Abstract
Objective:
Identification of aneurysms at risk for rupture is important and challenging. We sought to evaluate if intracranial vessel wall (IVW) imaging characteristics of unruptured aneurysms correlate with clinical risk factors for rupture.
Methods:
Patients with unruptured intracranial aneurysms were prospectively recruited and underwent a multi contrast 3D IVW protocol between April 6, 2016 and August 29, 2017. Two independent raters, blinded to aneurysm vulnerability, evaluated each aneurysm for wall enhancement, extent of enhancement in terms of the numbers of quadrants enhancing circumferentially, intensity of enhancement, and qualitative wall thinning. PHASES score was calculated for each aneurysm. Univariate logistic regression analysis was used to compare IVW characteristics between aneurysms at higher clinical risk for rupture (PHASES score > 3) and lower clinical risk for rupture (PHASES score ≤ 3).
Results:
45 patients with 65 unruptured aneurysms were analyzed; 38 aneurysms with PHASES score > 3 (58%) and 27 aneurysms with PHASES score ≤ 3 (42%). Aneurysms with PHASES score > 3 were more likely to demonstrate enhancement (42.1% vs 14.8%, p = 0.022), greater extent of enhancement (mean: 2.9 vs 2.2 quadrants, p = 0.063), and wall thinning (9.2% vs 0%, p = 0.044). Inter-reader agreement was moderate-to-good for the presence (κ = 0.64), extent (κ = 0.64), and intensity of enhancement (κ = 0.60) but relatively low for wall thinning (κ = 0.25).
Conclusion:
Aneurysms at higher risk of rupture by PHASES score are more likely to demonstrate wall enhancement, more diffuse enhancement, and wall thinning on IVW.
Advances in knowledge:
This study prospectively compares IVW-detected wall enhancement and thinning between unruptured aneurysms stratified into high and low risk groups by clinical scores (PHASES) of vulnerability.
Introduction
Intracranial aneurysms affect 2–4% of the population.1–3 Recent advancements in intracranial vessel wall (IVW) MRI have improved our ability to characterize intracranial vascular diseases4–6 including aneurysms, particularly with the use of 3D Variable Refocusing Flip Angle (VRFA) techniques.7,8 Vasa vasorum, not normally present in intracranial arteries, is thought to develop in larger aneurysms, which may leak, resulting in inflammation, aneurysm enlargement, and wall thinning. It has been suggested that enhancement of the vessel wall is due to the presence of vasa vasorum or associated inflammation,9 and prior studies have shown that enhancement is readily detectable on IVW, and seen more frequently in ruptured or vulnerable aneurysms.10–15 Wall thinning may also be an important factor in aneurysm rupture,16 and IVW can qualitatively evaluate aneurysm wall thickness.17,18
Aneurysm rupture is associated with devastating consequences,19–21 but both endovascular and surgical treatments pose their own risks22–24—therefore optimized patient selection is critical. Treatment algorithms for unruptured aneurysms have traditionally focused on size,25 however, natural history studies of unruptured aneurysms have described a 5-year risk of rupture of 4.5% for aneurysms smaller than 10 mm, as well as reporting rupture in 4.1% of patients with aneurysms smaller than 5 mm in a cohort followed for an average of 4.3 years.26 Therefore, with size alone a limited predictor of rupture, this necessitated the development of more complex predictive models such as the PHASES score,27 which incorporates size as well as location and clinical data to stratify risk of rupture. However, this score requires clinical data which are not always available or reliable, and while it makes use of luminal imaging, it does not take into account physiological/morphological changes of the vessel wall. Therefore, in order to determine if aneurysmal wall enhancement and thinning detected on IVW may correlate with clinical predictors of aneurysm vulnerability, we sought to compare these IVW characteristics between unruptured aneurysms at higher risk of rupture against those at lower risk of rupture, as determined by PHASES score.
Methods and materials
Patients
After institutional review board approval, luminal imaging was used to prospectively identify adult patients (>18 years of age) with unruptured, untreated intracranial aneurysms. Patients were then consented and underwent MRI with a 3D multi contrast IVW protocol. Previously ruptured or treated aneurysms were not included in the analysis. Patients were excluded if they had any contraindication to MRI or gadolinium contrast, or if their IVW study was of poor quality.
