Abstract
In recent years, there have been major advances in the imaging of myeloma with whole body MRI incorporating diffusion-weighted imaging, emerging as the most sensitive modality. Imaging is now a key component in the work-up of patients with a suspected diagnosis of myeloma. The International Myeloma Working Group now specifies that more than one focal lesion on MRI or lytic lesion on whole body low-dose CT or fludeoxyglucose (FDG) PET/CT fulfil the criteria for bone damage requiring therapy. The recent National Institute for Health and Care Excellence myeloma guidelines recommend imaging in all patients with suspected myeloma. In addition, there is emerging data supporting the use of functional imaging techniques (WB-DW MRI and FDG PET/CT) to predict outcome and evaluate response to therapy. This review summarises the imaging modalities used in myeloma, the latest guidelines relevant to imaging and future directions.
Background
Myeloma is a haematological malignancy arising from plasma cells characterised by clonal proliferation of plasma cells and excessive monoclonal protein in the blood and/ or urine. There is a wide spectrum of disease with both biological and clinical heterogeneity. It evolves from a pre-malignant asymptomatic precursor condition, monoclonal gammopathy of uncertain significance (MGUS), to smouldering/ asymptomatic myeloma, to symptomatic disease. It is the most common primary malignancy of the skeleton with an incidence in the UK of approximately 10/10,0000.1
Myeloma typically presents as multiple lytic lesions and/ or diffuse bone marrow disease and occasionally with extramedullary disease. The diagnosis of active myeloma as defined by the International Myeloma Working Group (IMWG) criteria, is based on histological confirmation of plasma cell infiltration of the bone marrow or extra medullary plasmacytoma (Table 1) and evidence of end organ damage as defined by CRAB-criteria (hyperCalcaemia, Renal insufficiency, Anaemia, Bone lesions).2 This criteria incorporates imaging evidence of more than one unequivocal focal bone lesion as a defining myeloma-related event, indicating need for therapy. Untreated myeloma can cause significant morbidity from immunosuppression and complications of end organ damage including renal impairment and lytic bone destruction with associated bone pain and pathological fractures.
Table 1.
| The IMWG updated criteria for the diagnosis of multiple myeloma 2: |
| Clonal bone marrow plasma cells > 10% or biopsy-proven bony or extramedullary plasmacytoma AND- |
| Any one or more of the following myeloma defining events: Evidence of end organ damage
|
Any one or more of the following biomarkers of malignancy:
CT—one or more osteolytic lesions (≥5 mm). a 18F FDG PET/CT—one or more osteolytic lesions (≥5 mm). Increased FDG uptake alone is not sufficient; evidence of osteolytic bone destruction is needed on the CT component of the study. a MRI—> 1 focal lesion of a diameter ≥5 mm. b Diffuse marrow abnormality does not qualify. Note: Bone densitometry studies are not sufficient to determine presence of multiple myeloma. The presence of osteoporosis or vertebral compression fractures in the absence of lytic lesions is no longer sufficient evidence of bone disease. |
FDG, fludeoxyglucose; IMWG, International Myeloma Working Group.
Care should be taken to avoid over interpretation of equivocal or tiny lucencies seen only on CT or PET-CT; For equivocal lesions a repeat study in 3–6 months should be done before a diagnosis of multiple myeloma is made. Such patients might be followed up closely at 1–3 month intervals before systemic therapy is started.
In cases of equivocal small lesions, a second MRI should be performed after 3 to 6 months, and if there is progression on MRI, the patient should be treated as having symptomatic myeloma.
Imaging now forms an important central role in the diagnosis and work-up of myeloma patients as the detection of lytic/ focal bone disease is part of the criteria for starting therapy and carries prognostic significance. In addition, early detection of the complications of myeloma such as osteoporosis and compression fractures is of value to help improve morbidity in myeloma patients. Furthermore, some patients have so called “non- or oligo-secretory” myeloma, whereby monitoring disease with blood or urine markers is limited. Imaging may be the only way of assessing extent of disease, response to therapy or disease relapse in this group.
In the past decade, there have been rapid advances in the use of modern therapies for myeloma which are expensive and some are associated with significant toxic effects. There is a clinical need for accurate diagnostic imaging tools to help select patients who will benefit from treatment and assess response. The recently revised IMWG consensus criteria for response assessment in myeloma also include imaging.3
For years, skeletal survey has formed the cornerstone of imaging in myeloma. However, over the past decade whole body low dose CT (WBLDCT) has emerged as an alternative to skeletal survey and functional imaging with diffusion weighted MRI and FDG PET/CT is increasingly being used. In particular, diffusion weighted MRI is the most sensitive imaging technique for detecting myeloma and therefore considered the first-line imaging modality in myeloma. However, in the UK, despite guidance from NICE which positioned whole body MRI as the first-line diagnostic imaging test, it is currently performed at only a few centres due to limited MRI capacity.
This review covers the imaging modalities available in myeloma, the current myeloma imaging guidance and the use of these imaging modalities in suspected myeloma, staging myeloma, response assessment and suspected relapsed disease.
The current imaging guidelines from IMWG, NICE and the British Society of Haematology are summarised inTable 2.
Table 2.
Imaging guidelines in myeloma
| NICE 2016 5 | BSH 2017 6 | IMWG 2014 2015 2,7 | |
| Suspected myeloma | First-line WB-MRI second-line WBLDCT third line SS Note: bone scintigraphy is not recommended |
First-line WB-MRI second-line MRI spine & pelvis if WB-MRI cannot be performed third line WBLDCT |
MRI has high sensitivity for the early detection of marrow infiltration compared with other radiographic methods |
| Smouldering/ asymptomatic myeloma a | Spine MRI or WB-MRI or WBLDCT or or FDG PET/CT |
First-line WB-MRI or FDG PET/CT second-line WBLDCT |
WB-MRI or MRI spine or WBLDCT or FDG PET/CT b (if they have more than one focal lesion of a diameter ≥5 mm accompanied by lytic destruction on the CT component, should be considered to have symptomatic disease that requires therapy. Diffuse disease does not qualify). |
| Solitary plasmacytoma (to exclude other sites of disease) | WB-MRI or FDG PET/CT |
WB-MRI or FDG PET/CT |
WDLBCT or MRI |
| Newly diagnosed myeloma | WB-MRI or WBLDCT or FDG PET/CT |
First-line WB-MRI second-line MRI spine & pelvis if WB-MRI cannot be performed third line WBLDCT |
|
| Treatment response/ relapse | WB-MRI or MRI spine or FDG PET/CT (Note WBLDCT not indicated) |
First-line WB-MRI second-line FDG PET/CT c |
WB-MRI or FDG PET/CT |
| Suspected cord compression | MRI spine CT spine for stability/ if considering vertebral kyphoplasty/ surgery |
First-line MRI spine second-line CT spine |
FDG, fludeoxyglucose; WBLDCT, whole body low dose CT; WB-MRI, whole body MRI.
10–60% plasma cells on trephine biopsy or bone marrow aspirate or M protein >30 g l–1 (BSH).
Dependent on availability and resources.
DW-WB MRI is recommended in response assessment, but in patients with non/oligosecretory or extramedullary disease DW-WB MRI or FDG PET/CT can be performed (BSH). Changes in FDG avidity can provide an earlier evaluation of response to therapy compared to MRI & predict outcome especially in patients eligible for autologous stem cell transplant.
Role of imaging in myeloma
Diagnosis
Skeletal survey
Skeletal survey (SS) has for decades been the imaging modality used to detect lytic bone disease as it is widely available, simple to perform and report, low cost and relatively low radiation dose. Table 3 Standard views obtained include:
Table 3.
