230 kHz center frequency; ExAblate Neuro, InSightec, Ltd., Tirat Carmel, Israel); one for enhanced chemotherapy delivery to brain tumors (NCT02343991) and another in patients with early AD24 (NCT02986932). A third safety trial in patients with amyotrophic lateral sclerosis (ALS) is currently ongoing (NCT03321487). Researchers in France have concurrently demonstrated that repeated ultrasound exposures with microbubbles in circulation using a device implanted within the skull (10 mm diameter unfocused single-element transducer, 1.05 MHz center frequency; SonoCloud, CarThera, Inc., Paris, France) is safe and well-tolerated in patients with recurrent glioblastoma25 (NCT02253212), and there is an ongoing trial in patients with mild AD using this invasive applicator (NCT03119961).
It has been known since the 1950’s that ultrasound exposure to the brain can lead to increased BBB permeability, when Bakay et al noted regions of trypan blue extravasation at the periphery of FUS-induced lesions in cat brain.26 Subsequent studies indicated that increases in BBB permeability can be achieved in the absence of tissue damage via high-intensity FUS alone (i.e. without circulating microbubbles), however the approach was found to be inconsistent and unpredictable.27,28 Both thermal and non-thermal mechanisms were hypothesized to contribute to the FUS-mediated BBB permeabilization observed in these early studies.27,28 It has since been demonstrated that the dose threshold for thermal-mediated increases in BBB permeability is above that for brain tissue damage.29 More recently, inadvertent increases in BBB permeability have been observed in acute stroke patients following transcranial Doppler sonography without microbubbles,30 presumably due to skull heating effects.31
Following the seminal work of Hynynen et al.1 (i.e. frequency = 1.63 MHz frequency, peak negative pressure ≈ 1 MPa in situ, pulse length = 10 ms, pulse repetition frequency = 1 Hz, exposure duration = 20 s, bolus of 50 µl kg–1 Optison® microbubbles), a wide range of treatment parameters have been investigated for inducing transient increases in BBB permeability via FUS and microbubbles (e.g. see reviews for details).32,33 With appropriate treatment parameters, which include factors related to the ultrasound pulsing scheme and microbubble administration protocol,34–40 increased microvascular permeability can be achieved without evident tissue damage upon histological examination.22,41,42 Under these conditions, barrier functionality is generally restored within a few hours1,43,44 independent of the treatment volume.45 However, unsuitable parameters can result in a lack of desired effect or, conversely, extended durations of increased BBB permeability (e.g. 3–5 days46,47), vascular damage48,49 or substantial neuroinflammation.40,50 Treatment parameter selection is complicated by the application of FUS exposures through the human skull, as it is difficult to predict the acoustic pressure levels in the brain due to substantial variations in skull transmission efficiency,51–53 imperfect trans-skull focusing,54 as well as the potential for standing wave formation within the skull cavity.55,56 Furthermore, the local vascularity and resulting microbubble concentration differ considerably between brain structures, and are thought to impact the obtained biological outcomes.22,57,58 These sources of variability, combined with the relatively narrow window of parameters for which increased BBB permeability can be achieved without causing apparent tissue damage,22,41,42 necessitate the development of systems and methods for real-time treatment monitoring and control.
MRI is commonly used to assess the outcome of FUS procedures. For instance, gadolinium-based contrast-enhanced T 1-weighted sequences can detect changes in microvascular permeability, whereas T 2- and T 2*-weighted sequences can monitor for the presence of unwanted edema or hemorrhage induced by the sonications, respectively.1,22,24 However, the potential for a delayed onset of increased BBB permeability following FUS exposures (e.g. 5–15 min post-sonication),59 coupled with the low temporal resolution of MRI (acquisition times ≈ seconds-to-minutes) relative to the timescales over which the relevant ultrasound–microbubble–vessel interactions take place (microseconds-to-milliseconds), restricts the use of MRI to post-treatment evaluations in this context. Further, repeated gadolinium-based MRI contrast agent injections can lead to longlasting brain deposits depending on the class of contrast agent that is administered, which may pose a risk to patients.60 MR-thermometry, which is routinely employed for online monitoring of thermal FUS brain treatments, has limited utility during FUS BBB permeabilization procedures as the macroscopic temperature elevations generated are insignificant.1,23
Since spectral characteristics of the acoustic emissions generated from ultrasound-stimulated microbubbles can uncover features of the underlying bubble oscillation dynamics, remote detection of these signals may provide a means of guiding microbubble-mediated FUS treatments. The acoustic signals detected during FUS exposures with microbubbles for increased BBB permeability have been identified as potential indicators of treatment outcome41,42,61,62 and have since been investigated for online monitoring and control of the procedures.63–68 Multielement detector arrays can provide spatial information regarding microbubble activity in the brain69–76 that can be exploited for acoustic exposure level calibration,74 and element-specific aberration corrections can be incorporated into the reconstruction process to improve image quality in transcranial scenarios.70–72,77–79 This review gives a basic overview of the oscillation dynamics, acoustic emissions, and biological effects associated with ultrasound-stimulated microbubbles in vivo, and provides a summary of recent advances in acoustic-based strategies for detecting, controlling, and mapping microbubble activity in the brain during transcranial FUS procedures.
Acoustic cavitation: dynamics, emissions, and bioeffects
Acoustic cavitation refers to the interaction of acoustic waves with gas or vapor-filled cavities. At sufficiently high rarefactional pressures, cavitation and its accompanying bioeffects can be induced directly within tissue80 and the vasculature81 through nucleation of bubbles from the absorbed gases present in vivo and subsequent interactions of the nucleated bubbles with the incident acoustic field. Alternatively, encapsulated microbubbles, long used as contrast agents in diagnostic ultrasound,82 can be injected into circulation to provide additional cavitation nuclei and reduce cavitation thresholds within the vasculature.83 Commercially available ultrasound contrast agents consist of an air or perfluorocarbon core coated with a protein, polymer, or phospholipid shell to increase stability (i.e. circulation lifetime) at the sizes (<10 µm) required for successful entry into systemic circulation upon intravenous injection.84
In the context of biomedical ultrasound, cavitation activity is commonly separated into two distinct phenomenological regimes. Bubbles driven acoustically at low pressure amplitudes undergo periodic, volumetric oscillations about their equilibrium state that are dominated by the compressibility of the gas core, and under sufficient driving pressures non-spherical surface mode vibrations can develop. This type of bubble activity is often termed stable cavitation.85 The biological effects on the vasculature induced by stable cavitation are mainly attributed to a combination of acoustic microstreaming of the medium surrounding the bubble, which can exert large shear stresses on nearby cells or vessel walls,86 and stretching of the capillary vessel walls during the expansion and contraction states of the bubble oscillation.87 In addition, acoustic radiation forces can promote the translation of bubbles toward vessel walls88 and enhance the stresses experienced by nearby endothelial cells. These highly localized mechanical forces are thought to contribute to the ultrasound-triggered cell wall and vessel wall permeability observed in the presence of cavitating microbubbles,89 and may also play a role in FUS-mediated increases in BBB permeability through transient widening of the tight junctions.57,90 There is also evidence to suggest that bubbles undergoing stable oscillations can generate sufficient stresses to rupture capillaries.87
If the acoustic pressure is increased above a certain threshold value, bubbles can rapidly expand and violently collapse during the compression phase of the incident acoustic wave. Because the resulting chaotic collapse is dominated by the inertia of the surrounding liquid, this behavior is often referred to as inertial cavitation.85 Inertial cavitation can generate shock waves, high-velocity liquid jets, as well as extreme thermodynamic conditions that can lead to chemical reactions as well as light production. Encapsulated microbubbles can also be disrupted through fragmentation91 or due to the loss of shell material.92 Inertial bubble collapse has been associated with several biological effects in vivo, including apoptosis,93 tissue necrosis,94 as well as blood vessel rupture.49
Bubbles scatter or reradiate pressure waves in response to ultrasound stimulation.85 These acoustic emissions can be detected remotely using piezoelectric transducers, providing a method for monitoring cavitation non-invasively. The pressure waves emitted by a bubble undergoing stable and inertial cavitation have markedly different acoustical signatures, which is most evident from differences in the spectral frequency content of the two scenarios. Under sufficient driving pressure, a bubble undergoing stable cavitation within an ultrasound field can emit harmonics (i.e. (n + 1)f; n = 1,2,3…) as well as subharmonics (i.e. f/(n + 1); n = 1,2,3…) and ultraharmonics (i.e. (2n + 1)f/2; n = 1,2,3…) of the excitation frequency (f). Theoretical models have shown that subharmonic and ultraharmonic behavior can only exist when the driving pressure exceeds certain threshold values, both for free95,96 and encapsulated97 bubbles. For free bubbles, the threshold for the first subharmonic (i.e. f/2) is minimized when the bubble is driven at twice its resonance frequency (i.e. f = 2f 0). For encapsulated microbubbles, rapid changes in the nonlinear shell elasticity and viscosity are thought to contribute to the enhancement of subharmonic behavior.97,98 Optical evidence suggests that the maximal subharmonic response from encapsulated microbubbles is obtained when 1.7f 0 < f < 2.4f 0, depending on the bubble size and transmit pressure employed.99 Non-spherical modes of oscillation may also contribute to nonlinear bubble emissions.100 As the driving pressure is further increased and the inertial cavitation threshold is approached, broadband continuous noise, also visible to some extent during stable cavitation, becomes more prominent.85 Spectral analysis of cavitation emissions can therefore be used to characterize aspects of the underlying bubble dynamics, and this approach is under investigation for FUS treatment monitoring and control.
