Abstract
Context:
Type-2 diabetes mellitus (DM2) requires an adequate glycemic control to avoid diabetic complications. The best parameter available is glycosylated hemoglobin (HbA1c), as it gives us an overview of an individuals’ glycemic control of the previous 4 months. Salivary biomarkers used as a diagnostic tool can indicate the control or degree of progression of diseases. Studies indicate that salivary alpha-2-macroglobulin (A2MG) levels are elevated in diabetes patients.
Aims:
To study the relationship of salivary A2MG with glycosylated Hba1c among patients with DM2.
Settings and Design:
A total of 87 patients of DM2, age 35–65 years were recruited.
Materials and Methods:
The routine oral cavity examination and dental check-up was done to rule out any dental disease. The patients with hepatic diseases and inflammatory diseases of oral cavity and body were excluded. The values of HbA1c were collected from the records of patients. Salivary A2MG levels were estimated by enzyme-linked immunosorbent assay. Levels of fasting and postprandial blood sugar, serum creatinine, and A2MG were compared with the HbA1c groups (<7 and ≥ 7).
Statistical Analysis Used:
Descriptive statistics (Software SPSS version 20.0). Nonparametric Pearson correlation test was used to assess the correlation between HbA1c and A2MG.
Results:
A positive correlation between salivary levels of A2MG and blood levels of HbA1c in blood was observed in this study. Results showed that there was also a significant correlation in mean values of fasting and postprandial blood sugar, serum creatinine, and salivary A2MG in diabetic subjects.
Conclusion:
Measurement of A2MG in saliva represents a promising noninvasive alternative method to evaluate glycemic index and consequently avoiding comorbidities.
Keywords: Alpha 2 macroglobulin, diabetes mellitus, salivary biomarker
INTRODUCTION
Diabetes mellitus is the third leading cause of death and due to limited access to the quality health care, the diabetic patients are more prone to develop complications. Screening requires measurement of blood sugar levels. The best parameter is glycosylated hemoglobin (HbA1c), which gives an overview of an individual's glycemic control of previous 4 months. However, this procedure is invasive and requires blood sample.[1,2] The noninvasive method of estimation of diabetic biomarker in salivary samples is required. Saliva is a source of the alpha-2-macroglobulin (A2MG) other than blood. In type 1 and type 2 diabetes mellitus (DM), the A2MG levels are increased in blood.[3] A2MG synthesis is enhanced in diabetic patients. A2MG is produced by liver and acts as a plasma antiproteinase. It traps and inhibits proteolytic enzymes which participate in inflammation and homeostasis.[4] The high serum A2MG decreases the bioavailability of insulin, leading to impairment of blood sugar control.[5,6] Saliva as a source of the A2MG biomarker and its association with glycemic control in DM2 will be useful especially in rural India where expertise of blood sample collection and its proper transport is not available.
MATERIALS AND METHODS
The study was initiated after obtaining ethical clearance. This cross-sectional was study done in 2 months’ duration from August to October 2018. A total of 87 patients of DM2 between 35 and 65 years after dental check-up were taken. The patients with chronic liver disease, history of chronic alcohol consumption, pre-existing hepatobiliary disease, acute disease affecting hepatic functions within past 6 months and inflammatory conditions like acute pancreatitis, inflammatory bowel disease, inflammatory conditions of oral cavity like periodontitis, chronic kidney disease (CKD) of stage IV and V, severe anemia, autoimmune diseases/collagen vascular disorder involving oral cavity were excluded.
Levels of HbA1c were measured using High Performance Liquid Chromatography (HPLC) method on Bio-Rad D-10. Detailed information about the collection procedure of saliva was given to the participants. They had to wash their mouths with tap water and spit two to three times, after which they had to spit saliva pooled in their mouths for the following 5 min into the sterile container. The sample containers were centrifuged at 3800 rpm for 10 min at 4°C, and then the saliva supernatant was collected and stored at −80°C. A2MG levels in saliva were estimated using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's (Biocodon Technologies, Kansas, USA) instructions. The kit used was human alpha-2 macroglobulin ELISA Kit. Samples and standard protein were added on plates, previously coated with a capture antibody A2MG and incubated for 1 hour. Plates were then washed five times. Then chromogen reagents were added. Following 10 min of incubation, the reaction was stopped using stop solution and plates were read at 450 nm. Levels of A2MG were calculated from the optical density obtained from ELISA readings. The values of HbA1c were collected from the records of patients who underwent routine check-up in last 3 months.