Typical protocols, advantages and disadvantages of the various imaging modalities
| Imaging modality | Protocol | Advantages | Disadvantages |
| Skeletal survey | Standard plain radiograph series |
|
|
| WB- Low dose CT | Protocols should be optimised locally but typical parameters are: 120 kV <100 mAs dose modulation and iterative reconstruction Vertex to knees |
|
|
| WB- MRI | Typical protocol: Sagittal T 1 and T 2 spine. Axial DIXONS Axial Diffusion (b 50 & b900) Post processing-ADC map, knitting of axial sequences (automated by some vendors), inverted b 900 MIP Total body – vertex to toes |
|
|
| FDG PET/CT | Standard preparation. Total body vertex to toes arms down CT parameters - bone reconstructions as well as soft tissue |
|
|
ADC, apparent diffusion coefficient;DWI, diffussion-weighted imaging; FDG, fludeoxyglucose.
Posteroanterior (PA) view of the chest
Anteroposterior (AP) and lateral views of the spine, humeri and femora
Lateral views of the skull
AP view of the pelvis
However, it has limited sensitivity as the detection of lytic bone disease is only demonstrated when 30–50% of trabecular bone is lost.8 Detection of lytic lesions, particularly in the axial skeleton, can be challenging due to overlying structures and false positives may occur in the pelvis due to overlying bowel loops mimicking disease.9 The uncertainties in reporting skeletal survey have been demonstrated with inferior intraclass correlation coefficients compared with whole body MRI. Whole body MRI also demonstrates more lesions than SS in all regions apart from the skull which is also a challenging site for WBLDCT and FDG PET/CT.10,11 In some large patients, the humeri may not be fully included in the field of view on WB-MRI or WB-CT so additional regional plain radiographs may be required particularly if local symptoms are reported.
Whole body low dose CT
Advances in CT technology allows the use of low dose CT protocols, first introduced by Horger and colleagues, which lower the dose necessary to image the skeletal system whilst preserving sensitivity and image detail.12 In their study, the effective radiation dose of MDCT calculated at a tube current time product of 40 mAs was only 1.7-fold higher than the mean radiation dose of conventional X-ray (4.1 vs 2.4 mSv).
WBLDCT has been shown to have superior sensitivity than SS.13–15 In a study comparing SS to WBLDCT the detection rates and diagnostic confidence were significantly higher with CT and CT led to a change in management in 18.2% of cases.13 In another study, WBLDCT led to change in staging in 61% (18 out of 38 cases) compared to skeletal survey.14 In a further study comparing SS to WBLDCT in 212 smouldering myeloma/ myeloma patients, 25.5% of cases with negative SS had lytic lesions on CT.15 In the cohort with smouldering myeloma the WBLDCT would have changed management in 20% cases. However, it was noted that SS can be complimentary in detecting humeral lesions in cases where the arms were at the edge of the field of view.
Low dose CT protocols are performed without contrast and the body coverage suggested in the literature varies but should include skull vertex to below knees.6 Low dose CT algorithms should be optimised locally but diagnostic images can be obtained with parameters such as 120 kV<100 mAs, dose modulation and iterative reconstruction.6,9 Radiation doses for WBLDCT using 1000kVp, an effective tube current-time product of 100 mAs and automatic dose modulation give an estimated effective dose of WBLDCT (4.8 mSv) which is reasonable compared to SS (1.7 mSv) given the gain in diagnostic accuracy.13
However, there are limitations of WBLDCT, including the ability to detect diffuse and focal marrow based/ early lytic bone disease. Extraosseous disease may not be readily demonstrated but careful review of images on soft tissue windows or brain windows may result in detection (Figure 1). Furthermore, during follow up it cannot discriminate between active and treated disease, such that it is not recommended for response assessment.5
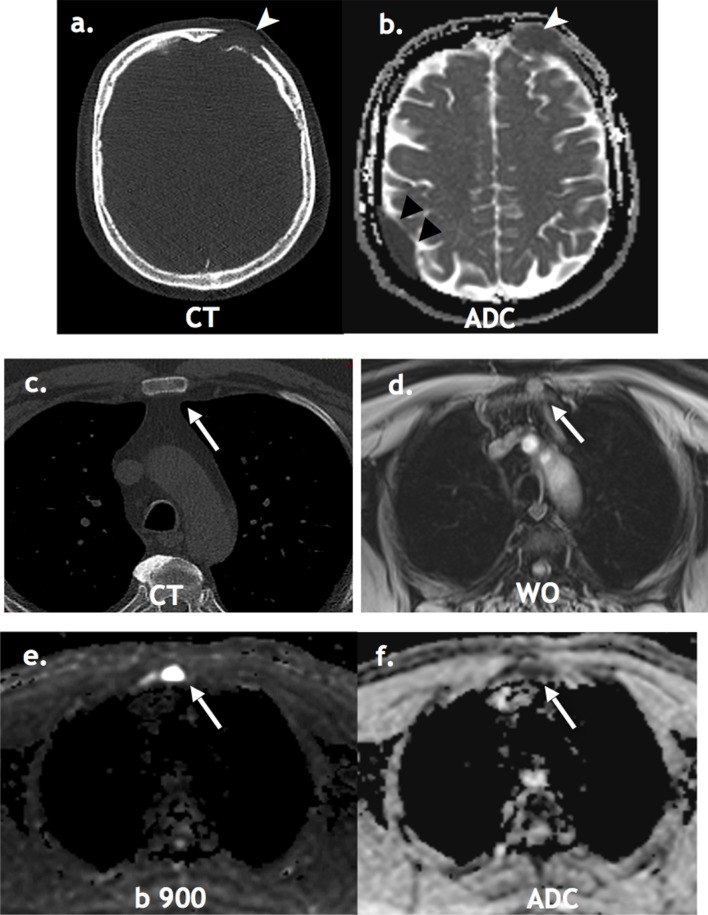
Figure 1.
Limitation of WBLDCT: A 58-year-old male staging WBLDCT (a) detected lytic left frontal lesion only (white arrowheads). WB-MRI (b, d, e, f) detected additional active extra osseous dural disease (black arrowheads) and osseous disease in sternum (white arrows), high signal in the water only DIXON sequence (d), high signal on the b900 (e) with restricted diffusion on the ADC map (f). This was occult on CT (c). ADC, apparent diffusion coefficient; WBLDCT, whole body low dose CT.
MRI
MRI has high sensitivity for the early detection of marrow infiltration by myeloma cells compared to SS and WBLDCT which only detect the secondary effects of myeloma once osteolysis has occurred. MRI provides distinct information about the bone marrow and patterns of bone marrow infiltration which have been shown to correlate with bone marrow biopsy.16 Different infiltration patterns (number of focal lesions and type of diffuse bone marrow infiltration) on WB-MRI (T 1 and STIR) have been reported to differ between the different stages of plasma cell disease (MGUS, smouldering myeloma and multiple myeloma) and correlate with M-protein and plasma cell percentage in bone marrow.16 In addition, MRI spine is the first-line investigation for suspected spinal cord compression/ neural compromise.
MRI detects bone involvement in patients with myeloma much earlier than the myeloma-related bone destruction, with no radiation exposure. An early large study of 611 patients showed MRI detected more focal lesions than SS and resolution of focal lesions correlated with survival.17 In a study comparing WBLDCT and WB-MRI (T 1 and STIR), MRI detected more lesions and 27% cases were under staged by WBLDCT.18 A study comparing WB-MRI to spine MRI showed up to 50% of lesions would be missed by imaging the spine alone.19 However, if patients cannot tolerate WB-MRI or WB-MRI is unavailable, MRI spine and pelvis could be considered as an alternative.6
Diffusion-weighted imaging (DWI), a functional MRI technique which assesses movement of water molecules within tissues providing information about the microarchitecture and tumour cellularity without the use of contrast agents, has emerged as a particularly sensitive technique to detect focal myeloma lesions and background marrow infiltration (Figure 2). DWI has been shown to be superior to STIR sequences for detecting focal disease.20 Emerging data suggest further improvement in sensitivity with the addition of DWI.21,22 Quantitative apparentdiffusion coefficient (ADC) measurement may act as an additional tool for the diagnosis of diffuse infiltration on MRI.23 In addition, DWI permits differentiation between active and treated disease in cases of disease relapse (Figure 3).