Magnetic resonance imaging
Scanning was performed on a 3T Philips Ingenia MRI system (Philips Healthcare, Best, the Netherlands) with a 32-channel head coil or Siemens Prisma MRI system (Siemens Healthineers, Erlangen, Germany) using a 64-channel neurovascular coil. Patients underwent a multi contrast variable flip-angle turbo spin-echo three-dimensional (3D) IVW protocol on both Siemens and Philips platforms. VRFA techniques with 3D T 1 sampling perfection with application optimized contrast using different flip angle evolutions [SPACE] were obtained on Siemens and volumetric isotropic turbo spin echo acquisition (VISTA), (0.56 mm3 acquired resolution) was obtained on Philips. The protocol included pre- and post-contrast 3D T 1 SPACE or VISTA, together with MRA and 3D T 122 SPACE or VISTA.28 3D time-of-flight MRA was performed as well as contrast enhanced MRA. Sequence parameters are available in Supplementary Material 1.
Clinical information
Clinical and demographic data were collected from each patient’s electronic medical record including age, gender, race, hypertension (diagnosed as systolic blood pressure over 140, diastolic pressure over 90, or the use of antihypertensive medications), diabetes mellitus, hyperlipidemia, vitamin B12 deficiency, sleep apnea, history of or current tobacco use, illicit drug use and type of drug (if ever indicated in patient’s chart), any prior ruptured intracranial aneurysm with subarachnoid hemorrhage, current aneurysm size and location, and the total number of aneurysms in each patient. For patients with multiple aneurysms, each was analyzed separately, and a PHASES score (Population, Hypertension, Age, Size, Earlier subarachnoid hemorrhage, Site) was calculated for each individual aneurysm.
Image analysis
Two raters underwent training in evaluation of intracranial aneurysms with IVW through an iterative literature and case review process (using five cases not included in the current analysis), with creation of a set of criteria for defining what represented enhancement and wall thinning.
By the criteria of this training, enhancement was defined as exceeding normal arterial wall enhancement, and was required to match the presumed location of the aneurysm wall between pre-contrast IVW, post-contrast IVW, and MRA. For cavernous sinus lesions or lesions adjacent to the cavernous sinus, enhancement was required to clearly differ in signal from cavernous sinus enhancement, with the aneurysm wall clearly delineated from the surrounding environment. Because aneurysm rupture is a focal process, the evaluation of wall thinning concentrated on focal wall thinning rather than global characteristics. Wall thinning was defined as being evident on both T 1 pre- and post-contrast IVW, appearing as the absence or marked thinning of aneurysm wall within a quadrant, tapering of the wall to a region of non-visualization of the wall or significant wall thinning, and without apposition of brain tissue in the region of apparent wall non-visualization. In cases where enhancement or wall thinning extended across quadrants, both quadrants were counted as abnormal.
Raters then independently reviewed IVW sequences of each aneurysm. Aneurysms were rated independently in patients with multiple aneurysms. Raters selected a single slice which best demonstrated aneurysm wall enhancement and wall thinning. Both readers used the same plane of review, which was recorded. The raters evaluated each aneurysm for the presence of wall enhancement above normal wall enhancement (y/n), intensity of enhancement relative to the pituitary infundibulum (0 = none or enhancement equivalent to expected for normal arterial wall, 1 = less than infundibulum, 2 = equal or greater than infundibulum), and qualitative presence of wall thinning (as described above). The circumferential extent of enhancement and wall thinning was also recorded in terms of the number of quadrants involved. Raters were blinded to relevant clinical data and each other’s ratings.
Statistical analysis
For analysis, aneurysms were divided into two groups: those with PHASES score >3 (higher risk for aneurysm rupture), and those with PHASES score ≤3 (lower risk for aneurysm rupture), with this threshold selected based on a prior study.29 Inter-reader agreement was evaluated using unweighted Cohen’s κ for binary variables and linearly weighted Cohen’s κ for ordinal variables. Logistic regression was used to compare each IVW feature individually between the higher and lower risk groups with an adjustment for reader. The generalized estimating equations approach was used to account for the non-independence between multiple aneurysms from the same patient and two ratings per aneurysm. Permutation tests were used to test for associations with IVW features when this could not be done using logistic regression due to very few or no cases in a group. The permutation tests were clustered by patient to account for non-independence between multiple ratings from the same patient. P-values were not adjusted for the number of tests. All statistical calculations were conducted with the statistical computing language R (v. 3.1.1; R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-sided tests were used, with statistical significance defined as p < 0.05.