Statistical analysis
Descriptive statistics (Software SPSS version 20.0) of saliva levels of A2MG and HbA1c was analyzed in terms of mean with standard deviation (SD). Pearson correlation coefficient was used to find the relationship between saliva levels of A2MG and HbA1c and its significance using t-test. Quantitative variables were expressed as mean ± SD. Nonparametric Pearson correlation test was used to assess the correlation between HbA1c and A2MG.
RESULTS
The study population consisted of 87 patients of type 2 diabetes, females-- 44 (50.6%) and males −43 (49.4%), mean age = 52.4 ± 8.1 years (range 35–65), 34 (39%) had HbA1c level lower than 7 and considered adequate glycemic control, while 53 (60.9%) had HbA1c level more than 7 and hence were considered inadequate glycemic control [Table 1]. There was no difference in male and female population (P = 0.915). There was no difference in age group (52.43 ± 9.28 and 52.48 ± 7.33) between two HbA1c groups (<7, ≥7, P = 0.978). There was no gender bias between two HbA1c groups (P = 0.315). Furthermore, we compared fasting blood glucose (FBS) levels, postprandial blood glucose (PPBS) levels, creatinine, A2MG with the HbA1c groups, results showed that there was significant difference in mean values of fasting blood sugars (132.37 ± 25.58 and 235.44 ± 77.16, P < 0.001), Postprandial blood sugar levels (172.83 ± 39.96 and 290 ± 96.13, P = 0.001), creatinine (1.29 ± 0.52 and 1.84 ± 0.43, P < 0.001) and A2MG (772.54 ± 118.32 and 2017.42 ± 575.13, P < 0.001) [Table 1, Figures 1 and 2].
Table 1.
Distribution of demographic and clinical values between HbA1c groups
| Parameters | Number of patients | HbA1c | Mean | SD | T | P |
|---|---|---|---|---|---|---|
| Age (years) | 35 | <7 | 52.43 | 9.284 | 0.029 | 0.978 |
| 52 | ≥7 | 52.48 | 7.328 | |||
| FBS (mg/dL) | 35 | <7 | 132.37 | 25.583 | 7.61 | <0.001 |
| 52 | ≥7 | 235.44 | 77.160 | |||
| PPBS (mg/dL) | 35 | <7 | 172.83 | 39.955 | 6.85 | <0.001 |
| 52 | ≥7 | 290.58 | 96.126 | |||
| Serum creatinine (mg/dL) | 35 | <7 | 1.286 | 0.5148 | 5.38 | <0.001 |
| 52 | ≥7 | 1.836 | 0.4338 | |||
| A2MG (ng/mL) | 35 | <7 | 772.54 | 118.324 | 12.60 | <0.001 |
| 52 | ≥7 | 2017.42 | 575.133 |
Independent samples t test used, P<0.05 significant
Figure 1.
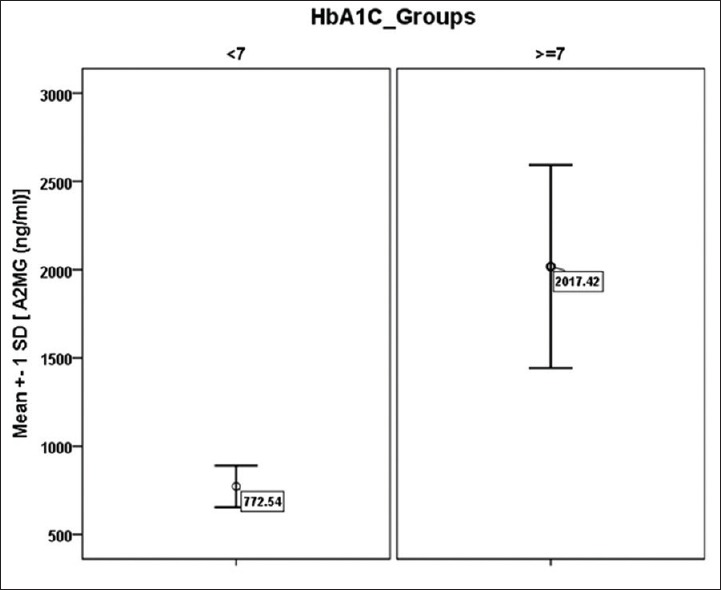
Distribution of A2MG values of the study patients in HbA1c groups
Figure 2.
