Abstract
Objective:
To evaluate the added value of diffusion-weighted imaging (DWI) to T 2 weighted imaging (T 2WI) for detection of extramural venous invasion (EMVI) in patients with primary rectal cancer.
Methods:
79 patients (50 men, 29 females, mean age 67.4 years, range 37–87 years) who had undergone rectal MRI and subsequently received surgical resection were included. The rectal MRI consisted of T 2WI in three planes and axial DWI (b-values, 0, 1000 s mm–2). Two radiologists blinded to the pathologic results independently reviewed the T 2WI first, and then the combined T 2WI and DWI 4 weeks later. They recorded their confidence scores for EMVI on a 5-point scale (0: definitely negative and 4: definitely positive). The diagnostic performance of each reading session for each reader was compared by pairwise comparison of receiver operating characteristic curves. The area under the ROC curve (AUC) was considered as the diagnostic performance. The result of a histopathological examination served as the reference standard for EMVI.
Results:
For both readers, the diagnostic performance was not significantly different between the two image sets (for reader 1, AUC, 0.828 and 0.825, p = 0.9426 and for reader 2, AUC, 0.723 and 0.726, p = 0.9244, respectively).
Conclusion:
There was no added value of DWI to T2WI for detection of EMVI in patients with primary rectal cancer.
Advances in knowledge:
High-resolution T2WI alone is sufficient to assess EMVI and a supplementary DWI has no added value in patients with primary rectal cancer.
INTRODUCTION
Extramural venous invasion (EMVI) refers to the presence of cancer cells/tissue within the veins beyond the muscularis propria of the rectal wall. EMVI is regarded as a bad prognostic indicator in terms of local recurrence, distant metastasis, as well as overall patient survival.1 In patients with rectal cancer, EMVI can provide a pathway for hematogenous dissemination of tumor cells, and is an independent risk factor for synchronous metastasis.1,2 Moreover, EMVI, which is detected on MRI, is assumed to be helpful in predicting disease relapse.2–4
Although EMVI is based on post-operative histologic diagnosis of surgical specimens, recent advances in high-resolution MRI allow for identification of EMVI before surgery.2 EMVI is typically seen on T 2 weighted imaging (T 2WI) as intermediate tumor–signal intensity within normal-caliber or slightly expanded extramural vessels contiguous to the primary tumor.5 Various studies have shown a wide range of MRI sensitivity (28–90%) and specificity (41–94%) for detection of EMVI.2,3,6,7 Since the time of EMVI’s introduction for rectal cancer with MRI, the accuracy of pre-operative assessment has been known to be approximately 70–80% at best.2,3,7,8 Lim et al reported diagnostic-predictive values of MRI in identifying EMVI in primary rectal cancer; specifically, the sensitivity, specificity and accuracy were 28, 94, and 80%, respectively.2
Diffusion-weighted imaging (DWI) has played an important role in tumor detection, characterization, monitoring and prediction of therapeutic response in rectal cancer, having shown promising results in those diagnostic tasks.9–13 However, to the best of our knowledge, the added value of DWI to T2WI for detection of EMVI has not yet been investigated in cases of primary rectal cancer.
Therefore, the purpose of our study was to determine whether DWI-added T2WI could provide any additional benefit for detection of EMVI in primary rectal cancer compared with T2WI alone.
methods and Materials
This retrospective study was approved by the relevant institutional review board, and informed consent was waived.
Study population
Between November 2010 and February 2018, 102 consecutive patients for whom documentation of histologically confirmed rectal adenocarcinoma was available and who had undergone preoperative rectal MRI and subsequent surgical resection were initially enrolled. 21 patients who had undergone neoadjuvant chemoradiation therapy prior to surgery were excluded. Among the remaining 81 patients, 2 patients without an adequate DWI sequence also were excluded. Finally, 79 patients (50 males, 29 females, mean age 67.4 years, range 37–87 years) were included in the study cohort and analyzed (Figure 1).
Figure 1.
Flowchart of the case enrollment process.
MRI
All of the MRI scans were performed using a 3.0 T MR scanner (Achieva TX; Philips Medical Imaging, Best, Netherlands) equipped with a phased-array torso coil (USA Instruments, Aurora, OH). To obtain optimal distension of the rectum, the rectal gel filling was made with approximately 50–80 ml of ultrasound gel (Ecosonic, Sanipia, Korea) just prior to the examination. The MRI protocol consisted of axial, coronal and sagittal T 2W turbo spin echo sequences, and DWI with a single-shot echo planar imaging sequence. The scout sagittal T 2WI acquisition was performed first in order to plan the axial and coronal images, which were orthogonal and parallel to the longitudinal tumor axis. Immediately after the acquisition of T 2WI, axial DWI (bvalues of 0 and 1000 s mm– 2) acquisition was performed. The detailed parameters of each sequence are summarized in Table 1.