Cavitation detection
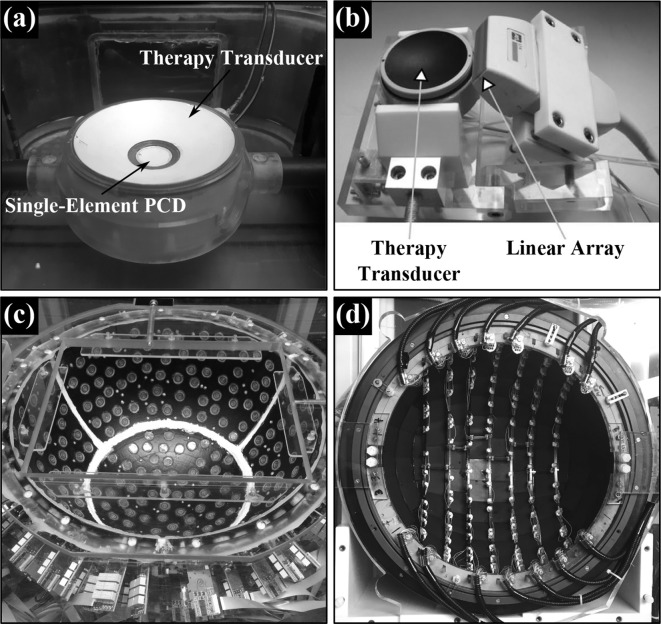
Perhaps the most common acoustic-based method for detecting cavitation remotely is through the use of a single-element passive cavitation detector (PCD), namely, a receive-only transducer that is separate from the therapy applicator81,94 (Figure 1a). With this approach, cavitation activity can be identified by an increase in the scattered signal in the time domain, or by changes in spectral content (e.g. harmonic, subharmonic, ultraharmonic, and broadband emission bands) in the frequency domain. The size, shape, and piezoelectric material of the detector can have a major impact on its spatial sensitivity profile and on the spectral frequency characteristics of the measured signals, and these factors should be carefully considered when interpreting acoustic emissions data.103 For example, detectors made of piezoelectric polymers (e.g. polyvinylidene fluoride) typically provide a broader frequency response than those made of piezoelectric ceramics (e.g. lead zirconate titanate) with equivalent geometry, with the trade-off of reduced levels of sensitivity. Single-element passive cavitation detection is now a well-established technique that is widely employed in both research and clinical settings for monitoring cavitation activity during the application of FUS. The existing clinical transcranial FUS brain systems (ExAblate Neuro, InSightec, Ltd., Tirat Carmel, Israel) have incorporated single-element PCDs for improved treatment safety, with sonications terminated automatically if signatures of significant inertial cavitation activity are observed.23,104
Figure 1.
Various detector types used to monitor acoustic cavitation remotely. (a) Single-element PCD potted in the middle of a spherically curved therapy transducer (FUS Instruments, Inc., Toronto, ON). (b) 1D linear array aligned with a spherically curved therapy transducer101 (© 2014 IEEE. Reprinted, with permission, from IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control). (c) Multifrequency sparse hemispherical transmit/receive ultrasound phased array clinical prototype.74 (d) Sparse hemispherical receiver array integrated within a clinical MRI-guided FUS brain system102 (© Institute of Physics and Engineering in Medicine. Reproduced by permission of IOP publishing. All rights reserved).
Several pre-clinical studies on FUS-mediated increased BBB permeability have reported correlations between spectral characteristics of the acoustic emissions captured using a single-element PCD and the resulting treatment outcomes.39,41,42,58,61,62,68,105–107 For example, it has been shown that increased vascular permeability can be achieved without detecting broadband emissions or red blood cell (RBC) extravasations,41,42 both evidence of inertial cavitation activity. These initial studies also reported significant increases in the strength of harmonic signals when exposure levels resulting in increased BBB permeability were employed, suggesting that harmonic emissions may be a useful metric for indicating treatment progress. Numerous reports have since attempted to correlate aspects of measured cavitation emissions with the resulting biological effects in a retrospective fashion, including the magnitude of increased BBB permeability, the BBB closure time, as well as the presence/absence of damage.39,58,61,62,68,105–107 For instance, exposure parameters that result in detectable broadband emissions are frequently associated with vascular, parenchymal, or neuronal damage.22,41,42,61,63,65,66,105 However, some studies have suggested that the detection of low-level broadband noise does not always result in damage,39,42,65,66 whereas in other cases RBC extravasations have been observed in the treated zone without detecting any significant wideband signals.39,64 The feasibility of monitoring contrast agent microbubble emissions transcranially with a single-element PCD has been demonstrated in large animal models through both primate22,58 and human23 skulls during pre-clinical testing of FUS-mediated increased BBB permeability, and initial clinical feasibility has recently been demonstrated.24,108,109
Cavitation control
The retrospective studies described in the previous section have prompted research that has attempted to exploit the acoustic emissions detected during FUS-mediated increased BBB permeability for real-time treatment feedback to achieve the desired biological effects while preventing unwanted damage. In one study, Arvanitis et al63 monitored changes in the combination of the first three harmonics of the transmit frequency during transcranial exposures in macaques using two separate detectors. The applied pressure level for each sonication was held constant, and was adjusted manually based on the spectral content of the received signals relative to baseline (i.e. without microbubbles in circulation) in previous exposures at the same target location for a given animal. The authors found that increasing the exposure level until a strong increase in harmonic emissions was detected relative to baseline, while staying below the threshold for substantial broadband noise, was an effective way of ensuring safe increases in BBB permeability. Expanding on this work, Sun et al66 developed a closed-loop feedback controller that sustains increased levels of harmonic emissions and suppresses broadband noise, both relative to their respective baseline levels. This control paradigm has been shown to enable reliable delivery of pre-defined, therapeutically relevant drug concentrations in both healthy and tumor bearing rats without causing apparent tissue damage.
Tsai et al carried out constant pressure sonications in rodents with a confocal dual-frequency transducer setup designed to terminate exposures automatically if subharmonic emissions were detected, and concluded that the detection of subharmonic activity was a good predictor of increased BBB permeability.65 With the current implementation, this approach65 lacks the flexibility to modulate the exposure level to account for variable in situ pressures within the brain. A recent pre-clinical study has proposed a closed-loop control scheme where a pre-determined level of ultraharmonic emissions is maintained throughout the entire exposure duration, however, safety assessments (e.g. histological examination) are necessary to determine whether this approach can lead to increased BBB permeability without causing tissue damage.67 Finally, O’Reilly et al increased the transmit pressure incrementally on a burst-by-burst basis until either the first or second ultraharmonic signals were detected by a single sensor, at which point the pressure was reduced to a percentage of the value required to induce this microbubble activity for the remainder of the sonication.64 In that study, sonications carried out at the 50% target level were shown to consistently produce safe increases in BBB permeability; lower target levels (i.e. 0 and 25%) were less likely to result in permeability enhancement, whereas at higher target levels (i.e. 75%) gross tissue damage was observed occasionally. Although ultraharmonics were analyzed in the original publication,64 subsequent work has utilized subharmonic signals within the same general approach.12 A modified version of this calibration method is currently being tested clinically during sonications in patients with early AD using a commercial transcranial FUS brain system.24
Although single-element PCDs can provide useful information for FUS treatment monitoring and control, the approach is limited by the sensor's fixed receive sensitivity profile, which inhibits spatially resolved cavitation detection from being carried out over an extended region. An inherent trade-off exists between the volume of sensitivity and spatial specificity of a single-element detector; focused receivers provide higher levels of sensitivity over a more confined spatial volume than do unfocused receivers of the same aperture size and spectral frequency characteristics. Focused detectors are therefore prone to missing cavitation events that originate from outside of their spatial sensing volume, whereas unfocused detectors cannot localize acoustic emissions with a high degree of spatial specificity and may fail to detect weaker cavitation events due to reduced levels of sensitivity. Unfocused detectors are thus more susceptible to false-positive detection events, such as the occurrence of cavitation activity in the coupling medium between the subject and the therapeutic transducer,64 though it may be possible to ascertain whether or not acoustic emissions have originated from within the brain based on the relative spectral content of different non-linear frequency components.110 Furthermore, when attempting to monitor cavitation activity in the brain, the location and orientation of the detector relative to the skull bone can greatly influence the nature of the recorded signals due to various effects such as local variations in attenuation, reflection/refractive effects, standing wave fields, and shear mode conversion.52,56,111,112 Thus, despite their widespread use in pre-clinical and clinical settings, single-element PCDs suffer from a number of limitations, particularly when employed in transcranial scenarios.