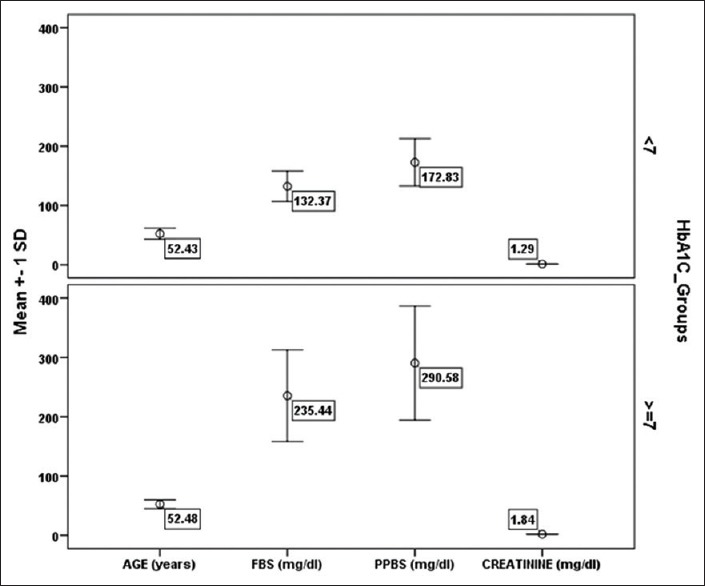
Distribution of Demographic and clinical Values of the study patients in HbA1c groups
Correlation between saliva levels of A2MG and HbA1c
Using Pearson correlation analysis, we found correlation between saliva levels of A2MG and HbA1c (r = 0.994 and P =0.001) in DM2. HbA1c groups highly correlated with A2MG. Similarly, Pearson correlation coefficient was calculated for HbA1c and A2MG [Figure 1]. It showed that there was good linear correlation between HbA1c and A2MG (r = 0.977, P < 0.001) r = correlation, R2 = regression prediction capacity; coefficient of determination R2 = 0.955 = 95.5% [Figure 3].
Figure 3.
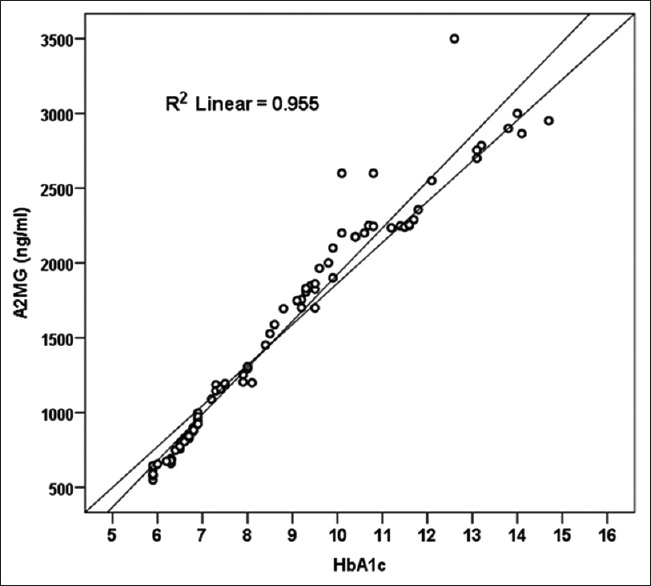
Scatter plot showing linear relationship between HbA1c and A2MG with coefficient of determination of the HbA1c to predict the A2MG
When we used HbA1c to project the A2MG value, our model of accuracy is 95.5% (R2) A2MG is dependent variable (outcome variable), while HbA1c is working as a predictor (independent variable) of A2MG. There is significant correlation in other variables (FBS, PPBS, and serum creatinine), as well as with A2MG with HbA1c. Similar correlation was obtained when analysis was done with A2MG and other variables [Table 2, Figure 2].
Table 2.