Results
Patient population
46 patients with 66 aneurysms were enrolled and imaged between April 6, 2016 and August 29, 2017. Nine patients were scanned on a 3T Philips Ingenia MRI system with a VISTA protocol, and 37 patients were scanned on a 3T Siemens Prisma MRI system (Siemens Healthineers; Erlangen, Germany) with a SPACE protocol. 1 patient was excluded due to poor image quality, therefore, 45 patients with a total of 65 aneurysms were included in the analysis. None of the aneurysms demonstrated visible thrombus adherent to the walls. A majority of patients (68.9%) had only a single aneurysm, while 11.1% had three or more aneurysms. There were 26 (40.6%) aneurysms involving the internal carotid artery, 14 (21.9%) involving the middle cerebral artery (MCA), and 24 (37.5%) involving the anterior cerebral artery (ACA), posterior communicating artery (PcoA), or posterior cerebral artery. See Table 1 for additional patient characteristics.
Table 1.
Characteristics of 45 patients with 65 aneurysms
| Variable | Value a | |
| Maximum aneurysm diameter (mm) | 4 (1–28) | |
| Maximum PHASES score | Please remove this box and reduce to 1 entry for maximum phases score | |
| 5 (0–12) | ||
| Male sex | 18 (40.0) | |
| Age, years | 57 (21–83) | |
| Race | White | 35 (83.3) |
| Black | 4 (9.5) | |
| Asian | 3 (7.1) | |
| Hypertension | 31 (68.9) | |
| Hyperlipidemia | 19 (42.2) | |
| Sleep apnea | 8 (17.8) | |
| B12 deficiency | 4 (8.9) | |
| Ever smoker | 26 (57.8) | |
| Illicit drug use | 11 (24.4) | |
| Prior Subarachnoid Hemorrhage | 3 (6.7) | |
| No. of aneurysms | 1 | 31 (68.9) |
| 2 | 9 (20.0) | |
| 3 | 4 (8.9) | |
| 4 | 1 (2.2) |
Values are no. (%) or median (range);
Race was missing or unknown in three patients.
Inter-reader agreement
Inter-reader agreement is summarized in Table 2. Inter reader agreement was moderate-to-good for the presence of enhancement (κ = 0.64), extent of enhancement (κ = 0.64), and intensity of enhancement (κ = 0.60) while agreement was relatively low for wall thinning (κ = 0.25).
Table 2.
Inter-reader agreement on vessel wall MRI features of aneurysm.
| Reader a | Agreement | ||||
| Variable | HW | JS | κ b | (95% CI) | |
| Any enhancement | 17 (26.2) | 23 (35.4) | 0.64 | (0.41, 0.83) | |
| No. of quadrants with enhancement | 0 | 48 (73.8) | 42 (64.6) | 0.64 | (0.41, 0.79) |
| 1 | 3 (4.6) | 5 (7.7) | |||
| 2 | 5 (7.7) | 5 (7.7) | |||
| 3 | 2 (3.1) | 3 (4.6) | |||
| 4 | 7 (10.8) | 10 (15.4) | |||
| Maximum intensity of enhancement | None | 48 (73.8) | 42 (64.6) | 0.60 | (0.37, 0.78) |
| <infundibulum | 10 (15.4) | 10 (15.4) | |||
| ≥infundibulum | 7 (10.8) | 13 (20.0) | |||
| Any appreciable wall thinning | 4 (6.2) | 3 (4.6) | 0.25 | (−0.07, 0.79) | |
CI, confidence interval.
Values are no. (%);
Unweighted Cohen’s κ for binary variables and linearly weighted Cohen’s κ for ordinal variables.
Vessel wall imaging correlation with PHASES score
Association between PHASES scores and aneurysm characteristics are detailed in Table 3. Data from the two raters were pooled for analysis, resulting in n = 130 evaluations of 65 aneurysms. Aneurysms with higher PHASES scores were significantly more likely to demonstrate enhancement [odds ratio (OR) = 4.2, 95% confidence interval (CI) (1.2–14.7), p = 0.022)] (Figure 1). Of those aneurysms with enhancement (n = 40, 30%), the number of quadrants trended toward association with higher PHASES scores (OR = 1.6 per 1-quadrant increase, 95% CI 1.0–2.6, p = 0.063). The intensity of enhancement (when present) was not statistically significantly different between the two groups (p = 0.53). Aneurysms with PHASES >3 were more likely to demonstrate wall thinning (9.2% vs 0%, p = 0.044) (Figure 2).