Cavitation mapping
Spatial information regarding microbubble activity would improve the safety of cavitation-mediated FUS procedures by allowing confirmation that the intended bioeffects are localized at the target(s) of interest while simultaneously monitoring for potential off-target microbubble behavior. During trans-skull FUS brain therapy, cavitation activity can be generated outside of the intended focal zone in sidelobes of the acoustic field112 or as a result of intracranial reverberations,55,56 and is more likely to occur in regions with a reduced cavitation threshold (e.g. tissue–ventricle interfaces).113 Multiple pre-clinical studies have observed FUS-induced bioeffects outside of the intended treatment region following exposures with microbubbles in circulation,112,114,115 including a recent study on FUS-mediated increased BBB permeability.66 Mechanical translation and/or rotation of a single-element detector can enable spatial mapping of cavitation distributions,116 though this approach requires highly stable cavitation fields over the course of signal acquisition and is impractical when such information is desired over large regions.
Pulse-echo imaging of ultrasound contrast agents via non-linear imaging sequences117,118 or filtering techniques119 can provide spatial information regarding microbubble activity in vivo. However, in the context of monitoring cavitation activity originating from within the brain, the acoustical properties (e.g. density, sound speed, attenuation) of human skull bone severely limit the use of typical B-mode frequencies.51 The application of low frequencies and shear-mode conversion may provide a solution for bubble cloud imaging in patients in the future.111,120
Multielement passive sensor arrays can generate spatial information regarding source fields via beamforming of the acquired signals, and this approach has been utilized for a wide variety of applications in the field of acoustics.121–125 Distribution of the receivers over a two-dimensional (2D) surface enables three-dimensional (3D) source field mapping.122 Over the past decade, passive sensor arrays have become increasingly investigated within the therapeutic ultrasound community for spatial mapping of cavitation activity.126–131 Passive acoustic imaging techniques have several important differences from traditional active imaging schemes (e.g. B-mode, Doppler) that make them particularly well-suited for ultrasound therapy guidance. Because passive imaging approaches rely on the therapy applicator for the excitation source, cavitation activity can be monitored continuously throughout the exposures without needing to gate the therapy burst to send interleaved imaging sequences. Passive beamforming methods typically form images based on correlations between the signals received on different elements of the array125,128 without using absolute time-of-flight information (n .b. synchronized passive acoustic imaging has been achieved in vitro 101 and in vivo 73,76 using absolute time-of-flight information, and has been shown to improve the axial resolution of one-dimensional (1D) arrays when short excitation bursts are used), whereas traditional line-by-line active imaging exploits absolute time-of-flight to encode image depth. As a result, the spatial resolution obtained via conventional delay, sum, and integrate passive beamforming algorithms (e.g. see Refs. 71,125,128 for details) is primarily dictated by the source's spectral characteristics and the receiver array’s diffraction pattern, and, contrary to traditional active imaging schemes is independent of burst length.129 This feature can be beneficial from a therapy monitoring perspective as it allows for continuous imaging throughout long therapy bursts without having to sacrifice spatial resolution. The use of long bursts also provides the opportunity to perform temporal averaging for improving the image signal-to-noise ratio (SNR) and temporal frequency resolution, the latter of which can aid in differentiating between specific types of cavitation activity, with the associated trade-off of reduced temporal resolution.77 However, if the SNR of the individual detectors is sufficient, short-time analysis can provide spatial cavitation maps as a function of time within a given therapy burst at high temporal resolution. In the context of sonothrombolysis in vitro without exogenous cavitation nuclei, megahertz-rate processing has been shown to uncover details regarding the evolution of cavitation activity that can be missed when temporal averaging is carried out over the duration of ultrasound on-time.132 Ultrafast 3D acoustic imaging has also been employed in vivo for spatiotemporal monitoring of contrast agent microbubbles during cavitation-mediated brain therapy.133 In the future, high volume-rate 3D microbubble imaging is expected to aid in the development of FUS treatment control strategies,74 and may also have application in bubble tracking for ultrasound angiography beyond the diffraction limit.134,135
The incorporation of passive acoustic imaging during cavitation-mediated FUS brain therapies would undoubtedly make the procedures more practical, however, ultrasound imaging in the human brain is complicated by the presence of the intervening skull. One approach to utilizing passive beamforming methods in the brain is to image through acoustic windows (e.g. temporal or suboccipital windows) using a narrow-aperture array, such as a 1D linear diagnostic probe69,73,75,76,78 (Figure 1b). With this configuration, the local variations in skull morphology over the region of acoustic signal collection are small, and transcranial passive acoustic imaging can be achieved at sufficiently low frequencies without the need for element-specific aberration corrections on receive.69,73,75,76,78 However, the limited range of acceptance angles from which source signals can be acquired using such a narrow-aperture device leads to poor spatial resolution, particularly in the axial direction (i.e. along the acoustic axis of the transducer), when employing delay, sum, and integrate passive beamforming methods.71,125,128 Furthermore, the effective imaging region with a stationary small-aperture 1D receiver array is confined to a 2D area located near the central axis of the device. Lastly, considerable inter- and intrapatient variability exists in the availability of acoustic windows within human skull bone, and each acoustic window has a limited field-of-view within the brain.136
Alternatively, the use of large-aperture 2D receiver arrays allows source signals of interest to be acquired over a greater solid angle, and enables 3D acoustic imaging with improvements in spatial resolution, receive sensitivity, and the effective imaging volume during passive beamforming.77 For monitoring cavitation activity within the brain in a clinical setting, the use of a receiver array covering the maximal available skull surface area would thus greatly improve upon the case of imaging through acoustic bone windows with a narrow-aperture device. Indeed, such a large-aperture design is employed in the transmit arrays that are currently used for transcranial FUS brain therapy in the clinic.23,24 However, element-specific corrections will become necessary on receive to account for skull-induced aberrations, particularly at higher source frequencies, analogous to the case of transcranial transmit beam focusing with large-aperture arrays. It has been shown through both numerical simulations77,78 and experimental measurements70–72,79,134,137 that aberration corrections can provide improvements in image quality (e.g. positional error, main lobe volume, peak sidelobe ratio, image SNR) when employing passive acoustic imaging through human calvaria. Similar to the transmit focusing case, trans-skull aberration corrections can be obtained for imaging purposes through invasive measurements with an emitting/receiving transducer,70,72 or via non-invasive approaches, such as CT-based ultrasound propagation modeling71,77–79 or analysis of single bubble emissions originating from within the skull cavity.134,137
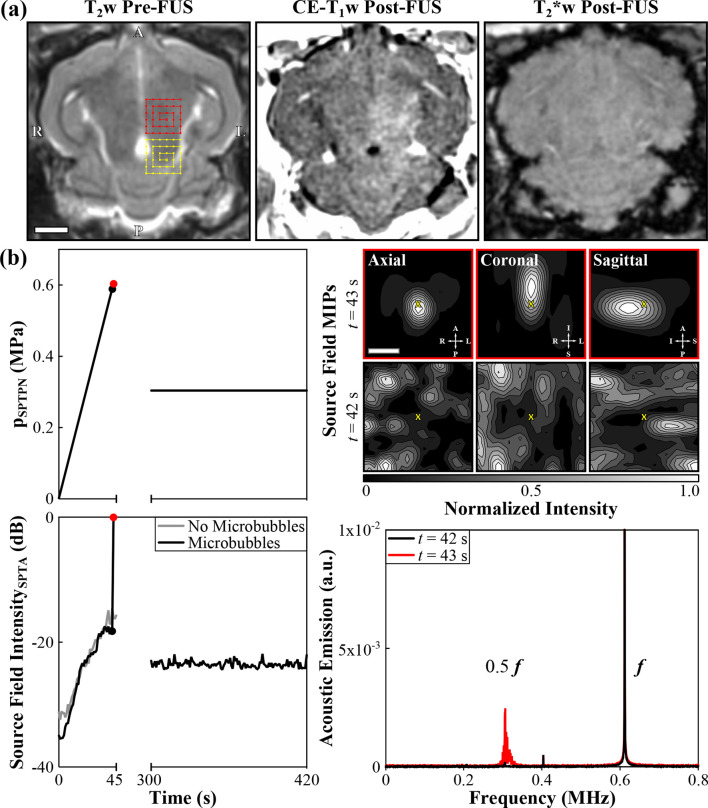
The FUS group at Sunnybrook Research Institute (Toronto, ON) has developed large-aperture transmit/receive hemispherical ultrasound arrays for microbubble-mediated transcranial therapy delivery and simultaneous 3D cavitation mapping.70,72,74 Numerical simulations were carried out to inform the various device designs,77 as transducer layout is known to play a major role in the obtainable transmit focusing quality and imaging point spread function of sparse array systems. In the initial work, O’Reilly et al integrated a sparse hemispherical receiver array within an existing fully populated transcranial phased array prototype.70 Using this dual-mode system, the feasibility of 3D bubble cloud mapping in the brain during exposures carried out at pressure levels below and above the threshold for FUS-mediated increases in BBB permeability was demonstrated in rodents70 with an intervening ex vivo human skullcap.71 The same group has also fabricated multifrequency FUS brain prototype systems (Figure 1c) with increased flexibility from both therapy delivery and treatment monitoring standpoints.72,74 Using one of these clinical-scale prototypes, it has been shown in both rabbits74 and pigs138 that 3D transcranial subharmonic imaging can be used to actively calibrate exposure levels for volumetric FUS-mediated increases in BBB permeability without causing apparent tissue damage (Figure 2). Researchers at Brigham and Womens Hospital (Boston, MA) have recently designed a sparse hemispherical array to fit within a clinical MRI-guided FUS brain system (Figure 1d), and have presented results of 3D bubble cloud mapping during non-thermal ablation in the brain of a macaque with this device.102 A lab in Taiwan has also constructed a dual-mode ultrasound phased array system for microbubble-mediated FUS brain therapy.139
Figure 2.