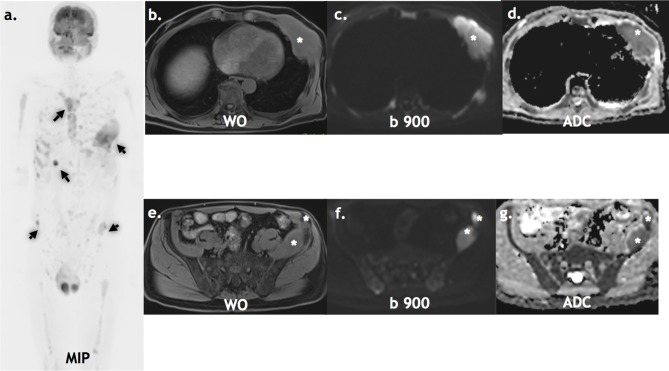
Figure 2.
Staging WB-MRI: A 65-year-old with non-secretory myeloma staging WB-MRI. The inverse grey scale b900 MIP (a), axial water only DIXON (b, c), axial b900 (d, e) and axial ADC (f, g) show multiple active lesions (arrows & asterisks). ADC, apparent diffusion coefficient; MIP, maximum intensity projection.
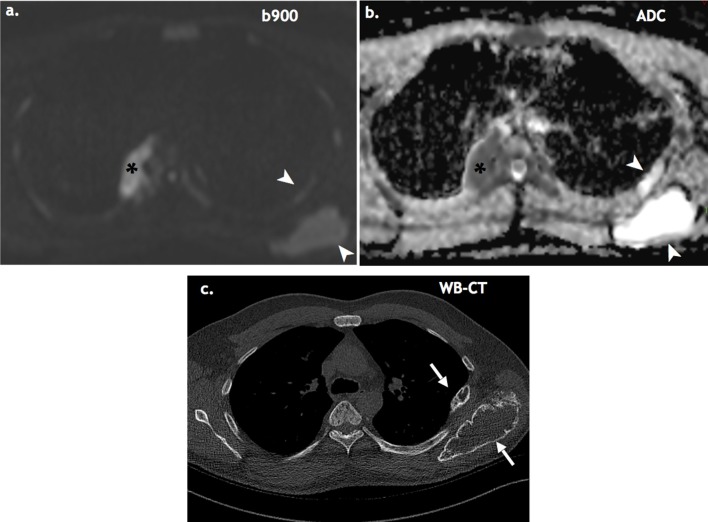
Figure 3.
Active vs treated WB-MRI: A 49-year-old with suspected relapse post autologous SCT. Restaging WB-MRI (a, b) shows mixed active (asterisks) and treated disease (white arrowheads). WBLDCT (c) cannot distinguish treated vs active disease (white arrows). WBLDCT, whole body low dose CT; WB-MRI, whole body MRI.
Imaging protocols for WB-MRI vary. However, international consensus on WB-MRI for metastatic prostate cancer are already published (MET-RADS) and a similar initiative in myeloma is underway.24,25 Most protocols include sagittal spine sequences (T 1, T 2 ± STIR) which contribute to disease detection and provide essential assessment for complications such as compression fractures and/ or neural/ cord compromise Table 3. Axial anatomical detail is provided by DIXON sequences which also facilitate disease detection and quantification in the form of fat fraction maps. Axial diffusion weighted imaging (typically b50 and b900) is the most sensitive sequence for disease detection and permits formation of ADC maps.26 Inverse grey scale maximum intensity projection (MIP) reconstructions produced from b900 DW images are useful for displaying disease distribution and as a review for disease detection but should never be interpreted in isolation from the source images. WB-MRI takes approximately 45 min to perform in our institution. A clear benefit for the use of intravenous contrast in the clinical setting has not been demonstrated. Furthermore, given that myeloma patients are often in an older age group with renal impairment it is not routinely administered.
Surveys have shown that although the scan times are relatively long, most patients find scans acceptable with the additional advantage of no contrast or radiation dose.21
However, there are some disadvantages including cost, limited MRI capacity, time and expertise required to perform and report, and interpretation challenges associated with lack of cortical bone detail.
Clinicians should be aware that not all patients may tolerate MRI, particularly those in pain or with claustrophobia, but with the help of dedicated MRI radiographers and effective analgesic planning, light sedation or tailored sequences, most patients can be scanned successfully.
FDG PET/CT
FDG PET/CT permits whole body assessment of glucose metabolism which can be used to assess the extent of both skeletal and extra medullary disease and response to therapy. Standard preparation for FDG PET/CT is followed and typically the field of view for the PET will include total body from vertex to toes.27 The CT component of the study is usually a low dose CT of similar parameters to the WBLDCT protocols. A major strength of FDG PET/CT is the ability to distinguish between metabolically active and inactive sites of disease such that it is recommended by the IMWG as the “gold standard” method for evaluating and monitoring response to therapy27 (Figure 4).
Figure 4.
FDG PET/CT Active vs treated disease: A 61-year-old with suspected relapse post autologous stem cell transplant. FDG PET/CT MIP (a) shows multiple lesions (black arrows). On the CT (b, c) and fused (d, e), there is a mixture of active (white arrowheads) and treated disease lytic on CT but not avid (white arrows). FDG, fludeoxyglucose; MIP, maximum intensity projection.
However, limitations include low sensitivity for detection of small lytic lesions due to limited spatial resolution and the unreliable assessment of diffuse marrow involvement on FDG PET/CT.21 Also, a small subset of patients may have non-FDG avid disease. The mechanistic explanation for this feature has been recently been provided by a study of 227 newly diagnosed myeloma patients in which FDG PET/CT was reported to be false negative in 11% cases. In this subset, the gene coding for hexokinase-2, which catalyzes the first step of glycolysis, was expressed significantly less in PET false-negative cases (5.3-fold change, p < 0.001).28
Prognosis
Several studies have shown that data derived from FDG PET/CT is prognostic. The number of FDG avid bone lesions29–31 and the intensity of uptake based on SUVmax 30,32,33 and presence of extramedullary disease31,33 have been shown to predict survival. Metabolic response during/ post therapy has also been shown to be useful indexes to stratify patients into different prognostic groups with different outcomes to therapy.29,30,34–36 Metabolic tumour volume (MTV) at baseline FDG PET/CT has been reported to be prognostic for disease progression and death, independent from other established prognostic factors such as percentage plasma cell infiltration of bone marrow and haemoglobin levels.37
WB-MRI vs FDG PET/CT
A recent systematic review of diagnostic performance of WB-MRI, WBLDCT and FDG PET/CT in myeloma suggests that WB-MRI detects more lesions than FDG PET/CT (sensitivity 68–100% vs 47–100%), but was less specific (specificity 36–83% vs 62–85.7%).38 However, it is noted most studies lacked a reference standard and good prospective studies comparing WB-MRI with DWI and FDG PET/CT are lacking.