Table 3.
Univariate analysis of associations between the PHASES score and vessel wall MRI features of aneurysms using 130 ratings of 65 aneurysm by two readers
| PHASES score a | Univariate model b | ||||||
| Variable | Group | No. |
0–
3
(N = 54) |
>3
(N = 76) |
OR | (95% CI) | p-value |
| Any enhancement | All | 130 | 8 (14.8) | 32 (42.1) | 4.2 | (1.2, 14.7) | 0.022 |
| No. of quadrants with enhancement (0–4), per 1-quadrant increase | All | 130 | 0.3 | 1.2 | 1.7 | (1.1, 2.7) | 0.020 |
| With enhancement | 40 | 2.2 | 2.9 | 1.6 | (1.0, 2.6) | 0.063 | |
| Maximum intensity of enhancement (0–2), per 1-level increase | All | 130 | 0.2 | 0.6 | 2.6 | (1.0, 6.9) | 0.050 |
| With enhancement | 40 | 1.4 | 1.5 | 2.0 | (0.2, 17.6) | 0.53 | |
| Any appreciable wall thinning | All | 130 | 0 (0.0) | 7 (9.2) | ∞ | - | 0.044 |
CI, confidence interval; OR, odds ratio.
Values are no. (%) or mean;
Logistic regression model containing a single IVW feature for predicting PHASES score, adjusted for reader.
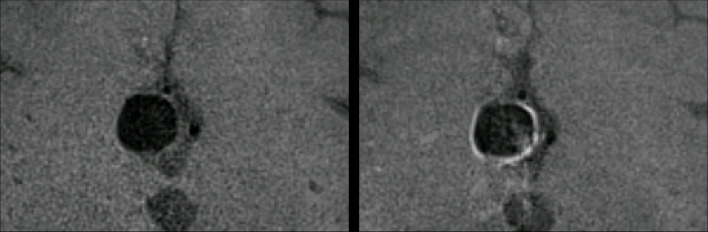
Figure 1.
Demonstrates a 12 mm anterior communicating artery aneurysm on pre- and post-contrast 3D T 1 SPACE sequences. The post-contrast image (right) demonstrates enhancement in all four quadrants of the aneurysm wall. PHASES score for this aneurysm was 12. 3D, three-dimensional.
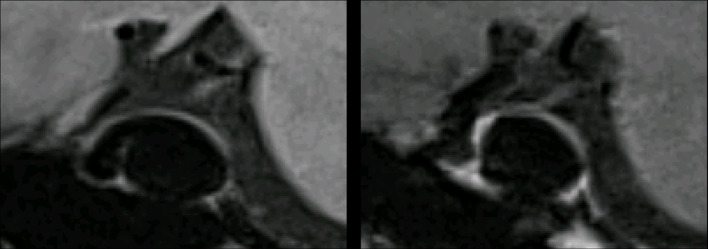
Figure 2.
Demonstrates an 18 mm paraclinoid segment right internal carotid artery aneurysm on pre- and post-contrast 3D T 1 SPACE sequences. On pre- and post-contrast images there is thinning of the posterior wall. PHASES score for this aneurysm was 7. 3D, three-dimensional.
Wall thinning was more common in aneurysms with enhancement (12.5% vs 2.2%, p = 0.042), though thinning was not significantly associated with the extent of enhancement (p = 0.62). Of the 38 aneurysms (58.5%) with PHASES >3, 16 had maximum size ≥7 mm (≥3 points in PHASES), 24 were located in the ACA/PcoA/posterior circulation (four points in PHASES) and 5 had both risk factors. Only three had other risk factors that combined exceeded three points. Aneurysms ≥ 7 mm were more likely to demonstrate wall enhancement (75.0% vs 16.3%, p < 0.001) and wall thinning (21.9% vs 0.0%, p < 0.001) than aneurysms < 7 mm. However, aneurysms in the ACA/PcoA/posterior circulation had similar rates of enhancement (29.2% vs 31.7%, p = 0.85) and wall thinning (6.1% vs 4.2%, p = 0.72) compared to aneurysms in other locations. Rates of wall enhancement and thinning did not differ significantly between the other risk factor groups comprising PHASES (Supplementary Material 1).