Example of 3D transcranial subharmonic microbubble imaging for calibrating acoustic exposure levels for inducing volumetric FUS-mediated BBB permeabilization in the thalamus of a New Zealand White rabbit (male, 3 kg) without causing apparent tissue damage. The data correspond to an animal from the “treatment group” of the study described in Ref.74 (a) MRI data. (Left) Axial T 2w MR image acquired pre-FUS. Two non-overlapping target volumes (6 × 6 point grids, 1.0 mm point spacing, “X” symbol denotes calibration point) and their respective electronic beam steering trajectories are illustrated. (Center) Axial CE-T 1w MR image acquired immediately post-FUS. Regions of CE-T 1w MRI signal hyperintensity were induced by the exposures, indicating increased BBB permeability within the target volumes. (Right) Axial T 2*w MR image acquired 15 min post-FUS. No obvious regions of T 2*w MRI signal hypointensity were induced by the exposures. Histological examination of hematoxylin-eosin stained tissue sections revealed no RBC extravasations in this animal,74 confirming that safe exposure levels were employed. Scale bar indicates 5 mm. (b) Ultrasound data corresponding to the posterior-most target volume (yellow). (Top Left) Estimated in situ spatial-peak temporal-peak negative pressure (p SPTPN) and (Bottom Left) SPTA source field intensity of 3D acoustic reconstructions during a calibration sonication with microbubbles in circulation and subsequent therapeutic exposure at the 50% target level (f = 612 kHz, burst length = 10 ms, burst repetition frequency = 1 Hz). Baseline values from a pressure ramp at the same target location acquired without microbubbles in circulation are also shown. The received signals were captured using the array elements tuned to the subharmonic (f/2 = 306 kHz). A delay of 5 min between sonications allowed the bubbles to mostly clear from the circulation. (Top Right) Normalized MIP contour images (linear contours at 10% intervals) of temporal-average source field intensity volume distributions for the bursts 42 s (pSPTPN = 0.59 MPa, no subharmonic activity) and 43 s (pSPTPN = 0.60 MPa, subharmonic activity) into the calibration sonication. The imaging field-of-view is centered on the calibration point (“X” symbols). Scale bar indicates 3 mm. (Bottom Right) Frequency spectrum of the beamformed signal at the location of SPTA source field intensity for the bursts 42 and 43 s into the calibration sonication. The data are normalized to the spectral peak at the fundamental frequency for t = 43 s. Further details regarding the MRI and ultrasound protocols can be found in Ref.74
The continued development of systems and methods for transcranial therapy delivery and simultaneous 3D microbubble mapping/control are expected to improve the safety and efficacy of FUS-mediated BBB permeability enhancement procedures. Furthermore, as this general approach to treatment guidance is applicable to other cavitation-mediated therapies (e.g. non-thermal ablation,114,115 sonothrombolysis,140 histotripsy141) and would improve upon existing cavitation monitoring strategies during treatments that are intended to be purely thermal-based,53,104 these acoustic-based techniques stand to have a substantial impact on the clinical management of neurological disorders by making FUS brain interventions safe and practical.
Conclusion
Since the first pre-clinical experiments were published in 2001,1 the application of transcranial FUS in conjunction with intravenously circulating microbubbles for increased BBB permeability has been shown by multiple independent laboratories to enable the delivery of a wide range of therapeutics into the brain and improve outcomes in various animal disease models—work that ultimately culminated in initial clinical testing in patients with brain tumors (in 2015), early AD (in 2017), and ALS (in 2018). Continual improvements in therapeutic ultrasound system capabilities and image-guidance strategies are expanding the use of FUS in the brain, thereby increasing the number of neurological indications for which FUS is a viable and effective treatment option. In particular, the development of next-generation clinical brain systems capable of transcranial therapy delivery and simultaneous detection, control, and mapping of microbubble activity will make FUS-mediated BBB permeability enhancement procedures more practical, and will help facilitate the technique’s translation into routine clinical practice.
Footnotes
Acknowledgment: The authors would like to thank Meaghan O’Reilly and Dallan McMahon for proofreading the manuscript, as well as Calum Crake and Nathan McDannold for providing the image of their ultrasound system (Figure 1d).
Conflicts of Interest : RMJ and KH are inventors on issued patents and patent applications related to transcranial focused ultrasound technology. KH owns stock in FUS Instruments, which has licensed IP related to the methods described in this review for pre-clinical use.
Funding: Financial support was provided by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (R01 EB003268), the Canadian Institutes of Health Research (MOP 119312, FDN 154272), the W. Garfield Weston Foundation, and the Canada Research Chairs program.
Contributor Information
Ryan M Jones, Email: rmjones@sri.utoronto.ca.
Kullervo Hynynen, Email: khynynen@sri.utoronto.ca.
REFERENCES
- 1. Hynynen K , McDannold N , Vykhodtseva N , Jolesz FA . Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits . Radiology 2001. ; 220 : 640 – 6 . doi: 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]
- 2. Kinoshita M , McDannold N , Jolesz FA , Hynynen K . Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption . Proc Natl Acad Sci U S A 2006. ; 103 : 11719 – 23 . doi: 10.1073/pnas.0604318103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burgess A , Ayala-Grosso CA , Ganguly M , Jordão JF , Aubert I , Hynynen K . Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier . PLoS One 2011. ; 6 : e27877 . doi: 10.1371/journal.pone.0027877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baseri B , Choi JJ , Deffieux T , Samiotaki G , Tung Y-S , Olumolade O , et al. . Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood–brain barrier using focused ultrasound and microbubbles . Phys Med Biol 2012. ; 57 : N65 – N81 . doi: 10.1088/0031-9155/57/7/N65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burgess A , Huang Y , Querbes W , Sah DW , Hynynen K . Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression . J Control Release 2012. ; 163 : 125 – 9 . doi: 10.1016/j.jconrel.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Etame AB , Diaz RJ , O'Reilly MA , Smith CA , Mainprize TG , Hynynen K , et al. . Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound . Nanomedicine 2012. ; 8 : 1133 – 42 . doi: 10.1016/j.nano.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Treat LH , McDannold N , Zhang Y , Vykhodtseva N , Hynynen K . Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma . Ultrasound Med Biol 2012. ; 38 : 1716 – 25 . doi: 10.1016/j.ultrasmedbio.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alkins R , Burgess A , Ganguly M , Francia G , Kerbel R , Wels WS , et al. . Focused ultrasound delivers targeted immune cells to metastatic brain tumors . Cancer Res 2013. ; 73 : 1892 – 9 . doi: 10.1158/0008-5472.CAN-12-2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scarcelli T , Jordão JF , O'Reilly MA , Ellens N , Hynynen K , Aubert I . Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice . Brain Stimul 2014. ; 7 : 304 – 7 . doi: 10.1016/j.brs.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mooney SJ , Shah K , Yeung S , Burgess A , Aubert I , Hynynen K . Focused ultrasound-induced neurogenesis requires an increase in blood-brain barrier permeability . PLoS One 11 : e0159892 . doi: 10.1371/journal.pone.0159892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jordão JF , Thévenot E , Markham-Coultes K , Scarcelli T , Weng Y-Q , Xhima K , et al. . Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound . Exp Neurol 2013. ; 248 : 16 – 29 . doi: 10.1016/j.expneurol.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burgess A , Dubey S , Yeung S , Hough O , Eterman N , Aubert I , et al. . Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior . Radiology 2014. ; 273 : 736 – 45 . doi: 10.1148/radiol.14140245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leinenga G , Götz J . Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model . Sci Transl Med 2015. ; 7 : 278ra33 . doi: 10.1126/scitranslmed.aaa2512 [DOI] [PubMed] [Google Scholar]