Correlation between HbA1C, A2MG and other variables
| Variables | Variables | Age | FBS | PPBS | Serum creatinine | A2MG |
|---|---|---|---|---|---|---|
| Correlation (r) | HbA1C | 0.063 | 0.679 | 0.653 | 0.642 | 0.977 |
| P value | 0.561 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Correlation (r) | A2MG | 0.060 | 0.660 | 0.620 | 0.644 | -- |
| P value | 0.560 | <0.001 | <0.001 | <0.001 | -- |
Pearson correlation coefficient (r) calculated, P<0.05 significant
DISCUSSION
In our study, we have used A2MG as marker to evaluate glycemic control in diabetics. Aitken et al.[5] in their study showed higher levels of salivary A2MG in diabetic patients between the ages of 31–79 years (median = 61.6) in Western population; in our study, we had similar age group of 35–65 years and gender distribution, though, we had a different ethnic group which correlates to South Asian population.
Takada et al.[7] and Soman et al.[8] have shown that increased levels of A2MG in blood of diabetic patients. There is sufficient evidence establishing that the majority of compounds found in blood may also be found in saliva.[9] Saliva contains many serum-derived proteins in addition to secretions from major and minor salivary glands. A2MG could be transferred by exocytosis from blood to saliva when this protein is present at high levels in plasma.[10]
In this study, we have shown a strong association between glycemic index and saliva levels of A2MG in DM2. These results indicate that A2MG can be used as a diagnostic method for diabetes with high specificity. Our results are in line with other studies, which detected higher concentrations of A2MG in blood and saliva of prediabetic state subjects compared to healthy controls;[11,12,13,14] however, we have taken only DM2 patients, who already on treatment.
Periodontitis is also related with increased saliva levels of A2MG and the study by Ertugrul et al.[15] has described that A2MG levels in gingival crevicular fluid are significantly higher in patients with aggressive periodontitis. In our study, we have excluded inflammatory conditions in oral cavity in primary screening to rule out rise of A2MG in inflammatory conditions. Our findings indicate a positive correlation between levels of A2MG in saliva and HbA1c, showing a possible complementary strategy for diabetes screening using saliva. Salivary A2MG is good marker that correlates strongly with HbA1c. Aitken et al.[5] showed correlation between saliva levels of A2MG and HbA1c percentage (r = 0.774) where our study showed even a higher correlation value (r = 0.955). Our data further confirm that patients with DM2, salivary A2MG are good marker to guide us about their control level as compared to HbA1c.
Hyperglycemia is the immediate metabolic consequence of diabetes mellitus. Uncontrolled diabetes mellitus has wide range of complications like diabetic neuropathy, retinopathy, nephropathy, and cardiovascular diseases. Diabetic nephropathy is the most common clinical condition of the diabetic patients with progressive deterioration of renal tissues. In nephropathy, the biomarkers as serum creatinine are known to be raised, correlating with severity of kidney damage. Studies have indicated that A2MG is elevated in the blood of patients with type 1 and DM2, particularly in those with other complications due to diabetes, such as chronic renal failure or nephrotic syndrome,[11,12] though in our study we have excluded stages IV–V patients of CKD. Serum creatinine, fasting plasma glucose, and postprandial plasma glucose are biomarkers of diabetes mellitus.[16,17] Our study also showed that there was increase in fasting and postprandial glucose and serum creatinine in diabetic patients. These variables are well in correlation with increase in HbA1C and as well as with salivary A2MG.
CONCLUSION
Glycemic index control in DM2 has been shown to reduce microvascular disease and improve quality of life. The screening techniques to evaluate glycemic index in diabetes using HbA1c have been well established. Our findings indicate a positive correlation between levels of A2MG in saliva and HbA1c in blood, so may be used as a complementary strategy for diabetes screening using saliva. The measurement of A2MG in saliva represents a noninvasive promising alternative method to evaluate glycemic index and consequently avoiding comorbidities of diabetes mellitus. Findings of our study are limited because it represents only a one center study with a single measurement and small sample size with short duration work. To solve this issue, a future research with a larger sample size and longer study duration is required to determine the value of salivary A2MG for evaluating diabetes control and complications related to diabetes.