Overall, 42.1% of higher risk aneurysms and 14.8% of lower risk aneurysms demonstrated any enhancement, resulting in sensitivity of 42.1% and specificity of 85.2%. Wall thinning was only seen in aneurysms with PHASES score greater than 3.
Discussion
In this study we prospectively compared IVW detected wall enhancement and wall thinning between unruptured intracranial aneurysms at high- and low-risk for future rupture. We found that the presence of wall enhancement, more diffuse wall enhancement, and wall thinning were more likely to occur in aneurysms at higher clinical risk of rupture based on PHASES score. In addition, we also found a significant relationship between wall thinning and the presence of aneurysm wall enhancement. This suggests that these features, if present, may help predict the risk of future aneurysm rupture, and aid in deciding which aneurysms should undergo intervention vs observation.
Multiple studies have attempted to predict intracranial aneurysm rupture risk. In 2014, Greving et al27 incorporated data from multiple prospective cohort studies to develop the PHASES score as a model to predict aneurysm rupture. This score integrates ethnicity (with Finnish and Japanese populations at higher risk), the presence of hypertension, age over 70 years, aneurysm size, prior aneurysmal subarachnoid hemorrhage, and site of aneurysm (with MCA and ACA/Posterior communicating/posterior locations at higher risk). For patients who score 12 points or higher, the 5-year risk of aneurysm rupture is greater than 15%. Subsequent studies have validated the PHASES score, determining that higher scores are associated with aneurysm growth30 and worse outcomes when aneurysms rupture.31
While luminal imaging contributes to the PHASES score, multiple studies have suggested that IVW may allow more direct evaluation of pathophysiological predictors of vulnerability, because ruptured, growing, or symptomatic aneurysms are more likely to demonstrate enhancement. Edjlali et al11 examined 307 unruptured aneurysms with IVW, categorizing wall enhancement as none, focal, thin, or thick. They found that unstable aneurysms (those which were ruptured, symptomatic or changing) were more likely to show wall enhancement, with thick wall enhancement providing the greatest specificity. Texakalidis et al14 performed a meta-analysis of 6 studies comprising 505 ruptured and unruptured intracranial aneurysms and found that wall enhancement was associated with higher odds of instability and a lack of wall enhancement may predict aneurysm stability. Backes et al10 examined gadolinium enhancement in unruptured intracranial aneurysms using IVW techniques, and reported that 26/89 aneurysms demonstrated wall enhancement, and that size was the strongest predictor of enhancement.
Our study showed similar findings and expanded on this work. Our study differs from the above studies in that we only evaluated unruptured aneurysms and compared high- and low-risk aneurysms based on PHASES scores. Inclusion of ruptured and unruptured aneurysms in the vulnerable/unstable category presents difficulties, as the presence of enhancement in ruptured aneurysms may be the result of inflammation or vasa vasorum that led to rupture, or inflammation secondary to rupture.
Prior studies have also suggested that wall thinning may be an important factor in aneurysm rupture. Cebral et al16 examined the wall shear stress distribution of nine ruptured aneurysms using computational fluid dynamics simulations. They tested models with varied wall thickness and stiffness, and ultimately found that wall thinning and stiffness in areas of high wall shear stress correlated with known rupture sites. Tenjin et al18 evaluated wall thickness of 37 unruptured aneurysms and graded the wall as either thinned (and therefore invisible or almost invisible) or normal, then compared to surgical findings. In 78% of cases the MRI findings correctly matched the surgical findings, demonstrating that high resolution MRI could be employed to identify wall thinning.
Our study differs in that we used PHASES scores to identify which aneurysms were at higher clinical risk of rupture, and subsequently found that these aneurysms were more likely to demonstrate wall thinning. Because quantitative analysis of wall thinning is below the imaging resolution threshold of IVW,17 we used qualitative assessment of wall thinning, in which the imperceptibly thin wall appears as an imaging gap.