Table 1.
MRI sequence parameters
| Parameters | T 2 weighted axial, sagittal, and coronal TSE | DWI (b = 0, 1000 s mm– 2) |
| TR | 3727 | 9500 |
| TE | 90 | 65 |
| ETL | 17 | 73 |
| Slice thickness | 3 | 2 |
| Slice gap | 0.3 | 0 |
| Matrix size | 300 × 290 | 120 × 118 |
| NEX | 1 | 10 |
| FOV | 240 × 240 | 240 × 240 |
| Acquisition time | 2 min 30 s | 5 min 40 s |
| No. of slices | 40 | 70 |
ETL, echo train length; FOV, field of view; NEX, number of excitations; TE, echo time ; TR, repetition time; TSE, turbo spin echo.
Diffusion weighted imaging (DWI) was performed using the single-shot echo planar imaging technique.
Image analysis
All of the MRI scans were reviewed on a picture archiving and communication system workstation (m-view; Marotech, Seoul, Korea). The MRI scans were analyzed by two independent readers with 5 and 2 years of experience in assessing rectal MRI, respectively. Both readers were blinded to the histologic results. They first performed an interval assessment of T 2WI images, and 4 weeks later, analyzed the combined T 2WI with DWI image set to evaluate any added value for detection of EMVI. During the first reading session, the readers independently scored the probability of EMVI based on the 5-point scale suggested by Smith et al.3 Details on the scale are available in Table 2.
Table 2.
5-point scale on MRI for detection of EMVI a
| Score | Possibility of EMVI |
Imaging features |
| 0 | Definitely negative | No vessels adjacent to areas of tumor penetration |
| 1 | Probably negative | Minimal extramural stranding/nodular extension, but not in the vicinity of any vascular structure |
| 2 | Possibly negative | Stranding demonstrated in the vicinity of extramural vessels, but these are of normal caliber, and no definite tumor signal in vessel |
| 3 | Probably positive | Intermediate signal intensity apparent within vessels, although contour and caliber of these vessels is only slightly expanded |
| 4 | Definitely positive | Obvious irregular vessel contour or nodular expansion of vessel by definite tumor signal |
EMVI, extramural venous invasion.
The 5-point scale that was adopted for this study had been suggested by Smith et al.3
During the second reading session, the patient order was reshuffled to avoid recall bias. The EMVI on DWI was considered to be positive when intermediate or high tumor-signal intensity was found within normal or slightly expanded extramural vessels neighboring the primary tumor on DWI. Thus, T 2WI and DWI images were presented side-by side-in a stack mode for convenient scrolling. The scoring rules for the combined image set were as follows; the two readers independently recorded a tentative confidence score for EMVI based on the same 5-point scale as used for T 2WI; immediately after reviewing T 2WI, they reviewed additional DWI based on the predefined criteria for EMVI positivity on DWI. The integration process was as follows; if the EMVI was considered to be positive on DWI, the final score was made one point higher than the tentative score on T 2WI; otherwise, the final confidence score was rendered one point lower than the tentative score on T 2WI.
Reference standard
The result of a histopathological examination served as the reference standard for EMVI. After surgical resection, the specimens were assessed by one dedicated gastrointestinal pathologist. All of the specimens were reviewed for the presence of EMVI, which was defined as tumor cells within endothelial cell-lined blood vessels beyond the muscularis propria. To facilitate radiologic–pathologic correlation, a reference standard EMVI map on T 2WI was made by an expert radiologist after referring to the pathologic results.
Statistical analysis
The diagnostic performance of each reading session for each reader was investigated using receiver operating characteristic (ROC) curve analyses. The areas under the ROC curve (AUCs) were calculated and regarded as diagnostic performance. To evaluate the added value of DWI to T 2WI for detection of EMVI, pairwise comparison of the ROC curves was used to determine whether there were significant differences between the two image sets. To express the statistical precision of result, 95% confidence intervals (CI) were used. Sensitivity, specificity, and diagnostic accuracy were calculated for both readers on the assumption that a confidence score of 3 or higher was positive for the diagnosis of EMVI. To evaluate the interobserver agreement in terms of the confidence score for the diagnosis of EMVI, the quadratic-weighted κ values were calculated. The strength of interobserver agreement was defined as poor (<0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), and excellent (0.81–1.00).