An early study comparing WB-MRI (coronal STIR not DW sequences) to FDG PET/CT reported superior sensitivity and specificity of WB-MRI.39 A study comparing WB DW-MRI and FDG PET/CT showed that DW-MRI had significantly superior detection rates for both diffuse and multifocal disease.21 A further prospective study of 56 patients reported WB-MRI is more sensitive than FDG PET/CT in the diagnosis of myeloma before treatment (WB-MRI sensitivity 94% vs FDG PET/CT 75%; whilst both had same specificity of 80%). However, for the detection of residual abnormalities post-treatment FDG PET/CT was more specific (specificity 86% FDG PET/CT vs 43% WB-MRI, both sensitivity of 75%).40
A recent prospective study of FDG PET/CT and WB DW-MRI reported DWI was more sensitive than FDG PET/CT in detecting myeloma lesions in a mixed population of primary and pre-treated MM patients.22 However, when primary untreated cases were considered separately FDG PET and DWI had equal sensitivities. The higher sensitivity of DWI was in pre-treated cases (following ASCT) which may reflect the fact that FDG PET/CT becomes negative earlier in the course of therapy than MRI, in which treated lesions can remain visible.
Overall, evidence shows MRI has superior detection of lesions compared to other imaging modalities. This is reflected in the guidelines where WB-MRI is generally the preferred imaging investigation in a range of clinical settings (Table 2).2,5,6 MRI spine/ pelvis can be performed if WB-MRI with DWI is not available, but up to 10% of lesions in the appendicular skeleton may be missed.6 In cord/ neural compromise, MRI spine is the modality of choice.5,6 Alternatively, imaging with WBLDCT and FDG PET/CT may be considered (Table 2), with evidence suggesting that FDG PET/CT may have a more valued role in response assessment, particularly in those suitable for autologous stem cell transplant.3,27 However, the optimum modality for response assessment requires further study as good comparative studies are lacking and ideally in individual patients it is desirable to stick to the same modality throughout their treatment pathway.
Smouldering myeloma
In smouldering myeloma, an intervening phase between MGUS and myeloma with a broad spectrum of behaviour, studies have shown MRI to have prognostic significance. The presence of focal lesions on MRI and the number of lesions have been shown to be the strongest adverse predictors for progression of asymptomatic myeloma into symptomatic myeloma in multivariate analysis.41 A diffuse infiltration pattern in MRI, a monoclonal protein of 40 g l−1 or greater, and a plasma cell infiltration in bone marrow of 20% or greater were other adverse prognostic factors for progression-free survival in univariate analysis. A more recent study of serial WB-MRI in a smouldering myeloma series showed patients who progressed on MRI had significant risk of clinical progression, whilst patients with stable disease (even with focal lesions on initial MRI) had no higher risk of progression.42 Furthermore a keynote study showed that early intervention for patients with high-risk smouldering myeloma delays progression to active disease and increases overall survival.43 Positive results from ongoing trials would support the use of early treatment for patients with high-risk disease in the near future.44
FDG PET/CT has been shown to be prognostic in smouldering myeloma with a probability of progression to MM within 2 years of 75% in patients with a positive PET-CT observed without therapy compared with 30% in patients with a negative PET-CT; median time to progression was 21 vs 60 months, respectively, p = 0.0008.45
Based on evidence to date, the International Myeloma Working Group (IMWG) recommended that in smouldering or asymptomatic myeloma, all patients should undergo whole-body MRI (WB-MRI; or spine and pelvic MRI if WB-MRI is not available) or FDG PET/CT or WBLDT, and if they have >one focal lesion of a diameter >5 mm (for FDG PET/CT with associated lytic destruction on CT), they should be considered to have symptomatic disease that requires therapy. In cases of equivocal small lesions, a second MRI should be performed after 3 to 6 months, and if there is progression on MRI, the patient should be treated as having symptomatic myeloma7 (Table 1).
Although diffuse marrow involvement on MRI is prognostic it has not been included in the criteria to start treatment since assessment of diffuse disease can be challenging and subjective. False positives may occur for example with G-CSF therapy. WB DW-MRI can be used to judge if posterior iliac crest trephine, which facilitates the diagnosis of diffuse infiltration, is likely to be representative.26 Imaging with WBLDCT and FDG PET/CT may also be considered with the presence of a lytic lesion on WBLDCT or on the CT component of the PET/CT required as an indication to treat.2,6
Solitary plasmacytoma
Solitary plasmacytoma is a localised proliferation of monoclonal plasma cells in bone or soft tissue with no features of myeloma but with risk of progression to multiple myeloma. Imaging has an important role to confirm solitary nature as detection of other sites of disease would change management from localised radiotherapy to systemic therapy.
Initial MRI studies with MRI spine and pelvis demonstrated detection of additional sites compared to SS in up to a third of cases.46 The use of WB DW-MRI has not yet been reported in solitary plasmacytoma but is expected to be useful due to the supreme sensitivity of this technique.
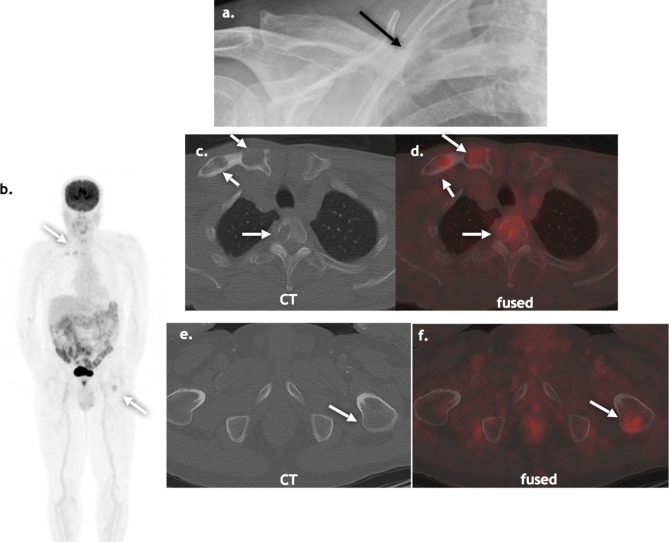
FDG PET/CT may show additional lesions and change therapy in 33–35% of cases47–49 (Figure 5). A recent study assessed the impact of FDG PET/CT and MRI (spine and pelvis) on transformation of conventionally defined solitary plasmacytoma to multiple myeloma in 43 subjects.50 Two or more focal lesions where detected in 33% cases with FDG PET/CT and 20% cases with MRI (albeit spine ad pelvis only). The presence of at least two hypermetabolic lesions on PET/CT and an abnormal serum-free light chain value were predictive of progression to multiple myeloma and may potentially change management from surgery and/ or radiotherapy to systemic myeloma therapy.50
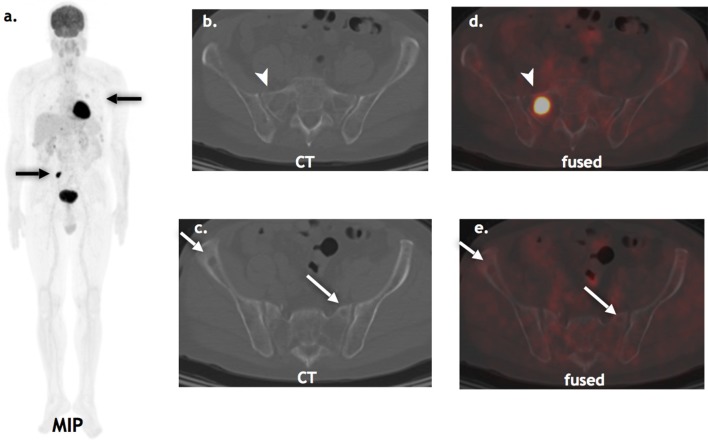
Figure 5.