Our study is limited by the use of PHASES scores to identify the future risk of aneurysm rupture, which is an estimate of aneurysm vulnerability and risk of rupture, not a true endpoint, however, other studies have validated the PHASES score.30,31 Multiple factors for aneurysm vulnerability were considered individually such as aneurysm size and location, however, these criteria contribute heavily to PHASES score, and thus are incorporated in terms of overall risk assessment. It could be considered a limitation that two different MRI systems were used, however we strove to create nearly identical protocols with similar sequences and parameters on the two platforms, and the heterogeneity of imaging environment further supports the significance of our results, as they survived any minimal introduced noise. The small sample size prevented us from performing a reliable multivariate analysis, and p-values were not adjusted for the number of tests, so the results should be considered principally hypothesis-generating. Inter-reader agreement was moderate-to-good for enhancement characteristic evaluations. However, evaluating aneurysms using IVW has a steep learning curve, and this is reflected in our relatively limited inter-reader agreement for the presence of wall thinning. In part this could be because wall thinning was only seen in less than 7% of aneurysms, limiting our analysis of this feature. Additionally, the evaluation of qualitative characteristics is inherently expected to result in lower κ values. The training of raters was brief, which may have contributed to limited agreement, however both raters have extensive experience in IVW review and are expert general reviewers. While these early results are not sufficient to conclude that IVW adds value to the PHASES score, future multicenter, prospective trials correlating pathology and flow simulations with IVW findings would be beneficial to confirm vulnerable features that correlate with imaging findings.
Conclusions
Unruptured intracranial aneurysms at higher risk of rupture based on PHASES score are more likely to demonstrate wall enhancement, more diffuse wall enhancement, and wall thinning on IVW. Future studies would be helpful to determine if these IVW findings may help supplement or supplant PHASES scores in the pretreatment evaluation of unruptured intracranial aneurysms.
Footnotes
Funding: The authors report no conflicts of interest. This work was supported by the Philips Pilot Project Program and National Institutes of Health grant [grant R01NS092207 01A1]. The authors have no additional disclosures. Presented at ASNR 2018, Vancouver, British Columbia, under the title, “Intracranial Aneurysms at Higher Clinical Risk for Rupture Demonstrate Increased Wall Enhancement on Vessel Wall MRI.”
Contributor Information
Jason Brett Hartman, Email: jhrtmn@uw.edu.
Hiroko Watase, Email: hiroko7@uw.edu.
Jie Sun, Email: sunjie@uw.edu.
Daniel S Hippe, Email: dhippe@uw.edu.
Louis Kim, Email: ljkim1@neurosurgery.washington.edu.
Michael Levitt, Email: mlevitt@neurosurgery.washington.edu.
Laligam Sekhar, Email: lsekhar@neurosurgery.washington.edu.
Niranjan Balu, Email: ninja@uw.edu.
Thomas Hatsukami, Email: tomhat@uw.edu.
Chun Yuan, Email: cyuan@uw.edu.
Mahmud Mossa-Basha, Email: mmossab@uw.edu.
REFERENCES
- 1. Etminan N , Rinkel GJ . Unruptured intracranial aneurysms: development, rupture and preventive management . Nat Rev Neurol 2016. ; 12 : 699 – 713 . doi: 10.1038/nrneurol.2016.150 [DOI] [PubMed] [Google Scholar]
- 2. Rinkel GJ , Djibuti M , Algra A , van Gijn J . Prevalence and risk of rupture of intracranial aneurysms: a systematic review . Stroke 1998. ; 29 : 251 – 6 . [DOI] [PubMed] [Google Scholar]
- 3. Vlak MH , Algra A , Brandenburg R , Rinkel GJ . Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis . Lancet Neurol 2011. ; 10 : 626 – 36 . doi: 10.1016/S1474-4422(11)70109-0 [DOI] [PubMed] [Google Scholar]