All of the statistical analyses were carried out using MedCalc software for Windows (MedCalc Software v.n 12.7.1.0, Mariakerke, Belgium). A p-value less than 0.05 was considered to indicate a statistically significant difference.
Results
Demographics of study population
The mean interval between the preoperative rectal MRI and surgery was 8 days (range 1–20). Among the 79 patients, 20 (25%) had histopathological documentation of positive EMVI. Perineural invasion was pathologically confirmed in 34 patients. Nine patients had associated findings of focal or partly (less than 30% of whole tumor volume) mucin production at histopathological examination. The final histopathological stages of the study population are summarized in Table 3.
Table 3.
Pathologic stages of study population
| T1 | T2 | T3 | T4 | Total | |
| N0 | 7 | 16 | 20 | 3 | 46 |
| N1 | 5 | 12 | 17 | ||
| N2 | 14 | 2 | 16 | ||
| Total | 7 | 21 | 46 | 5 | 79 |
Comparison of diagnostic performance
For Reader 1, the diagnostic performance was not significantly different between the two image sets (AUC for T 2WI, 0.828 [95% CI (0.726–0.903)]; for combined T 2WI and DWI, 0.825 [95% CI (0.724–0.902)], p = 0.9426). The sensitivity and specificity of T 2WI were 85 and 75%, respectively, and those of the combined image set were 90 and 66%, respectively. The estimated accuracy, positive predict value (PPV) and negative predict value (NPV) of T 2WI were 77, 53 and 94%, respectively, and those of the combined image set were 72, 47 and 95%, respectively.
For Reader 2, the diagnostic performance was not significantly different between the two image sets {AUC, 0.723 [95% CI (0.611–0.818)] and 0.726 [95% CI (0.614–0.821)], respectively, p = 0.9244}, either. The sensitivity and specificity of T 2WI were 80 and 53%, respectively, and those of the combined image set were 90 and 54%, respectively. The estimated accuracy, PPV and NPV of T 2WI were 59, 36 and 89%, respectively, and those of the combined image set were 63, 40 and 94%, respectively.
For the combined T 2WI and DWI image set, the numbers of false-positives and false-negatives were 20 and 2, respectively, for Reader 1, and 27 and 2, respectively, for Reader 2. The two readers had 15 false-positives in common. For the T 2WI image set, the numbers of false-positives and false-negatives were 15 and 3, respectively, for Reader 1, and 28 and 4, respectively, for Reader 2.
Interobserver agreement
The interobserver agreements regarding confidence score for EMVI were good for both the first (T2WI alone) and the second reading session (combined T2WI and DWI) between the readers (κ = 0.704 and 0.611, respectively).
Discussion
Our results demonstrated that adding DWI to T 2WI showed no additional diagnostic benefit for evaluation of EMVI. The stationary diagnostic performance even after adding DWI to T 2WI could be attributed to the fact that when diagnosing EMVI, it is important to identify the neighboring peritumoral venous structure, which appears as a signal-void tubular structure on T 2WI. In any case, DWI has inherent limitations such as a low signal-to-noise ratio and suppression of signals from background anatomical structures.14 Therefore, it was difficult to identify the intermediate or high signal intensity of the tumor portion impacted in the dilated peritumoral venous structure on DWI. Moreover, EMVI involving small venules less than 3 mm in diameter could be easily missed due to the limited spatial resolution, particularly on the z-axis, even with the recent advances in MRI technology. Consequently, these intrinsic limitations might incur an increased number of false-positive cases on the combined T 2WI and DWI image set, as observed in Reader 1. A little benefit from DWI was a reduction in the number of false-negatives (by 1 for Reader 1 and by 2 for Reader 2), albeit at the cost of an increase in the number of false-positives (by 5 for Reader 1). In other words, the minimally increased NPV from 94 to 95% after additional reading of DWI was achieved at the cost of decreased PPV from 53 to 47%. Although DWI increased the confidence level for EMVI in case of EMVI positive case on T 2WI, it was statistically insignificant (Figure 2). The most common cause of false-positive cases was difficulty in differentiating EMVI from peritumoral fibrosis (desmoplastic reaction) in dirty perirectal fat infiltration on T 2WI, and overestimation of peritumoral high signal intensity on DWI (Figure 3). Other causes of false-positive included obliteration of signal void in rectal veins, tumoral encasement of rectal veins, and tumor deposition on T 2WI. The most common cause of false-negative cases was microscopic EMVI that was beyond the level of visual assessment on T 2WI and DWI. Therefore, we believe that current high-resolution T2WI is good enough and that supplementation by DWI for EMVI assessment is not necessary.