Financial support and sponsorship
ICMR Short Term Studentship Funding.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P, Xu X, et al. Saliva in the diagnosis of diseases. Int J Oral Sci. 2016;8:133–7. doi: 10.1038/ijos.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naing C, Mak JW. Salivary glucose in monitoring glycaemia in patients with type 1 diabetes mellitus: A systematic review. J Diabetes Metab Disord. 2017;16:2–9. doi: 10.1186/s40200-017-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamoto I, Morimoto K, Takeshita T, Tada M. Correlation between saliva glycated and blood glycated proteins. Environ Health Prev Med. 2003;8:95–9. doi: 10.1007/BF02897922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao PV, Laurie A, Bean ES, Roberts CT, Nagalla SR. Salivary protein glycosylation as a noninvasive biomarker for assessment of glycemia. J Diabetes Sci Technol. 2015;9:97–104. doi: 10.1177/1932296814554414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitken JP, Ortiz C, Morales-Bozo I, Rojas-Alcayaga G, Baeza M, Beltran C, et al. α-2-Macroglobulin in saliva is associated with glycemic control in patients with type 2 diabetes mellitus? Dis Markers. 2015;2015:128653. doi: 10.1155/2015/128653. doi: 10.1155/2015/128653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sathyapriya S, Bharani GO, Nagalingam M, Jayanthi M, Kanagavalli U. Potential of salivary protein as a biomarker in prognosis of diabetes mellitus. J Pharm Res. 2011;4:2228–9. [Google Scholar]
- 7.Takada T, Kodera Y, Matsubara M, Kawashima Y, Maeda T, Fujita Y, et al. Serum monomeric alpha2-macroglobulin as a clinical biomarker in diabetes. Atherosclerosis. 2013;228:270–6. doi: 10.1016/j.atherosclerosis.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Soman S, Manju CS, Rauf AA, Indira M, Rajamanickam C. Role of cardiac isoform of alpha-2 macroglobulin in diabetic myocardium. Mol Cell Biochem. 2011;350:229–35. doi: 10.1007/s11010-010-0702-4. [DOI] [PubMed] [Google Scholar]
- 9.Puy L, Carmen M. The role of saliva in maintaining oral health and as an aid to diagnosis. Medicina Oral Patol Oral Cir Bucal. 2006;11:E449–55. [PubMed] [Google Scholar]
- 10.Wilmarth PA, Riviere MA, Rustvold DL, Lauten JD, Madden TE, David LL. Two-dimensional liquid chromatography study of the human whole saliva proteome. J Proteome Res. 2004;3:1017–23. doi: 10.1021/pr049911o. [DOI] [PubMed] [Google Scholar]
- 11.Rao PV, Reddy AP, Lu X, Dasari S, Krishnaprasad A, Biggs E, et al. Proteomic identification of salivary biomarkers of type-2 diabetes. J Proteome Res. 2009;8:239–45. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 12.Chung T. Association of salivary alpha 2-macroglobulin levels and clinical characteristics in type 2 diabetes. J Diabetes Investig. 2016;7:190–6. doi: 10.1111/jdi.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad J, Singh M, Saleemuddin M. A study of plasma alpha-2-macroglobulin levels in type 2 diabetic subjects with microalbuminuria. J Assoc Physicians India. 2001;49:1062–5. [PubMed] [Google Scholar]
- 14.James K, Merriman J, Gray RS, Duncan LJ, Herd R. Serum α 2-macroglobulin levels in diabetes. J Clin Pathol. 1980;33:163–6. doi: 10.1136/jcp.33.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertugrul AS, Sahin H, Dikilitas A, Alpaslan N, Bozoglan A. Evaluation of beta-2 macroglobulin and alpha-2 macroglobulin levels in patients with different periodontal diseases. Aus Dent J. 2013;58:170–5. doi: 10.1111/adj.12022. [DOI] [PubMed] [Google Scholar]
- 16.Shaker YM, Soliman HA, Ezzat E, Hussein NS, Ashour E, Donia A, et al. Serum and urinary transforming growth factor beta 1 as biochemical markers in diabetic nephropathy patients. Beni-Suef Uni J of Bsc and Appl Sci. 2014;3:16–23. [Google Scholar]
- 17.Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: Present and future. World J Diabetes. 2014;5:763–76. doi: 10.4239/wjd.v5.i6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]