- 4. de Havenon A , Yuan C , Tirschwell D , Hatsukami T , Anzai Y , Becker K , et al. . Nonstenotic culprit plaque: the utility of high-resolution vessel wall MRI of intracranial vessels after ischemic stroke . Case Reports in Radiology 2015. ; 2015 : 1 – 4 . doi: 10.1155/2015/356582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mossa-Basha M , de Havenon A , Becker KJ , Hallam DK , Levitt MR , Cohen WA , et al. . Added value of vessel wall magnetic resonance imaging in the differentiation of moyamoya vasculopathies in a non-Asian cohort . Stroke 2016. ; 47 : 1782 – 8 . doi: 10.1161/STROKEAHA.116.013320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mossa-Basha M , Shibata DK , Hallam DK , de Havenon A , Hippe DS , Becker KJ , et al. . Added value of vessel wall magnetic resonance imaging for differentiation of Nonocclusive intracranial vasculopathies . Stroke 2017. ; 48 : 3026 – 33 . doi: 10.1161/STROKEAHA.117.018227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander MD , Yuan C , Rutman A , Tirschwell DL , Palagallo G , Gandhi D , et al. . High-resolution intracranial vessel wall imaging: imaging beyond the lumen . J Neurol Neurosurg Psychiatry 2016. ; 87 : 589 – 97 . doi: 10.1136/jnnp-2015-312020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qiao Y , Steinman DA , Qin Q , Etesami M , Schär M , Astor BC , et al. . Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla . J Magn Reson Imaging 2011. ; 34 : 22 – 30 . doi: 10.1002/jmri.22592 [DOI] [PubMed] [Google Scholar]
- 9. Portanova A , Hakakian N , Mikulis DJ , Virmani R , Abdalla WM , Wasserman BA . Intracranial vasa vasorum: insights and implications for imaging . Radiology 2013. ; 267 : 667 – 79 . doi: 10.1148/radiol.13112310 [DOI] [PubMed] [Google Scholar]
- 10. Backes D , Hendrikse J , van der Schaaf I , Algra A , Lindgren AE , Verweij BH , et al. . Determinants of Gadolinium-Enhancement of the aneurysm wall in unruptured intracranial aneurysms . Neurosurgery 2018. ; 83 : 719 – 25 . doi: 10.1093/neuros/nyx487 [DOI] [PubMed] [Google Scholar]
- 11. Edjlali M , Guédon A , Ben Hassen W , Boulouis G , Benzakoun J , Rodriguez-Régent C , et al. . Circumferential thick enhancement at vessel wall MRI has high specificity for intracranial aneurysm instability . Radiology 2018. ; 289 : 181 – 7 . doi: 10.1148/radiol.2018172879 [DOI] [PubMed] [Google Scholar]
- 12. Hu P , Yang Q , Wang DD , Guan SC , Zhang HQ . Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm . Neuroradiology 2016. ; 58 : 979 – 85 . doi: 10.1007/s00234-016-1729-3 [DOI] [PubMed] [Google Scholar]
- 13. Liu P , Qi H , Liu A , Lv X , Jiang Y , Zhao X , et al. . Relationship between aneurysm wall enhancement and conventional risk factors in patients with unruptured intracranial aneurysms: a black-blood MRI study . Interv Neuroradiol 2016. ; 22 : 501 – 5 . doi: 10.1177/1591019916653252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Texakalidis P , Hilditch CA , Lehman V , Lanzino G , Pereira VM , Brinjikji W . Vessel wall imaging of intracranial aneurysms: systematic review and meta-analysis . World Neurosurg 2018. ; 117 : 453 – 8 . doi: 10.1016/j.wneu.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 15. Wang GX , Wen L , Lei S , Ran Q , Yin JB , Gong ZL , et al. . Wall enhancement ratio and partial wall enhancement on MRI associated with the rupture of intracranial aneurysms . J Neurointerv Surg 2018. ; 10 : 566 – 70 . doi: 10.1136/neurintsurg-2017-013308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cebral JR , Vazquez M , Sforza DM , Houzeaux G , Tateshima S , Scrivano E , et al. . Analysis of hemodynamics and wall mechanics at sites of cerebral aneurysm rupture . J Neurointerv Surg 2015. ; 7 : 530 – 6 . doi: 10.1136/neurintsurg-2014-011247 [DOI] [PubMed] [Google Scholar]
- 17. Sherif C , Kleinpeter G , Loyoddin M , Mach G , Plasenzotti R , Haider T . Aneurysm wall thickness measurements of experimental aneurysms: in vivo high-field MR imaging versus direct microscopy . Acta neurochirurgica Supplement 2015. ; 120 : 17 – 20 . [DOI] [PubMed] [Google Scholar]