- 14. McMahon D , Mah E , Hynynen K . Angiogenic response of rat hippocampal vasculature to focused ultrasound-mediated increases in blood-brain barrier permeability . Sci Rep 2018. ; 8 : 12178 . doi: 10.1038/s41598-018-30825-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu H-L , Hua M-Y , Chen P-Y , Chu P-C , Pan C-H , Yang H-W , et al. . Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment . Radiology 2010. ; 255 : 415 – 25 . doi: 10.1148/radiol.10090699 [DOI] [PubMed] [Google Scholar]
- 16. Aryal M , Vykhodtseva N , Zhang YZ , Park J , McDannold N . Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model . J Control Release 2013. ; 169 ( 1-2 ): 103 – 11 . doi: 10.1016/j.jconrel.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nisbet RM , Van der Jeugd A , Leinenga G , Evans HT , Janowicz PW , Götz J . Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model . Brain 2017. ; 140 : 1220 – 30 . doi: 10.1093/brain/awx052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Reilly MA , Jones RM , Barrett E , Schwab A , Head E , Hynynen K . Investigation of the Safety of Focused Ultrasound-Induced Blood-Brain Barrier Opening in a Natural Canine Model of Aging . Theranostics 2017. ; 7 : 3573 – 84 . doi: 10.7150/thno.20621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samiotaki G , Acosta C , Wang S , Konofagou EE . Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood--brain barrier opening in vivo . J Cereb Blood Flow Metab 2015. ; 35 : 611 – 22 . doi: 10.1038/jcbfm.2014.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan CH , Ting CY , Lin CY , Chan HL , Chang YC , Chen YY , et al. . Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of parkinson's disease . Sci Rep 2016. ; 6 : 19579 . doi: 10.1038/srep19579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mead BP , Kim N , Miller GW , Hodges D , Mastorakos P , Klibanov AL , et al. . Novel focused ultrasound gene therapy approach noninvasively restores dopaminergic neuron function in a rat Parkinson’s disease model . Nano Lett 2017. ; 17 : 3533 – 42 . doi: 10.1021/acs.nanolett.7b00616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDannold N , Arvanitis CD , Vykhodtseva N , Livingstone MS . Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques . Cancer Res 2012. ; 72 : 3652 – 63 . doi: 10.1158/0008-5472.CAN-12-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Y , Alkins R , Schwartz ML , Hynynen K . Opening the blood-brain barrier with MR imaging-guided focused ultrasound: preclinical testing on a trans-human skull porcine model . Radiology 2017. ; 282 : 123 – 30 . doi: 10.1148/radiol.2016152154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lipsman N , Meng Y , Bethune AJ , Huang Y , Lam B , Masellis M , et al. . Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound . Nat Commun 2018. ; 9 : 2336 . doi: 10.1038/s41467-018-04529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carpentier A , Canney M , Vignot A , Reina V , Beccaria K , Horodyckid C , et al. . Clinical trial of blood-brain barrier disruption by pulsed ultrasound . Sci Transl Med 2016. ; 8 : 343re2 . doi: 10.1126/scitranslmed.aaf6086 [DOI] [PubMed] [Google Scholar]
- 26. Bakay L , BALLANTINE HT , HUETER TF , Sosa D . Ultrasonically produced changes in the blood-brain barrier . AMA Arch Neurol Psychiatry 1956. ; 76 : 457 – 67 . doi: 10.1001/archneurpsyc.1956.02330290001001 [DOI] [PubMed] [Google Scholar]
- 27. Vykhodtseva NI , Hynynen K , Damianou C . Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo . Ultrasound Med Biol 1995. ; 21 : 969 – 79 . doi: 10.1016/0301-5629(95)00038-S [DOI] [PubMed] [Google Scholar]
- 28. Mesiwala AH , Farrell L , Wenzel HJ , Silbergeld DL , Crum LA , Winn HR , et al. . High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo . Ultrasound Med Biol 2002. ; 28 : 389 – 400 . doi: 10.1016/S0301-5629(01)00521-X [DOI] [PubMed] [Google Scholar]
- 29. McDannold N , Vykhodtseva N , Jolesz FA , Hynynen K . MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain . Magn Reson Med 2004. ; 51 : 913 – 23 . doi: 10.1002/mrm.20060 [DOI] [PubMed] [Google Scholar]
- 30. Reinhard M , Hetzel A , Krüger S , Kretzer S , Talazko J , Ziyeh S , et al. . Blood-brain barrier disruption by low-frequency ultrasound . Stroke 2006. ; 37 : 1546 – 8 . doi: 10.1161/01.STR.0000221813.27519.0b [DOI] [PubMed] [Google Scholar]
- 31. Connor CW , Hynynen K . Patterns of thermal deposition in the skull during transcranial focused ultrasound surgery . IEEE Transactions on Biomedical Engineering 2004. ; 51 : 1693 – 706 . doi: 10.1109/TBME.2004.831516 [DOI] [PubMed] [Google Scholar]
- 32. O'Reilly MA , Hynynen K . Ultrasound enhanced drug delivery to the brain and central nervous system . Int J Hyperthermia 2012. ; 28 : 386 – 96 . doi: 10.3109/02656736.2012.666709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aryal M , Arvanitis CD , Alexander PM , McDannold N . Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system . Adv Drug Deliv Rev 2014. ; 72 : 94 – 109 . doi: 10.1016/j.addr.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDannold N , Vykhodtseva N , Hynynen K . Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: a feasibility study . Ultrasound Med Biol 2007. ; 33 : 584 – 90 . doi: 10.1016/j.ultrasmedbio.2006.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDannold N , Vykhodtseva N , Hynynen K . Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption . Ultrasound Med Biol 2008. ; 34 : 930 – 7 . doi: 10.1016/j.ultrasmedbio.2007.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chopra R , Vykhodtseva N , Hynynen K . Influence of exposure time and pressure amplitude on blood-brain-barrier opening using transcranial ultrasound exposures . ACS Chem Neurosci 2010. ; 1 : 391 – 8 . doi: 10.1021/cn9000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi JJ , Feshitan JA , Baseri B , Wang S , , Borden MA , Tung YS , et al. . Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo . IEEE Trans Biomed Eng 2010. ; 57 : 145 – 54 . doi: 10.1109/TBME.2009.2034533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi JJ , Selert K , Gao Z , Samiotaki G , Baseri B , Konofagou EE . Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies . J Cereb Blood Flow Metab 2011. ; 31 : 725 – 37 . doi: 10.1038/jcbfm.2010.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McDannold N , Zhang Y , Vykhodtseva N . The effects of oxygen on ultrasound-induced blood-brain barrier disruption in mice . Ultrasound Med Biol 2017. ; 43 : 469 – 75 . doi: 10.1016/j.ultrasmedbio.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McMahon D , Hynynen K . Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose . Theranostics 2017. ; 7 : 3989 – 4000 . doi: 10.7150/thno.21630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McDannold N , Vykhodtseva N , Hynynen K . Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity . Phys Med Biol 2006. ; 51 : 793 – 807 . doi: 10.1088/0031-9155/51/4/003 [DOI] [PubMed] [Google Scholar]
- 42. Tung Y-S , Vlachos F , Choi JJ , Deffieux T , Selert K , Konofagou EE . In vivo transcranial cavitation threshold detection during ultrasound-induced blood–brain barrier opening in mice . Phys Med Biol 2010. ; 55 : 6141 – 55 . doi: 10.1088/0031-9155/55/20/007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mei J , Cheng Y , Song Y , Yang Y , Wang F , Liu Y , et al. . Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound . J Ultrasound Med 2009. ; 28 : 871 – 80 . doi: 10.7863/jum.2009.28.7.871 [DOI] [PubMed] [Google Scholar]