Suspected solitary plasmacytoma: A 75-year-old presenting with pathological fracture of the right mid-clavicle, presumed to be a solitary plasmacytoma on standard skeletal survey (a, black arrow). FDG PET/CT (b, c, d, e, f) detected additional lesions in the thoracic spine lytic on CT and left femur, occult on CT (white arrows). FDG, fludeoxyglucose, PET, positron emission tomography.
Based on this evidence the IMWG, BSH and recent European expert Panel recommend WB DW-MRI first-line if available or FDG PET/CT in presumed solitary plasmacytoma.6,27,51 One caveat of FDG PET/CT worth mentioning is in patients with solitary lytic lesions presenting with cord/ neural compromise in whom steroids have been administered, WB-MRI should be considered first-line as high dose steroids can reduce the sensitivity of FDG PET/CT.
Response assessment
The assessment of treatment response is predominately based on paraprotein and serum free light chain measurement. However, imaging has the potential for a larger role in this setting. The IMWG recently incorporated imaging into response assessment, with current evidence favouring the use of FDG PET/CT,3 although preliminary evidence for DW MRI is very promising.52,53
Whilst some studies suggest MRI is more sensitive at initial assessment, FDG PET/CT has advantages for follow-up post-chemotherapy induction, autologous stem cell transplantation (ASCT) and determination of remission status.54
In terms of ASCT response assessment, FDG PET/CT response correlates well with the clinical response and PET metabolic response precedes normalization in conventional MRI.29,30,32
A prospective study comparing MRI (spine and pelvis) and FDG PET/CT at diagnosis, after three cycles of chemotherapy and before maintenance therapy reported non-significant higher detection rates with MRI (95% MRI v s 91% FDG PET/CT). Normalization of MRI after three cycles of chemotherapy was not predictive of progression-free survival (PFS) or overall survival (OS); whilst normalization of FDG PET/CT after three cycles of chemotherapy was predictive of PFS and OS.55 Another study has reported changes in infiltration patterns on MRI pre- and post-ASCT predicts response.56
However, there is increasing evidence to suggest that DWI is useful for assessing response to therapy.52,53,57,58 During response to therapy, the increased extracellular spaces within a tumour manifest as increase distances of water motion and an increase in ADC.59 Quantitative ADC measurements (ADC min and ADC mean) at baseline and early changes during therapy may predict outcome. Fat fraction maps derived from the DIXON sequences can provide data regarding response as in responding lesions normal fat is restored.52 A recent study reported that early signal fat fraction changes 8 weeks post-chemotherapy was a biomarker for inferior response.60 Responders had a significant increase in signal fat fraction as the normal fat signal returned, whilst non-responders had no significant change.
There are some reports of potentially superior performance of FDG PET/CT over DW-MRI in early response assessment of combination therapy with monoclonal antibody, an immunomodulatory drug and dexamethasone in relapsed refractory MM.61 However, to date a large study comparing FDG PET/CT and WB DW-MRI in the setting of therapy response assessment has not been performed.
FDG PET/CT and WB DW-MRI are both attractive options for monitoring non-secretory/oligo-secretory disease in whom the standard clinical markers are not reliable.(13;25) Approximately, 1% of myeloma cases are non-secretory and up to 5% are oligo-secretory, meaning there is low intact monoclonal product or serum-free light chain relative to the tumour load measured either by bone marrow biopsy or imaging. Additionally, transformation to a non- or oligo-secretory state becomes more likely with disease progression. Currently, serial bone marrow biopsy is used to monitor disease status in this group which is invasive and painful and therefore imaging with DW-MRI or FDG PET/CT in this setting would be advantageous and is less prone to non-representative sampling errors.
Several studies have shown FDG PET/CT to be prognostic in the post induction setting, pre-ASCT and response during therapy. Bartel et al assessed seven variables from three imaging methods (FDG PET/CT, MRI and CT) in 239 untreated myeloma patients and reported presence of avid focal lesions on FDG PET/CT to most highly correlate to six prognostic variables (β−2-microglobulin, C-reactive protein, lactate dehydrogenase, gene expression profiling (GEP) parameters.29 The presence of focal lesions on FDG PET identified 30% patients in whom, despite having low risk disease as defined by GEP analysis, prognosis was inferior. In addition, complete metabolic response of focal lesions prior to transplantation conferred significantly better outcomes such that it was proposed that myeloma survival may be improved by altering treatments in patients in whom FDG suppression cannot be achieved after induction therapy.
A further study showed that the persistence of >3 focal lesions at day seven post induction chemotherapy was a predictor of significantly shorter PFS and OS.36 A large Italian study by Zamagni et al reported that baseline FDG PET/CT in newly diagnosed cases treated with upfront autologous transplantation was prognostic.30 In multivariate analysis, presence of extramedullary disease, SUV max >4.2 at baseline and persistence of FDG uptake after ASCT (SUV max >4.2) were independent variables adversely affecting PFS. Updated results in 282 patients from the same group, reported baseline SUV max >4.2 combined with ISS Stage III and failure to achieve complete response (CR) upon first-line treatment identified a subgroup of patients (10%) with very poor prognosis who might be candidates for alternative therapeutic regimes.35
******Other studies have reported that negative FDG PET/CT scans after ASCT are associated with improved PFS.62,63
In the context of minimal residual disease assessment, there is a clinical need for more sophisticated instruments to measure residual disease as a potential source of relapse.64 Minimal residual disease assessment has traditionally been performed on bone marrow biopsy, which is limited as it may not assess actual tumour burden.
PET/CT scans provided a more accurate definition of CR than conventional criteria. Serial PET/CT scans post-first-line therapy may detect skeletal progression in 12% cases with no additional criteria of progressive disease.35
A study comparing the diagnostic performance of FDG PET/CT and WB-MRI (T 1, T 2 and DCE, not DWI) to determine remission status post-stem cell transplantation, reported MRI was more often false-positive due to persistent non-viable lesions, but this study did not include DWI.54
The revised IMWG includes FDG PET/CT in the new response categories of minimal residual disease negativity, defined as disappearance of foci found at baseline or decrease to less than mediastinal blood pool or surrounding soft tissue.3
There is evidence to support the use of functional imaging in response assessment. Its role in myeloma imaging is rapidly evolving; however, there is currently no standardisation for its use in this setting. The lack of standardised protocols, consensus for image analysis and reporting together with limited MRI capacity are current barriers preventing widespread adoption into routine clinical practice. However, consensus UK WB-MRI recommendations are being developed.25
Future directions
There are moves to standardise protocols for WB-MRI to incorporate DWI and development of reporting criteria. Several different interpretation criteria have been proposed for FDG PET/CT, most recently an Italian group have developed interpretation criteria for FDG PET/CT in multiple myeloma (IMPeTUs) based on a 5-point visual scale.65 Initial assessment has shown high interobserver agreement at baseline, post-induction and end of treatment FDG PET/CT studies and a prospective validation study is underway. Both MRI and PET/CT techniques are likely to benefit from the application of machine learning algorithms performing automated lesion segmentation to measure disease burden and subsequent response. The Machine Learning in Myeloma Response (MALIMAR) study is currently assessing the application of machine learning in WB-MRI in the UK.66
Currently it seems that WB-MRI and FDG PET/CT will have complimentary roles which may alter throughout the patient pathway. PET/MRI scanners offer the strengths of both imaging techniques in one sitting but is currently limited to a few research centres.67
Conclusions
The role of SS in the imaging myeloma is limited and has been largely replaced with more sensitive imaging techniques.
WBLDCT is superior to skeletal survey for detecting focal lytic lesions but as it images the secondary effects of bone infiltration it cannot reliably assess marrow and early disease. In addition, it cannot discriminate between active and treated disease.
Advanced functional imaging techniques with WB DW-MRI and FDG PET/CT have an increasing role in the diagnosis, prognosis and response assessment of myeloma.