- 18. Tenjin H , Tanigawa S , Takadou M , Ogawa T , Mandai A , Nanto M , et al. . Relationship between preoperative magnetic resonance imaging and surgical findings: aneurysm wall thickness on high-resolution T1-weighted imaging and contact with surrounding tissue on steady-state free precession imaging . Neurol Med Chir 2013. ; 53 : 336 – 42 . doi: 10.2176/nmc.53.336 [DOI] [PubMed] [Google Scholar]
- 19. Broderick JP , Brott TG , Duldner JE , Tomsick T , Leach A . Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage . Stroke 1994. ; 25 : 1342 – 7 . doi: 10.1161/01.STR.25.7.1342 [DOI] [PubMed] [Google Scholar]
- 20. Tidswell P , Dias PS , Sagar HJ , Mayes AR , Battersby RDE . Cognitive outcome after aneurysm rupture: relationship to aneurysm site and perioperative complications . Neurology 1995. ; 45 : 876 – 82 . doi: 10.1212/WNL.45.5.876 [DOI] [PubMed] [Google Scholar]
- 21. van Gijn J , Rinkel GJ , diagnosis Subarachnoid haemorrhage . Causes and management . Brain : a journal of neurology 2001. ; 124 ( Pt 2 ): 249 – 78 . [DOI] [PubMed] [Google Scholar]
- 22. Alshekhlee A , Mehta S , Edgell RC , Vora N , Feen E , Mohammadi A , et al. . Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm . Stroke 2010. ; 41 : 1471 – 6 . doi: 10.1161/STROKEAHA.110.580647 [DOI] [PubMed] [Google Scholar]
- 23. Kotowski M , Naggara O , Darsaut TE , Nolet S , Gevry G , Kouznetsov E , et al. . Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis of the literature from 1990 to 2011 . J Neurol Neurosurg Psychiatry 2013. ; 84 : 42 – 8 . doi: 10.1136/jnnp-2011-302068 [DOI] [PubMed] [Google Scholar]
- 24. Smith TR , Cote DJ , Dasenbrock HH , Hamade YJ , Zammar SG , El Tecle NE , et al. . Comparison of the efficacy and safety of endovascular coiling versus microsurgical clipping for unruptured middle cerebral artery aneurysms: a systematic review and meta-analysis . World Neurosurg 2015. ; 84 : 942 – 53 . doi: 10.1016/j.wneu.2015.05.073 [DOI] [PubMed] [Google Scholar]
- 25. Brown RD , Broderick JP . Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening . Lancet Neurol 2014. ; 13 : 393 – 404 . doi: 10.1016/S1474-4422(14)70015-8 [DOI] [PubMed] [Google Scholar]
- 26. Tsutsumi K , Ueki K , Morita A , Kirino T . Risk of rupture from incidental cerebral aneurysms . Journal of Neurosurgery 2000. ; 340 : 550 – 3 . doi: 10.3171/jns.2000.93.4.0550 [DOI] [PubMed] [Google Scholar]
- 27. Greving JP , Wermer MJH , Brown RD , Morita A , Juvela S , Yonekura M , et al. . Development of the phases score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies . Lancet Neurol 2014. ; 13 : 59 – 66 . doi: 10.1016/S1474-4422(13)70263-1 [DOI] [PubMed] [Google Scholar]
- 28. Fan Z , Yang Q , Deng Z , Li Y , Bi X , Song S , et al. . Whole-brain intracranial vessel wall imaging at 3 tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo . Magn Reson Med 2017. ; 77 : 1142 – 50 . doi: 10.1002/mrm.26201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bijlenga P , Gondar R , Schilling S , Morel S , Hirsch S , Cuony J , et al. . Phases score for the management of intracranial aneurysm: a cross-sectional population-based retrospective study . Stroke 2017. ; 48 : 2105 – 12 . doi: 10.1161/STROKEAHA.117.017391 [DOI] [PubMed] [Google Scholar]
- 30. Brinjikji W , Pereira VM , Khumtong R , Kostensky A , Tymianski M , Krings T , et al. . Phases and ELAPSS scores are associated with aneurysm growth: a study of 431 unruptured intracranial aneurysms . World Neurosurg 2018. ; 114 : e425 – 32 . doi: 10.1016/j.wneu.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 31. Foreman PM , Hendrix P , Harrigan MR , Fisher WS , Vyas NA , Lipsky RH , et al. . Phases score applied to a prospective cohort of aneurysmal subarachnoid hemorrhage patients . J Clin Neurosci 2018. ; 53 : 69 – 73 . doi: 10.1016/j.jocn.2018.04.014 [DOI] [PubMed] [Google Scholar]