- 44. Marty B , Larrat B , Van Landeghem M , Robic C , Robert P , Port M , et al. . Dynamic study of blood-brain barrier closure after its disruption using ultrasound: a quantitative analysis . J Cereb Blood Flow Metab 2012. ; 32 : 1948 – 58 . doi: 10.1038/jcbfm.2012.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Reilly MA , Hough O , Hynynen K . Blood-brain barrier closure time after controlled ultrasound-induced opening is independent of opening volume . Journal of Ultrasound in Medicine 2017. ; 36 : 475 – 83 . doi: 10.7863/ultra.16.02005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Samiotaki G , Vlachos F , Tung Y-S , Konofagou EE . A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI . Magn Reson Med 2012. ; 67 : 769 – 77 . doi: 10.1002/mrm.23063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samiotaki G , Konofagou EE . Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo . IEEE Trans Ultrason Ferroelectr Freq Control 2013. ; 60 : 2257 – 65 . doi: 10.1109/TUFFC.2013.6644731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller DL , Quddus J . Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice . Proc Natl Acad Sci U S A 2000. ; 97 : 10179 – 84 . doi: 10.1073/pnas.180294397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aviv RI , Huynh T , Huang Y , Ramsay D , Van Slyke P , Dumont D , et al. . An in vivo, MRI-integrated real-time model of active contrast extravasation in acute intracerebral hemorrhage . AJNR Am J Neuroradiol 2014. ; 35 : 1693 – 9 . doi: 10.3174/ajnr.A3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kovacs ZI , Kim S , Jikaria N , Qureshi F , Milo B , Lewis BK , et al. . Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation . Proc Natl Acad Sci U S A 2017. ; 114 : E75 – E84 . doi: 10.1073/pnas.1614777114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fry FJ , Barger JE . Acoustical properties of the human skull . J Acoust Soc Am 1978. ; 63 : 1576 – 90 . doi: 10.1121/1.381852 [DOI] [PubMed] [Google Scholar]
- 52. Pichardo S , Sin VW , Hynynen K . Multi-frequency characterization of the speed of sound and attenuation coefficient for longitudinal transmission of freshly excised human skulls . Phys Med Biol 2011. ; 56 : 219 – 50 . doi: 10.1088/0031-9155/56/1/014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwartz ML , Yeung R , Huang Y , Lipsman N , Krishna V , Jain JD , et al. . Skull bone marrow injury caused by MR-guided focused ultrasound for cerebral functional procedures . J Neurosurg 2018. ; 4 : 1 – 5 . doi: 10.3171/2017.11.JNS17968 [DOI] [PubMed] [Google Scholar]
- 54. Pulkkinen A , Werner B , Martin E , Hynynen K . Numerical simulations of clinical focused ultrasound functional neurosurgery . Phys Med Biol 2014. ; 59 : 1679 – 700 . doi: 10.1088/0031-9155/59/7/1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baron C , Aubry J-F , Tanter M , Meairs S , Fink M . Simulation of intracranial acoustic fields in clinical trials of sonothrombolysis . Ultrasound Med Biol 2009. ; 35 : 1148 – 58 . doi: 10.1016/j.ultrasmedbio.2008.11.014 [DOI] [PubMed] [Google Scholar]
- 56. Song J , Pulkkinen A , Huang Y , Hynynen K , Pulkkinen A . Investigation of standing-wave formation in a human skull for a clinical prototype of a large-aperture, transcranial MR-guided focused ultrasound (MRgFUS) phased array: an experimental and simulation study . IEEE Trans Biomed Eng 2012. ; 59 : 435 – 44 . doi: 10.1109/TBME.2011.2174057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hosseinkhah N , Goertz DE , Hynynen K . Microbubbles and blood-brain barrier opening: a numerical study on acoustic emissions and wall stress predictions . IEEE Trans Biomed Eng 2015. ; 62 : 1293 – 304 . doi: 10.1109/TBME.2014.2385651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu SY , Sanchez CS , Samiotaki G , Buch A , Ferrera VP , Konofagou EE . Characterizing focused-ultrasound mediated drug delivery to the heterogeneous primate brain in vivo with acoustic monitoring . Sci Rep 2016. ; 6 : 37094 . doi: 10.1038/srep37094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cho EE , Drazic J , Ganguly M , Stefanovic B , Hynynen K . Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening . J Cereb Blood Flow Metab 2011. ; 31 : 1852 – 62 . doi: 10.1038/jcbfm.2011.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gulani V , Calamante F , Shellock FG , Kanal E , Reeder SB , International Society for Magnetic Resonance in Medicine . Gadolinium deposition in the brain: summary of evidence and recommendations . Lancet Neurol 2017. ; 16 : 564 – 70 . doi: 10.1016/S1474-4422(17)30158-8 [DOI] [PubMed] [Google Scholar]
- 61. Yang FY , Fu WM , Chen WS , Yeh WL , Lin WL . Quantitative evaluation of the use of microbubbles with transcranial focused ultrasound on blood-brain-barrier disruption . Ultrason Sonochem 2008. ; 15 : 636 – 43 . doi: 10.1016/j.ultsonch.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 62. O’Reilly MA , Hynynen K . A PVDF receiver for ultrasound monitoring of transcranial focused ultrasound therapy . IEEE Trans Biomed Engg 2012. ; 57 : 2286 – 94 . doi: 10.1109/TBME.2010.2050483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arvanitis CD , Livingstone MS , Vykhodtseva N , McDannold N . Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring . PLoS One 2012. ; 7 : e45783 . doi: 10.1371/journal.pone.0045783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Reilly MA , Hynynen K . Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller . Radiology 2012. ; 263 : 96 – 106 . doi: 10.1148/radiol.11111417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tsai C-H , Zhang J-W , Liao Y-Y , Liu H-L . Real-time monitoring of focused ultrasound blood-brain barrier opening via subharmonic acoustic emission detection: implementation of confocal dual-frequency piezoelectric transducers . Phys Med Biol 2016. ; 61 : 2926 – 46 . doi: 10.1088/0031-9155/61/7/2926 [DOI] [PubMed] [Google Scholar]
- 66. Sun T , Zhang Y , Power C , Alexander PM , Sutton JT , Aryal M , et al. . Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model . Proc Nat Aca Sci 2017. ; 114 : E10281 – E10290 . doi: 10.1073/pnas.1713328114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bing C , Hong Y , Hernandez C , Rich M , Cheng B , Munaweera I , et al. . Characterization of different bubble formulations for blood-brain barrier opening using a focused ultrasound system with acoustic feedback control . Sci Rep 2018. ; 8 : 7986 . doi: 10.1038/s41598-018-26330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kamimura HAS , Flament J , Valette J , Cafarelli A , Aron Badin R , Hantraye P , et al. . Feedback control of microbubble cavitation for ultrasound-mediated blood–brain barrier disruption in non-human primates under magnetic resonance guidance . Journal of Cerebral Blood Flow & Metabolism 2018. ; 10 : 0271678X1775351 . doi: 10.1177/0271678X17753514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Arvanitis CD , Livingstone MS , McDannold N . Combined ultrasound and MR imaging to guide focused ultrasound therapies in the brain . Phys Med Biol 2013. ; 58 : 4749 – 61 . doi: 10.1088/0031-9155/58/14/4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Reilly MA , Jones RM , Hynynen K . Three-dimensional transcranial ultrasound imaging of microbubble clouds using a sparse hemispherical array . IEEE Trans Biomed Eng 2014. ; 61 : 1285 – 94 . doi: 10.1109/TBME.2014.2300838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jones RM , O'Reilly MA , Hynynen K . Experimental demonstration of passive acoustic imaging in the human skull cavity using CT-based aberration corrections . Med Phys 2015. ; 42 : 4385 – 400 . doi: 10.1118/1.4922677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Deng L , O’Reilly MA , Jones RM , An R , Hynynen K . A multi-frequency sparse hemispherical ultrasound phased array for microbubble-mediated transcranial therapy and simultaneous cavitation mapping . Phys Med Biol 2016. ; 61 : 8476 – 501 . doi: 10.1088/0031-9155/61/24/8476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Burgess MT , Apostolakis I , Konofagou EE . Power cavitation-guided blood-brain barrier opening with focused ultrasound and microbubbles . Phys Med Biol 2018. ; 63 : 065009 . doi: 10.1088/1361-6560/aab05c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jones RM , Deng L , Leung K , McMahon D , O'Reilly MA , Hynynen K . Three-dimensional transcranial microbubble imaging for guiding volumetric ultrasound-mediated blood-brain barrier opening . Theranostics 2018. ; 8 : 2909 – 26 . doi: 10.7150/thno.24911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu S-Y , Aurup C , Sanchez CS , Grondin J , Zheng W , Kamimura H , et