WB DW-MRI is the most sensitive imaging technique for detecting focal disease and diffuse marrow infiltration. FDG PET/CT is less sensitive than WB-MRI for diffuse marrow infiltration but is prognostic.
FDG PET/CT is useful for detecting residual disease post-therapy. Functional MRI assessment in this setting is preliminary but promising.
It is likely that WB-DW-MRI and FDG PET/CT shall have an increasing role in response assessment particularly in non- or oligo-secretory disease patients, and to guide management/ alternative therapies in non-responding patients.
Footnotes
Acknowledgment: TB and AR acknowledge support from the NIHR Biomedical Research Centre to Imperial College London and Imperial CRUK centre. CM acknowledges CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC and Department of Health C1060/A10334, C1060/A16464 and NHS funding to the NIHR Biomedical Research Centre, Clinical Research Facility in Imaging and the Cancer Research Network. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Contributor Information
Tara Barwick, Email: tara.barwick@nhs.net.
Laure Bretsztajn, Email: laure.bretsztajn@nhs.net.
Kathryn Wallitt, Email: kathryn.wallitt@nhs.net.
Dimitri Amiras, Email: dimitri.amiras@nhs.net.
Andrea Rockall, Email: a.rockall@imperial.ac.uk.
Christina Messiou, Email: christina.messiou@icr.ac.uk.
REFERENCES
- 1. CRUK myeloma statistics . 2018. . Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma#heading-Three .
- 2. Rajkumar SV , Dimopoulos MA , Palumbo A , Blade J , Merlini G , Mateos MV , et al. . International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma . Lancet Oncol 2014. ; 15 : e538 – e548 . doi: 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 3. Kumar S , Paiva B , Anderson KC , Durie B , Landgren O , Moreau P , et al. . International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma . Lancet Oncol 2016. ; 17 : e328 – e346 . doi: 10.1016/S1470-2045(16)30206-6 [DOI] [PubMed] [Google Scholar]
- 4. CRUK myeloma survival statistics . 2018. . Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma/survival .
- 5. Myeloma: diagnosis and management. NICE guideline [NG35] . 2016. . Available from: https://www.nice.org.uk/guidance/ng35 .
- 6. Chantry A , Kazmi M , Barrington S , Goh V , Mulholland N , Streetly M , et al. . Guidelines for the use of imaging in the management of patients with myeloma . Br J Haematol 2017. ; 178 : 380 – 93 . doi: 10.1111/bjh.14827 [DOI] [PubMed] [Google Scholar]
- 7. Dimopoulos MA , Hillengass J , Usmani S , Zamagni E , Lentzsch S , Davies FE , et al. . Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement . J Clin Oncol 2015. ; 33 : 657 – 64 . doi: 10.1200/JCO.2014.57.9961 [DOI] [PubMed] [Google Scholar]
- 8. Edelstyn GA , Gillespie PJ , Grebbell FS . The radiological demonstration of osseous metastases. Experimental observations . Clin Radiol 1967. ; 18 : 158 – 62 . doi: 10.1016/S0009-9260(67)80010-2 [DOI] [PubMed] [Google Scholar]
- 9. Pianko MJ , Terpos E , Roodman GD , Divgi CR , Zweegman S , Hillengass J , et al. . Whole-body low-dose computed tomography and advanced imaging techniques for multiple myeloma bone disease . Clin Cancer Res 2014. ; 20 : 5888 – 97 . doi: 10.1158/1078-0432.CCR-14-1692 [DOI] [PubMed] [Google Scholar]
- 10. Giles SL , deSouza NM , Collins DJ , Morgan VA , West S , Davies FE , et al. . Assessing myeloma bone disease with whole-body diffusion-weighted imaging: comparison with X-ray skeletal survey by region and relationship with laboratory estimates of disease burden . Clin Radiol 2015. ; 70 : 614 – 21 . doi: 10.1016/j.crad.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Regelink JC , Minnema MC , Terpos E , Kamphuis MH , Raijmakers PG , Pieters-van den Bos IC , et al. . Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review . Br J Haematol 2013. ; 162 : 50 – 61 . doi: 10.1111/bjh.12346 [DOI] [PubMed] [Google Scholar]
- 12. Horger M , Claussen CD , Bross-Bach U , Vonthein R , Trabold T , Heuschmid M , et al. . Whole-body low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography . Eur J Radiol 2005. ; 54 : 289 – 97 . doi: 10.1016/j.ejrad.2004.04.015 [DOI] [PubMed] [Google Scholar]
- 13. Kröpil P , Fenk R , Fritz LB , Blondin D , Kobbe G , Mödder U , et al. . Comparison of whole-body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma . Eur Radiol 2008. ; 18 : 51 – 8 . doi: 10.1007/s00330-007-0738-3 [DOI] [PubMed] [Google Scholar]
- 14. Gleeson TG , Moriarty J , Shortt CP , Gleeson JP , Fitzpatrick P , Byrne B , et al. . Accuracy of whole-body low-dose multidetector CT (WBLDCT) versus skeletal survey in the detection of myelomatous lesions, and correlation of disease distribution with whole-body MRI (WBMRI . Skeletal Radiol 2009. ; 38 : 225 – 36 . doi: 10.1007/s00256-008-0607-4 [DOI] [PubMed] [Google Scholar]
- 15. Hillengass J , Moulopoulos LA , Delorme S , Koutoulidis V , Mosebach J , Hielscher T , et al. . Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: a study of the International Myeloma Working Group . Blood Cancer J 2017. ; 7 : e599 . doi: 10.1038/bcj.2017.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kloth JK , Hillengass J , Listl K , Kilk K , Hielscher T , Landgren O , et al. . Appearance of monoclonal plasma cell diseases in whole-body magnetic resonance imaging and correlation with parameters of disease activity . Int J Cancer 2014. ; 135 : 2380 – 6 . doi: 10.1002/ijc.28877 [DOI] [PubMed] [Google Scholar]
- 17. Walker R , Barlogie B , Haessler J , Tricot G , Anaissie E , Shaughnessy JD , et al. . Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications . J Clin Oncol 2007. ; 25 : 1121 – 8 . doi: 10.1200/JCO.2006.08.5803 [DOI] [PubMed] [Google Scholar]
- 18. Baur-Melnyk A , Buhmann S , Becker C , Schoenberg SO , Lang N , Bartl R , et al. . Whole-body MRI versus whole-body MDCT for staging of multiple myeloma . AJR Am J Roentgenol 2008. ; 190 : 1097 – 104 . doi: 10.2214/AJR.07.2635 [DOI] [PubMed] [Google Scholar]
- 19. Bäuerle T , Hillengass J , Fechtner K , Zechmann CM , Grenacher L , Moehler TM , et al. . Multiple myeloma and monoclonal gammopathy of undetermined significance: importance of whole-body versus spinal MR imaging . Radiology 2009. ; 252 : 477 – 85 . doi: 10.1148/radiol.2522081756 [DOI] [PubMed] [Google Scholar]
- 20. Pearce T , Philip S , Brown J , Koh DM , Burn PR . Bone metastases from prostate, breast and multiple myeloma: differences in lesion conspicuity at short-tau inversion recovery and diffusion-weighted MRI . Br J Radiol 2012. ; 85 : 1102 – 6 . doi: 10.1259/bjr/30649204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pawlyn C , Fowkes L , Otero S , Jones JR , Boyd KD , Davies FE , et al. . Whole-body diffusion-weighted MRI: a new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia 2016. ; 30 : 1446 – 8 . doi: 10.1038/leu.2015.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sachpekidis C , Mosebach J , Freitag MT , Wilhelm T , Mai EK , Goldschmidt H , et al. . Application of (18)F-FDG PET and diffusion weighted imaging (DWI) in multiple myeloma: comparison of functional imaging modalities . Am J Nucl Med Mol Imaging 2015. ; 5 : 479 – 92 . [PMC free article] [PubMed] [Google Scholar]