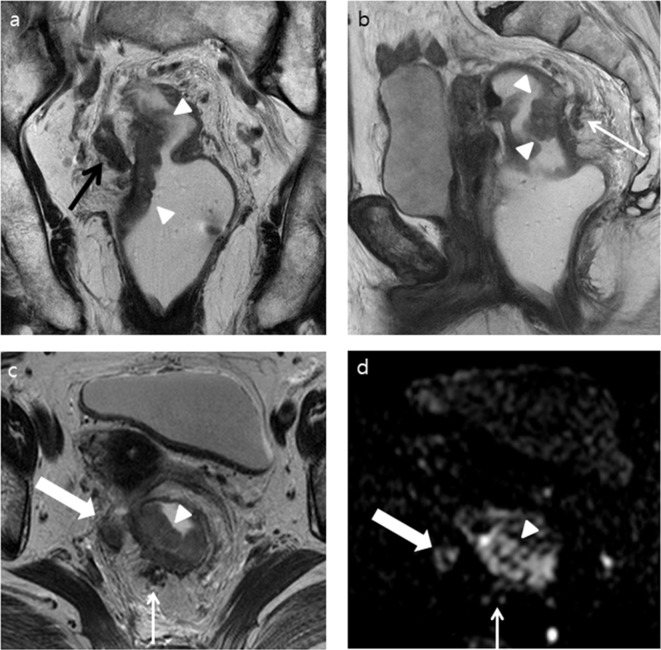
Figure 2.
A 81-year-old female with a pathologically confirmed rectal adenocarcinoma (pT3N2bM0) and positive EMVI. Both readers increased their confidence score for EMVI from 3 to 4 after adding DWI to T 2WI. (a) T 2WI coronal and (b) sagittal images show an ulcerofungating mass (white arrow heads) arising from the right posterolateral wall of the proximal rectum. Tumor invasion of the right mesorectal fascia is obvious (black arrow). Dilated peritumoral venous structure filled with tumor signal, located posterior to the tumor (white arrow), is also noted. (c) T2W axial image shows tumor (white arrow head) extending from the rectal muscularis propria to the neighboring serpiginous venous structure in the 7 o’clock direction (thin white arrow). The tumor penetrates into the right mesorectal fascia (thick white arrow) (d) DWI (b = 1000 s mm–2) shows a high signal intensity in the tumor (white arrow head) and in the presumed EMVI in the 7 o’clock direction (thin white arrow). An amorphous intermediate signal-intensity lesion corresponding to the tumor invading the right mesorectal fascia in the 9 o’clock direction (thick white arrow) is also noted. DWI, diffusion-weighted imaging; EMVI, extramural venous invasion; T 2WI, T 2 weighted imaging.
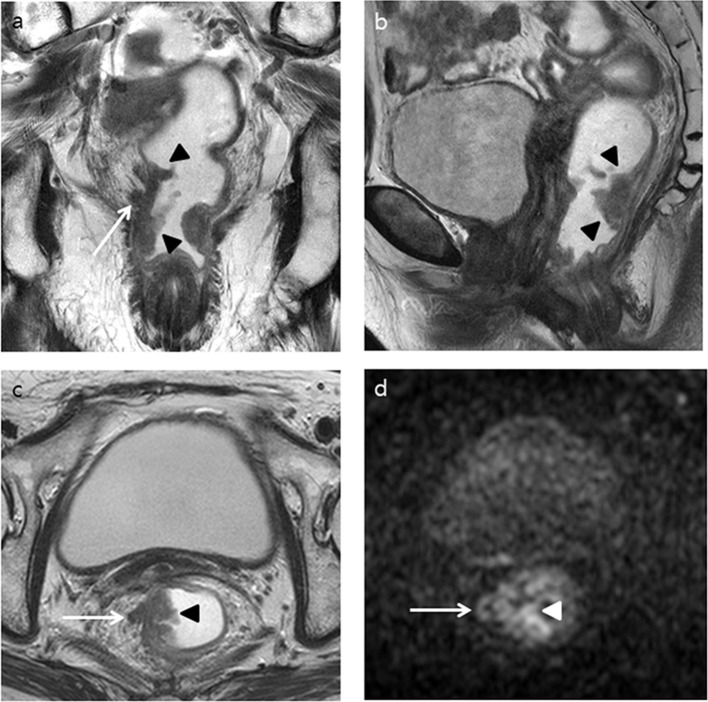
Figure 3.