al. . Efficient blood-brain barrier opening in primates with neuronavigation-guided ultrasound and real-time acoustic mapping . Sci Rep 2018. ; 8 : 7978 . doi: 10.1038/s41598-018-25904-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pouliopoulos AN , Burgess MT , Konofagou EE . Pulse inversion enhances the passive mapping of microbubble-based ultrasound therapy . Appl Phys Lett 2018. ; 113 : 044102 . doi: 10.1063/1.5036516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jones RM , O’Reilly MA , Hynynen K . Transcranial passive acoustic mapping with hemispherical sparse arrays using CT-based skull-specific aberration corrections: a simulation study . Phys Med Biol 2013. ; 58 : 4981 – 5005 . doi: 10.1088/0031-9155/58/14/4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Arvanitis CD , Clement GT , McDannold N . Transcranial assessment and visualization of acoustic cavitation: modeling and experimental validation . IEEE Trans Med Imag 2015. ; 34 : 1270 – 81 . doi: 10.1109/TMI.2014.2383835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jones RM , Hynynen K . Comparison of analytical and numerical approaches for CT-based aberration correction in transcranial passive acoustic imaging . Phys Med Biol 2016. ; 61 : 23 – 36 . doi: 10.1088/0031-9155/61/1/23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hynynen K . The threshold for thermally significant cavitation in dog's thigh muscle in vivo . Ultrasound Med Biol 1991. ; 17 : 157 – 69 . doi: 10.1016/0301-5629(91)90123-E [DOI] [PubMed] [Google Scholar]
- 81. Holland CK , Deng CX , Apfel RE , Alderman JL , Fernandez LA , Taylor KJW . Direct evidence of cavitation in vivo from diagnostic ultrasound . Ultrasound Med Biol 1996. ; 22 : 917 – 25 . doi: 10.1016/0301-5629(96)00083-X [DOI] [PubMed] [Google Scholar]
- 82. Feinstein SB , Shah PM , Bing RJ , Meerbaum S , Corday E , Chang B-L , et al. . Microbubble dynamics visualized in the intact capillary circulation . J Am Coll Cardiol 1984. ; 4 : 595 – 600 . doi: 10.1016/S0735-1097(84)80107-2 [DOI] [PubMed] [Google Scholar]
- 83. Miller D . Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation . Prog Biophys Mol Biol 2007. ; 93 ( 1-3 ): 314 – 30 . doi: 10.1016/j.pbiomolbio.2006.07.027 [DOI] [PubMed] [Google Scholar]
- 84. Stride E , Saffari N . Microbubble ultrasound contrast agents: A review . Proc Inst Mech Eng H 2003. ; 217 : 429 – 47 . doi: 10.1243/09544110360729072 [DOI] [PubMed] [Google Scholar]
- 85. Leighton TG . The acoustic bubble . San Diego, CA, USA: : The British Institute of Radiology. ; 1994. . [Google Scholar]
- 86. Lewin PA , Bjo/rno/ L . Acoustically induced shear stresses in the vicinity of microbubbles in tissue . J Acoust Soc Am 1982. ; 71 : 728 – 34 . doi: 10.1121/1.387549 [DOI] [Google Scholar]
- 87. Chen H , Kreider W , Brayman AA , Bailey MR , Matula TJ . Blood vessel deformations on microsecond time scales by ultrasonic cavitation . Phys Rev Lett 2011. ; 106 : 034301 . doi: 10.1103/PhysRevLett.106.034301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Caskey CF , Qin S , Dayton PA , Ferrara KW . Microbubble tunneling in gel phantoms . J Acoust Soc Am 2009. ; 125 : EL183 – EL189 . doi: 10.1121/1.3097679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Helfield B , Chen X , Watkins SC , Villanueva FS . Biophysical insight into mechanisms of sonoporation . Proc Natl Acad Sci U S A 2016. ; 113 : 9983 – 8 . doi: 10.1073/pnas.1606915113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sheikov N , McDannold N , Vykhodtseva N , Jolesz F , Hynynen K . Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles . Ultrasound Med Biol 2004. ; 30 : 979 – 89 . doi: 10.1016/j.ultrasmedbio.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 91. Chomas JE , Dayton P , Allen J , Morgan K , Ferrara KW . Mechanisms of contrast agent destruction . IEEE Trans Ultrason Ferroelectr Freq Control 2001. ; 48 : 232 – 48 . doi: 10.1109/58.896136 [DOI] [PubMed] [Google Scholar]
- 92. Borden MA , Kruse DE , Caskey CF , Zhao S , Dayton PA , Ferrara KW . Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction . IEEE Trans Ultrason Ferroelectr Freq Control 2005. ; 52 : 1992 – 2002 . doi: 10.1109/TUFFC.2005.1561668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vykhodtseva N , McDannold N , Hynynen K . Induction of apoptosis in vivo in the rabbit brain with focused ultrasound and Optison® . Ultrasound Med Biol 2006. ; 32 : 1923 – 9 . doi: 10.1016/j.ultrasmedbio.2006.06.026 [DOI] [PubMed] [Google Scholar]
- 94. Lele PP . Cavitation and its effects on organized mammalian tissues . : Fry F. J , Ultrasound: its applications in medicine and biology . Amsterdam, The Netherlands: : The British Institute of Radiology. ; 1978. . . 737 – 41 . [Google Scholar]
- 95. Neppiras EA . Subharmonic and other low‐frequency emission from bubbles in sound‐irradiated liquids . J Acoust Soc Am 1969. ; 46 ( 3B ): 587 – 601 . doi: 10.1121/1.1911735 [DOI] [Google Scholar]
- 96. Lauterborn W . Numerical investigation of nonlinear oscillations of gas bubbles in liquids . J Acoust Soc Am 1976. ; 59 : 283 – 93 . doi: 10.1121/1.380884 [DOI] [Google Scholar]
- 97. Prosperetti A . A general derivation of the subharmonic threshold for non-linear bubble oscillations . J Acoust Soc Am 2013. ; 133 : 3719 – 26 . doi: 10.1121/1.4802742 [DOI] [PubMed] [Google Scholar]
- 98. Sijl J , Dollet B , Overvelde M , Garbin V , Rozendal T , de Jong N , et al. . Subharmonic behavior of phospholipid-coated ultrasound contrast agent microbubbles . J Acoust Soc Am 2010. ; 128 : 3239 – 52 . doi: 10.1121/1.3493443 [DOI] [PubMed] [Google Scholar]
- 99. Faez T , Emmer M , Docter M , Sijl J , Versluis M , de Jong N . Characterizing the subharmonic response of phospholipid-coated microbubbles for carotid imaging . Ultrasound Med Biol 2011. ; 37 : 958 – 70 . doi: 10.1016/j.ultrasmedbio.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 100. Sijl J , Vos HJ , Rozendal T , de Jong N , Lohse D , Versluis M . Combined optical and acoustical detection of single microbubble dynamics . J Acoust Soc Am 2011. ; 130 : 3271 – 81 . doi: 10.1121/1.3626155 [DOI] [PubMed] [Google Scholar]
- 101. Gateau J , Aubry JF , Pernot M , Fink M , Tanter M . Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound . IEEE Trans Ultrason Ferroelectr Freq Control 2011. ; 58 : 517 – 32 . doi: 10.1109/TUFFC.2011.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Crake C , Brinker ST , Coviello CM , Livingstone MS , McDannold NJ . A dual-mode hemispherical sparse array for 3D passive acoustic mapping and skull localization within a clinical MRI guided focused ultrasound device . Phys Med Biol 2018. ; 63 : 065008 . doi: 10.1088/1361-6560/aab0aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Harris GR . Progress in medical ultrasound exposimetry . IEEE Trans Ultrason Ferroelectr Freq Control 2005. ; 52 : 717 – 36 . doi: 10.1109/TUFFC.2005.1503960 [DOI] [PubMed] [Google Scholar]
- 104. Jeanmonod D , Werner B , Morel A , Michels L , Zadicario E , Schiff G , et al. . Transcranial magnetic resonance imaging–guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain . Neurosurg Focus 2012. ; 41 : E1 . doi: 10.3171/2011.10.FOCUS11248 [DOI] [PubMed] [Google Scholar]
- 105. Sun T , Samiotaki G , Wang S , Acosta C , Chen CC , Konofagou EE . Acoustic cavitation-based monitoring of the reversibility and permeability of ultrasound-induced blood-brain barrier opening . Phys Med Biol 2015. ; 60 : 9079 – 94 . doi: 10.1088/0031-9155/60/23/9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Aryal M , Fischer K , Gentile C , Gitto S , Zhang YZ , McDannold N . Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles . PLoS One 2017. ; 12 : e0166061 . doi: 10.1371/journal.pone.0166061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tsai C-H , Chen K-T , Lin Y-X , Lin Y-C , Chen W-S , Inserra C . Acoustic emission-feedback planar ultrasound system for localized blood–brain barrier opening monitoring . J Med Biol Eng 2018. ; 24 . doi: 10.1007/s40846-018-0406-x [DOI] [Google Scholar]
- 108. Huang Y , Alkins R , Chapman M , Perry J , Sahgal A , Trudeau M . Initial experience in a pilot study of blood-brain barrier opening for chemo-drug delivery to brain tumors by MR-guided focused ultrasound . : ISMRM 24th Annual Meeting 2016 . Singapore: ; 2016. . http://archive.ismrm.org/2016/0450.html . [Google Scholar]