- 23. Koutoulidis V , Fontara S , Terpos E , Zagouri F , Matsaridis D , Christoulas D , et al. . Quantitative Diffusion-weighted Imaging of the Bone Marrow: An adjunct tool for the diagnosis of a diffuse MR imaging pattern in patients with multiple myeloma . Radiology 2017. ; 282 : 484 – 93 . doi: 10.1148/radiol.2016160363 [DOI] [PubMed] [Google Scholar]
- 24. Padhani AR , Lecouvet FE , Tunariu N , Koh DM , De Keyzer F , Collins DJ , et al. . ME tastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer . Eur Urol 2017. ; 71 : 81 – 92 . doi: 10.1016/j.eururo.2016.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barnes A , Alonzi R , Blackledge M , Charles-Edwards G , Collins DJ , Cook G , et al. . UK quantitative WB-DWI technical workgroup: consensus meeting recommendations on optimisation, quality control, processing and analysis of quantitative whole-body diffusion-weighted imaging for cancer . Br J Radiol 2018. ; 91 : 20170577 . doi: 10.1259/bjr.20170577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Messiou C , Kaiser M . Whole body diffusion weighted MRI--a new view of myeloma . Br J Haematol 2015. ; 171 : 29 – 37 . doi: 10.1111/bjh.13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cavo M , Terpos E , Nanni C , Moreau P , Lentzsch S , Zweegman S , et al. . Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group . Lancet Oncol 2017. ; 18 : e206 – e217 . doi: 10.1016/S1470-2045(17)30189-4 [DOI] [PubMed] [Google Scholar]
- 28. Rasche L , Angtuaco E , McDonald JE , Buros A , Stein C , Pawlyn C , et al. . Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma . Blood 2017. ; 130 : 30 – 4 . doi: 10.1182/blood-2017-03-774422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartel TB , Haessler J , Brown TL , Shaughnessy JD , van Rhee F , Anaissie E , et al. . F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma . Blood 2009. ; 114 : 2068 – 76 . doi: 10.1182/blood-2009-03-213280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zamagni E , Patriarca F , Nanni C , Zannetti B , Englaro E , Pezzi A , et al. . Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation . Blood 2011. ; 118 : 5989 – 95 . doi: 10.1182/blood-2011-06-361386 [DOI] [PubMed] [Google Scholar]
- 31. Moon SH , Choi WH , Yoo IR , Lee SJ , Paeng JC , Jeong SY , et al. . Prognostic Value of Baseline 18F-Fluorodeoxyglucose PET/CT in Patients with Multiple Myeloma: A Multicenter Cohort Study . Korean J Radiol 2018. ; 19 : 481 – 8 . doi: 10.3348/kjr.2018.19.3.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dimitrakopoulou-Strauss A , Hoffmann M , Bergner R , Uppenkamp M , Haberkorn U , Strauss LG . Prediction of progression-free survival in patients with multiple myeloma following anthracycline-based chemotherapy based on dynamic FDG-PET . Clin Nucl Med 2009. ; 34 : 576 – 84 . doi: 10.1097/RLU.0b013e3181b06bc5 [DOI] [PubMed] [Google Scholar]
- 33. Haznedar R , Akı SZ , Akdemir OU , Ozkurt ZN , Ceneli O , Yağcı M , et al. . Value of 18F-fluorodeoxyglucose uptake in positron emission tomography/computed tomography in predicting survival in multiple myeloma . Eur J Nucl Med Mol Imaging 2011. ; 38 : 1046 – 53 . doi: 10.1007/s00259-011-1738-8 [DOI] [PubMed] [Google Scholar]
- 34. Zamagni E , Nanni C , Gay F , Pezzi A , Patriarca F , Bellò M , et al. . 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease . Leukemia 2016. ; 30 : 417 – 22 . doi: 10.1038/leu.2015.291 [DOI] [PubMed] [Google Scholar]
- 35. Zamagni E , Nanni C , Mancuso K , Tacchetti P , Pezzi A , Pantani L , et al. . PET/CT Improves the Definition of Complete Response and Allows to Detect Otherwise Unidentifiable Skeletal Progression in Multiple Myeloma . Clin Cancer Res 2015. ; 21 : 4384 – 90 . doi: 10.1158/1078-0432.CCR-15-0396 [DOI] [PubMed] [Google Scholar]
- 36. Usmani SZ , Mitchell A , Waheed S , Crowley J , Hoering A , Petty N , et al. . Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3 . Blood 2013. ; 121 : 1819 – 23 . doi: 10.1182/blood-2012-08-451690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fonti R , Larobina M , Del Vecchio S , De Luca S , Fabbricini R , Catalano L , et al. . Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma . J Nucl Med 2012. ; 53 : 1829 – 35 . doi: 10.2967/jnumed.112.106500 [DOI] [PubMed] [Google Scholar]
- 38. Gariani J , Westerland O , Natas S , Verma H , Cook G , Goh V . Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or (18)F-fluorodeoxyglucose positron emission tomography/CT ((18)F-FDG PET/CT) in atients with myeloma: Systematic review of diagnostic performance . Crit Rev Oncol Hematol 2018. ; 124 : 66 – 72 . doi: 10.1016/j.critrevonc.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 39. Shortt CP , Gleeson TG , Breen KA , McHugh J , O'Connell MJ , O'Gorman PJ , et al. . Whole-Body MRI versus PET in assessment of multiple myeloma disease activity . AJR Am J Roentgenol 2009. ; 192 : 980 – 6 . doi: 10.2214/AJR.08.1633 [DOI] [PubMed] [Google Scholar]
- 40. Basha MAA , Hamed MAG , Refaat R , AlAzzazy MZ , Bessar MA , Mohamed EM , et al. . Diagnostic performance of (18)F-FDG PET/CT and whole-body MRI before and early after treatment of multiple myeloma: a prospective comparative study . Jpn J Radiol 2018. ; 36 : 382 – 93 . doi: 10.1007/s11604-018-0738-z [DOI] [PubMed] [Google Scholar]
- 41. Hillengass J , Fechtner K , Weber MA , Bäuerle T , Ayyaz S , Heiss C , et al. . Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma . J Clin Oncol 2010. ; 28 : 1606 – 10 . doi: 10.1200/JCO.2009.25.5356 [DOI] [PubMed] [Google Scholar]
- 42. Merz M , Hielscher T , Wagner B , Sauer S , Shah S , Raab MS , et al. . Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma . Leukemia 2014. ; 28 : 1902 – 8 . doi: 10.1038/leu.2014.75 [DOI] [PubMed] [Google Scholar]
- 43. Mateos MV , Hernández MT , Giraldo P , de la Rubia J , de Arriba F , López Corral L , et al. . Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma . N Engl J Med 2013. ; 369 : 438 – 47 . doi: 10.1056/NEJMoa1300439 [DOI] [PubMed] [Google Scholar]
- 44. Mateos MV , Hernández MT , Giraldo P , de la Rubia J , de Arriba F , Corral LL , et al. . Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial . Lancet Oncol 2016. ; 17 : 1127 – 36 . doi: 10.1016/S1470-2045(16)30124-3 [DOI] [PubMed] [Google Scholar]