A 83-year-old female with a pathologically confirmed rectal adenocarcinoma (pT3N2bM0) without EMVI. Both readers increased their confidence score for EMVI, from 2 to 3 and from 3 to 4, respectively, after adding DWI to T 2WI. (a) T 2WI coronal and (b) sagittal images show an ulcerofungating tumor (black arrowheads) arising from the right posterolateral wall of the lower rectum as well as tumor infiltration to perirectal fat (white arrow). (c) T 2WI axial image shows the ulcerofungating tumor (black arrowhead) penetrating the right wall to the perirectal fat layer like a thick spicule (white arrow). (d) DWI (b = 1000 s mm– 2 ) shows a high signal intensity in the tumor (white arrowhead) as well as tumor invasion to perirectal fat in a curvilinear–vascular-like appearance in the 9 o’clock direction (white arrow). DWI, diffusion-weighted imaging; EMVI, extramural venous invasion; T 2WI, T 2 weighted imaging.
In terms of any added value of DWI for evaluation of EMVI in patients with rectal cancer, similar studies have not yet been conducted. Therefore, direct comparison under the same or a similar setting was not possible; only a partial historical comparison could be made. Dam et al reported the diagnostic-predictive values of a combined DWI and T 2WI image set for detection of EMVI in 35 patients with primary sigmoid colon cancer. The sensitivity and specificity of the image set by Observer 1 were 67 and 81%, respectively, and those by Observer 2 were 56 and 62%, respectively.15
In the current study, the diagnostic performance of T 2WI alone for identification of EMVI was also evaluated. The sensitivity and specificity of T 2WI for detection of EMVI were within the range of 80–85% and 53–75%, respectively. In addition, the estimated accuracy was within the range of 59–77%. These results fall under those of the relevant previous studies.2,3,7 Liu et al reported that T 2WI showed a sensitivity of 62%, a specificity of 82%, a PPV of 55%, a NPV of 87%, and an overall accuracy of 77% for detection of EMVI in patients with primary rectal cancer.7 Lim et al reported the diagnostic-predictive values of T 2WI for detection of 39 EMVI-positive cases among 188 patients with primary rectal cancer. The sensitivity, specificity, accuracy, PPV, and NPV of T 2WI were 28, 94, 80, 55, and 83%, respectively.2
Several limitations of the present study should be noted. We did not measure the apparent diffusion coefficient (ADC) value of the tumor portion in EMVI.16 However, we considered that the measured ADC value of the tumor portion should be guaranteed by accurate identification of EMVI on the ADC map. Second, although the result of a histopathological examination served as the reference standard for EMVI, complete radiologic–pathologic 1:1 matching might not have been achievable given the retrospective study design. Therefore, mismatch between EMVI on T 2WI and on pathologic slides might have been incurred. However, prospective meticulous radiologic–pathologic matching is a challenging task for investigators worldwide, in lymph node studies in rectal cancer, for example. We believe that our reference standard EMVI map, as drawn by an expert radiologist after referring to the pathologic results, was the second-best option available.
In conclusion, there was no additional diagnostic benefit after adding DWI to T2WI for detection of EMVI in patients with primary rectal cancer.
Contributor Information
Ju Hee Ahn, Email: ebgmlwm@nate.com.
Seung Ho Kim, Email: radiresi@gmail.com.
Jung Hee Son, Email: lidiacubic@gmail.com.
Sung Jae Jo, Email: family5sw@naver.com.