- 109. Huang Y , Lipsman N , Meng Y , Bethune A , Lam B , Masellis M . Feasibility of blood-brain barrier opening in patients with Alzheimer’s disease by MR-guided focused ultrasound . : ISMRM 26th Annual Meeting 2018 . France: ; 2018. . https://www.ismrm.org/18/program_files/O82.htm . [Google Scholar]
- 110. Maimbourg G , Houdouin A , Santin M , Lehericy S , Tanter M , Aubry JF . Inside/outside the brain binary cavitation localization based on the lowpass filter effect of the skull on the harmonic content: a proof of concept study . Phys Med Biol 2018. ; 63 : 135012 . doi: 10.1088/1361-6560/aaca21 [DOI] [PubMed] [Google Scholar]
- 111. Clement GT , White PJ , Hynynen K . Enhanced ultrasound transmission through the human skull using shear mode conversion . J Acoust Soc Am 2004. ; 115 : 1356 – 64 . doi: 10.1121/1.1645610 [DOI] [PubMed] [Google Scholar]
- 112. Top CB , White PJ , McDannold NJ . Nonthermal ablation of deep brain targets: A simulation study on a large animal model . Med Phys 2016. ; 43 : 870 – 82 . doi: 10.1118/1.4939809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fry FJ , Kossoff G , Eggleton RC , Dunn F . Threshold ultrasonic dosages for structural changes in the mammalian brain . J Acoust Soc Am 1970. ; 48 ( 6B ): 1413 – 7 . doi: 10.1121/1.1912301 [DOI] [PubMed] [Google Scholar]
- 114. McDannold NJ , Vykhodtseva NI , Hynynen K . Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits . Radiology 2006. ; 241 : 95 – 106 . doi: 10.1148/radiol.2411051170 [DOI] [PubMed] [Google Scholar]
- 115. Arvanitis CD , Vykhodtseva N , Jolesz F , Livingstone M , McDannold N . Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model . J Neurosurg 2016. ; 14 : 1450 – 9 . doi: 10.3171/2015.4.JNS142862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Coleman AJ , Whitlock M , Leighton T , Saunders JE . The spatial distribution of cavitation induced acoustic emission, sonoluminescence and cell lysis in the field of a shock wave lithotripter . Phys Med Biol 1993. ; 38 : 1545 – 60 . doi: 10.1088/0031-9155/38/11/001 [DOI] [PubMed] [Google Scholar]
- 117. Burns PN . Harmonic imaging with ultrasound contrast agents . Clin Radiol 1996. ; 51 : 50 – 5 . [PubMed] [Google Scholar]
- 118. Simpson DH , Burns PN , Chin CT . Pulse inversion Doppler: a new method for detecting nonlinear echoes from microbubble contrast agents . IEEE Trans Ultrason Ferroelectr Freq Control 1999. ; 46 : 372 – 82 . doi: 10.1109/58.753026 [DOI] [PubMed] [Google Scholar]
- 119. Du J , Liu D , Ebbini ES . Nonlinear Imaging of Microbubble Contrast Agent Using the Volterra Filter: In Vivo Results . IEEE Trans Ultrason Ferroelectr Freq Control 2006. ; 63 : 2069 – 81 . doi: 10.1109/TUFFC.2016.2614430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lucht B , Hubbell A , Hynynen K . Contrast-enhanced transcranial two-dimensional ultrasound imaging using shear-mode conversion at low frequency . Ultrasound Med Biol 2013. ; 39 : 332 – 44 . doi: 10.1016/j.ultrasmedbio.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 121. Korpel A . Acoustic imaging and holography . IEEE Spectr 1968. ; 5 : 45 – 52 . doi: 10.1109/MSPEC.1968.5215380 [DOI] [Google Scholar]
- 122. Sato T , Nakamura Y , Sasaki K , Uemura K . Three dimensional passive acoustical imaging system using hemispherical array detectors . : Metherell AF , Acoustical imaging . Boston, MA: : The British Institute of Radiology. ; 1980. . . 187 – 200 . [Google Scholar]
- 123. Buckingham MJ , Berknout BV , Glegg SAL . Imaging the ocean with ambient noise . Nature 1992. ; 356 : 327 – 9 . doi: 10.1038/356327a0 [DOI] [Google Scholar]
- 124. Kruger RA , Liu P , Fang YR , Appledorn CR . Photoacoustic ultrasound (PAUS)--reconstruction tomography . Med Phys 1995. ; 22 : 1605 – 9 . doi: 10.1118/1.597429 [DOI] [PubMed] [Google Scholar]
- 125. Norton SJ , Won IJ . Time exposure acoustics . IEEE Transactions on Geoscience and Remote Sensing 2000. ; 38 : 1337 – 43 . doi: 10.1109/36.843027 [DOI] [Google Scholar]
- 126. Farny CH , Holt RG , Roy RA . Temporal and spatial detection of HIFU-induced inertial and hot-vapor cavitation with a diagnostic ultrasound system . Ultrasound Med Biol 2009. ; 35 : 603 – 15 . doi: 10.1016/j.ultrasmedbio.2008.09.025 [DOI] [PubMed] [Google Scholar]
- 127. Salgaonkar VA , Datta S , Holland CK , Mast TD . Passive cavitation imaging with ultrasound arrays . J Acoust Soc Am 2009. ; 126 : 3071 – 83 . doi: 10.1121/1.3238260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gyöngy M , Coussios CC . Passive spatial mapping of inertial cavitation during HIFU exposure . IEEE Trans Biomed Eng 2010. ; 57 : 48 – 56 . doi: 10.1109/TBME.2009.2026907 [DOI] [PubMed] [Google Scholar]
- 129. Haworth KJ , Mast TD , Radhakrishnan K , Burgess MT , Kopechek JA , Huang S-L , et al. . Passive imaging with pulsed ultrasound insonations . J Acoust Soc Am 2012. ; 132 : 544 – 53 . doi: 10.1121/1.4728230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Choi JJ , Carlisle RC , Coviello C , Seymour L , Coussios C-C . Non-invasive and real-time passive acoustic mapping of ultrasound-mediated drug delivery . Phys Med Biol 2014. ; 59 : 4861 – 77 . doi: 10.1088/0031-9155/59/17/4861 [DOI] [PubMed] [Google Scholar]
- 131. Lu S , Shi A , Jing B , Du X , Wan M . Real-time monitoring of controllable cavitation erosion in a vessel phantom with passive acoustic mapping . Ultrason Sonochem 2017. ; 39 : 291 – 300 . doi: 10.1016/j.ultsonch.2017.03.060 [DOI] [PubMed] [Google Scholar]
- 132. Acconcia CN , Jones RM , Goertz DE , O'Reilly MA , Hynynen K . Megahertz rate, volumetric imaging of bubble clouds in sonothrombolysis using a sparse hemispherical receiver array . Phys Med Biol 2017. ; 62 : L31 – L40 . doi: 10.1088/1361-6560/aa84d7 [DOI] [PubMed] [Google Scholar]
- 133. Jones RM , Hynynen K . Ultrafast three-dimensional imaging of contrast agent microbubble dynamics in vivo during pulsed ultrasound exposures in the brain . : International Symposium for Therapeutic Ultrasound . USA: ; 2018. . [Google Scholar]
- 134. O'Reilly MA , Hynynen K . A super-resolution ultrasound method for brain vascular mapping . Med Phys 2013. ; 40 : 110701 . doi: 10.1118/1.4823762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Errico C , Pierre J , Pezet S , Desailly Y , Lenkei Z , Couture O , et al. . Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging . Nature 2015. ; 527 : 499 – 502 . doi: 10.1038/nature16066 [DOI] [PubMed] [Google Scholar]
- 136. Postert T , Federlein J , Przuntek H , Büttner T . Insufficient and absent acoustic temporal bone window: Potential and limitations of transcranial contrast-enhanced color-coded sonography and contrast-enhanced power-based sonography . Ultrasound Med Biol 1997. ; 23 : 857 – 62 . doi: 10.1016/S0301-5629(97)00047-1 [DOI] [PubMed] [Google Scholar]
- 137. O’Reilly MA , Jones RM , Hynynen K . Investigating a method for non-invasive ultrasound aberration correction through the skull bone . Proc SPIE Med Imag 2014. ; 904013 . [Google Scholar]
- 138. Jones R , Deng L , Leung K , McMahon D , O’Reilly M , Hynynen K . Repeated hippocampal blood-brain barrier opening controlled via three-dimensional transcranial acoustic imaging: safety study in a porcine model . : IEEE International Ultrasonics Symposium . USA: ; 2017. . doi: https://doi.org/DOI: 10.1109/ULTSYM.2017.8091965 [Google Scholar]
- 139. Liu H-L , Tsai C-H , Jan C-K , Chang H-Y , Huang S-M , Li M-L , et al. . Design and implementation of a transmit/receive ultrasound phased array for brain applications . IEEE Trans Ultrason Ferroelectr Freq Control 2018. ; 65 : 1756 – 67 . doi: 10.1109/TUFFC.2018.2855181 [DOI] [PubMed] [Google Scholar]
- 140. Molina CA , Ribo M , Rubiera M , Montaner J , Santamarina E , Delgado-Mederos R , et al. . Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator . Stroke 2006. ; 37 : 425 – 9 . doi: 10.1161/01.STR.0000199064.94588.39 [DOI] [PubMed] [Google Scholar]
- 141. Schuster TG , Wei JT , Hendlin K , Jahnke R , Roberts WW . Histotripsy treatment of benign prostatic enlargement using the Vortx Rx system: initial human safety and efficacy outcomes . Urology 2018. ; 114 : 184 – 7 . doi: 10.1016/j.urology.2017.12.033 [DOI] [PubMed] [Google Scholar]