- 45. Siontis B , Kumar S , Dispenzieri A , Drake MT , Lacy MQ , Buadi F , et al. . Positron emission tomography-computed tomography in the diagnostic evaluation of smoldering multiple myeloma: identification of patients needing therapy . Blood Cancer J 2015. ; 5 : e364 . doi: 10.1038/bcj.2015.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moulopoulos LA , Dimopoulos MA , Weber D , Fuller L , Libshitz HI , Alexanian R . Magnetic resonance imaging in the staging of solitary plasmacytoma of bone . J Clin Oncol 1993. ; 11 : 1311 – 5 . doi: 10.1200/JCO.1993.11.7.1311 [DOI] [PubMed] [Google Scholar]
- 47. Nanni C , Rubello D , Zamagni E , Castellucci P , Ambrosini V , Montini G , et al. . 18F-FDG PET/CT in myeloma with presumed solitary plasmocytoma of bone . In Vivo 2008. ; 22 : 513 – 7 . [PubMed] [Google Scholar]
- 48. Schirrmeister H , Buck AK , Bergmann L , Reske SN , Bommer M . Positron emission tomography (PET) for staging of solitary plasmacytoma . Cancer Biother Radiopharm 2003. ; 18 : 841 – 5 . doi: 10.1089/108497803770418382 [DOI] [PubMed] [Google Scholar]
- 49. Kim PJ , Hicks RJ , Wirth A , Ryan G , Seymour JF , Prince HM , et al. . Impact of 18F-fluorodeoxyglucose positron emission tomography before and after definitive radiation therapy in patients with apparently solitary plasmacytoma . Int J Radiat Oncol Biol Phys 2009. ; 74 : 740 – 6 . doi: 10.1016/j.ijrobp.2008.08.037 [DOI] [PubMed] [Google Scholar]
- 50. Fouquet G , Guidez S , Herbaux C , Van de Wyngaert Z , Bonnet S , Beauvais D , et al. . Impact of initial FDG-PET/CT and serum-free light chain on transformation of conventionally defined solitary plasmacytoma to multiple myeloma . Clin Cancer Res 2014. ; 20 : 3254 – 60 . doi: 10.1158/1078-0432.CCR-13-2910 [DOI] [PubMed] [Google Scholar]
- 51. Caers J , Paiva B , Zamagni E , Leleu X , Bladé J , Kristinsson SY , et al. . Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel . J Hematol Oncol 2018. ; 11 : 10 . doi: 10.1186/s13045-017-0549-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Messiou C , Giles S , Collins DJ , West S , Davies FE , Morgan GJ , et al. . Assessing response of myeloma bone disease with diffusion-weighted MRI . Br J Radiol 2012. ; 85 : e1198 – e1203 . doi: 10.1259/bjr/52759767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Giles SL , Messiou C , Collins DJ , Morgan VA , Simpkin CJ , West S , et al. . Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma . Radiology 2014. ; 271 : 785 – 94 . doi: 10.1148/radiol.13131529 [DOI] [PubMed] [Google Scholar]
- 54. Derlin T , Peldschus K , Münster S , Bannas P , Herrmann J , Stübig T , et al. . Comparative diagnostic performance of ¹⁸F-FDG PET/CT versus whole-body MRI for determination of remission status in multiple myeloma after stem cell transplantation . Eur Radiol 2013. ; 23 : 570 – 8 . doi: 10.1007/s00330-012-2600-5 [DOI] [PubMed] [Google Scholar]
- 55. Moreau P , Attal M , Caillot D , Macro M , Karlin L , Garderet L , et al. . Prospective evaluation of magnetic resonance imaging and [(18)F]Fluorodeo by glucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study . J Clin Oncol 2017. ; 35 : 2911 – 8 . doi: 10.1200/JCO.2017.72.2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hillengass J , Ayyaz S , Kilk K , Weber MA , Hielscher T , Shah R , et al. . Changes in magnetic resonance imaging before and after autologous stem cell transplantation correlate with response and survival in multiple myeloma . Haematologica 2012. ; 97 : 1757 – 60 . doi: 10.3324/haematol.2012.065359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Horger M , Weisel K , Horger W , Mroue A , Fenchel M , Lichy M . Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring in multiple myeloma: preliminary results . AJR Am J Roentgenol 2011. ; 196 : W790 – W795 . doi: 10.2214/AJR.10.5979 [DOI] [PubMed] [Google Scholar]
- 58. Dutoit JC , Claus E , Offner F , Noens L , Delanghe J , Verstraete KL . Combined evaluation of conventional MRI, dynamic contrast-enhanced MRI and diffusion weighted imaging for response evaluation of patients with multiple myeloma . Eur J Radiol 2016. ; 85 : 373 – 82 . doi: 10.1016/j.ejrad.2015.11.040 [DOI] [PubMed] [Google Scholar]
- 59. Chenevert TL , Stegman LD , Taylor JM , Robertson PL , Greenberg HS , Rehemtulla A , et al. . Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors . J Natl Cancer Inst 2000. ; 92 : 2029 – 36 . doi: 10.1093/jnci/92.24.2029 [DOI] [PubMed] [Google Scholar]
- 60. Latifoltojar A , Hall-Craggs M , Rabin N , Popat R , Bainbridge A , Dikaios N , et al. . Whole body magnetic resonance imaging in newly diagnosed multiple myeloma: early changes in lesional signal fat fraction predict disease response . Br J Haematol 2017. ; 176 : 222 – 33 . doi: 10.1111/bjh.14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sachpekidis C , Dimitrakopoulou-Strauss A , Delorme S , Goldschmidt H . Functional Imaging with (18)F-FDG PET/CT and Diffusion Weighted Imaging (DWI) in Early Response Evaluation of Combination Therapy of Elotuzumab, Lenalidomide, and Dexamethasone in a Relapsed Multiple Myeloma Patient . LID - E61 [pii]LID - 10.3390/diagnostics7040061 [doi]. Diagnostics 2017. ; 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nanni C , Zamagni E , Celli M , Caroli P , Ambrosini V , Tacchetti P , et al. . The value of 18F-FDG PET/CT after autologous stem cell transplantation (ASCT) in patients affected by multiple myeloma (MM): experience with 77 patients . Clin Nucl Med 2013. ; 38 : e74 – e79 . doi: 10.1097/RLU.0b013e318266cee2 [DOI] [PubMed] [Google Scholar]
- 63. Patriarca F , Carobolante F , Zamagni E , Montefusco V , Bruno B , Englaro E , et al. . The role of positron emission tomography with 18F-fluorodeoxyglucose integrated with computed tomography in the evaluation of patients with multiple myeloma undergoing allogeneic stem cell transplantation . Biol Blood Marrow Transplant 2015. ; 21 : 1068 – 73 . doi: 10.1016/j.bbmt.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 64. Hillengass J , Merz M , Delorme S . Minimal residual disease in multiple myeloma: use of magnetic resonance imaging . Semin Hematol 2018. ; 55 : 19 – 21 . doi: 10.1053/j.seminhematol.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 65. Nanni C , Zamagni E , Versari A , Chauvie S , Bianchi A , Rensi M , et al. . Image interpretation criteria for FDG PET/CT in multiple myeloma: a new proposal from an Italian expert panel. IMPeTUs (Italian Myeloma criteria for PET USe . Eur J Nucl Med Mol Imaging 2016. ; 43 : 414 – 21 . doi: 10.1007/s00259-015-3200-9 [DOI] [PubMed] [Google Scholar]
- 66. Machine Learning in Myeloma Response (MALIMAR) study . 2018. . Available from: https://clinicaltrials.gov/ct2/show/NCT03574454 [ accessed 29/8/18 ].
- 67. Sachpekidis C , Hillengass J , Goldschmidt H , Mosebach J , Pan L , Schlemmer HP , et al. . Comparison of (18)F-FDG PET/CT and PET/MRI in patients with multiple myeloma . Am J Nucl Med Mol Imaging 2015. ; 5 : 469 – 78 . [PMC free article] [PubMed] [Google Scholar]