REFERENCES
- 1. KSAR Study Group for Rectal Cancer . Essential items for structured reporting of rectal cancer MRI: 2016 consensus recommendation from the Korean Society of abdominal radiology . Korean J Radiol 2017. ; 18 : 132 – 51 . doi: 10.3348/kjr.2017.18.1.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sohn B , Lim JS , Kim H , Myoung S , Choi J , Kim NK , et al. . MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer . Eur Radiol 2015. ; 25 : 1347 – 55 . doi: 10.1007/s00330-014-3527-9 [DOI] [PubMed] [Google Scholar]
- 3. Smith NJ , Barbachano Y , Norman AR , Swift RI , Abulafi AM , Brown G . Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer . Br J Surg 2008. ; 95 : 229 – 36 . doi: 10.1002/bjs.5917 [DOI] [PubMed] [Google Scholar]
- 4. Hung EHY , Dai EYL , Cho CCM . Magnetic resonance imaging for staging of primary rectal cancer: imaging prognosticators . Hong Kong J Radiol 2017. ; 20 : 259 – 71 . doi: 10.12809/hkjr1716937 [DOI] [Google Scholar]
- 5. Smith NJ , Shihab O , Arnaout A , Swift RI , Brown G . MRI for detection of extramural vascular invasion in rectal cancer . AJR Am J Roentgenol 2008. ; 191 : 1517 – 22 . doi: 10.2214/AJR.08.1298 [DOI] [PubMed] [Google Scholar]
- 6. Lee ES , Kim MJ , Park SC , Hur BY , Hyun JH , Chang HJ , et al. . Magnetic resonance Imaging-Detected extramural venous invasion in rectal cancer before and after preoperative chemoradiotherapy: diagnostic performance and prognostic significance . Eur Radiol 2018. ; 28 : 496 – 505 . doi: 10.1007/s00330-017-4978-6 [DOI] [PubMed] [Google Scholar]
- 7. Liu L , Liu M , Yang Z , He W , Wang Z , Jin E . Correlation of MRI-detected extramural vascular invasion with regional lymph node metastasis in rectal cancer . Clin Imaging 2016. ; 40 : 456 – 60 . doi: 10.1016/j.clinimag.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 8. Brown G , Radcliffe AG , Newcombe RG , Dallimore NS , Bourne MW , Williams GT . Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging . Br J Surg 2003. ; 90 : 355 – 64 . doi: 10.1002/bjs.4034 [DOI] [PubMed] [Google Scholar]
- 9. Ryu KH , Kim SH , Yoon J-H , Lee Y , Paik JH , Lim Y-J , et al. . Diffusion-weighted imaging for evaluating lymph node eradication after neoadjuvant chemoradiation therapy in locally advanced rectal cancer . Acta Radiol 2016. ; 57 : 133 – 41 . doi: 10.1177/0284185114568908 [DOI] [PubMed] [Google Scholar]
- 10. Heijnen LA , Lambregts DM , Mondal D , Martens MH , Riedl RG , Beets GL , et al. . Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes . Eur Radiol 2013. ; 23 : 3354 – 60 . doi: 10.1007/s00330-013-2952-5 [DOI] [PubMed] [Google Scholar]
- 11. Beets-Tan RG , Lambregts DM , Maas M , Bipat S , Barbaro B , Caseiro-Alves F , et al. . Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of gastrointestinal and abdominal radiology (ESGAR) consensus meeting . Eur Radiol 2013. ; 23 : 2522 – 31 . doi: 10.1007/s00330-013-2864-4 [DOI] [PubMed] [Google Scholar]
- 12. Beets-Tan RGH , Lambregts DMJ , Maas M , Bipat S , Barbaro B , Curvo-Semedo L , et al. . Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of gastrointestinal and abdominal radiology (ESGAR) consensus meeting . Eur Radiol 2018. ; 28 : 1465 – 75 . doi: 10.1007/s00330-017-5026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koh DM , Collins DJ . Diffusion-weighted MRI in the body: applications and challenges in oncology . AJR Am J Roentgenol 2007. ; 188 : 1622 – 35 . doi: 10.2214/AJR.06.1403 [DOI] [PubMed] [Google Scholar]
- 14. Dikaios N , Punwani S , Hamy V , Purpura P , Rice S , Forster M , et al. . Noise estimation from averaged diffusion weighted images: can unbiased quantitative decay parameters assist cancer evaluation? Magn Reson Med 2014. ; 71 : 2105 – 17 . doi: 10.1002/mrm.24877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dam C , Lindebjerg J , Jakobsen A , Jensen LH , Rahr H , Rafaelsen SR . Local staging of sigmoid colon cancer using MRI . Acta Radiologica Open 2017. ; 6 : 205846011772095 . doi: 10.1177/2058460117720957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coruh AG . The diagnostic performance of diffusion-weighted MRI and computed tomography in the detection of extramural venous invasion in rectal cancer . : Proceeding of the 29th Annual Meeting and Postgraduate Course of the European Society of Gastrointestinal & Abdominal Radiology . 2018 . Ireland. Dublin: : The British Institute of Radiology. ; 2018. . [Google Scholar]