Abstract
Thyroid dysfunction is associated with elevated cardiovascular risk factors and atherosclerosis. It could be suggested that, hyperthyroidism is related to a higher prevalence of arterial abnormalities. Therefore, evaluating the endothelial dysfunction (ED) related biomarkers seem to be an important issue. It is not clear whether endothelial cells are biologically responsive to thyroid hormones (THs) or how THs induces the production of endothelial cells (EC)-derived proinflammatory mediators. Hence, in this study the effects of thyroxine (T4) on ED and inflammatory related mediators were evaluated. Human umbilical vein endothelial cells was used as endothelial cell model which was treated with concentrations of 50, 100, 200 nmol/L of T4 in various exposure times. In the following, gene and protein expression levels of EC-related markers including intercellular adhesion molecule-1 (ICAM-1), vascular endothelial growth factor (VEGF), and E-selectin were determined using real time polymerase chain reaction (RT-PCR) and western blotting methods. Also, interleukin-6 (IL-6) and tumor necrosis factor (TNF-α) protein levels as proinflammatory cytokines were determined by enzyme linked immunosorbent assay (ELISA) method. Gene and protein expression analysis revealed that T4 treatments up regulated the levels of VEGF, ICAM-1, and E-selectin as ED markers. In addition, T4-treated cells had higher significant levels of IL-6 and TNF-α versus untreated cells in different incubation times. This study proposed the atherosclerotic effects of thyroid hormone. Based on our findings, T4 had strong effects on the gene and protein expression levels of pro-inflammatory, angiogenesis, and ED major mediators associated with atherosclerosis development.
Keywords: E-selectin, ICAM-1, Interleukin-6, Thyroxine, TNF-α, VEGF
INTRODUCTION
Thyroid hormones (THs) exert numerous effects on the cardiovascular system. It has been indicated that thyroid dysfunction is related to cardiovascular diseases, and both hyperthyroidism and hypothyroidism accelerate cardiovascular disorders. Although with the advances in the prevention and treatment of atherosclerosis, this disorder still stands as one of the main causes of morbidity and mortality worldwide (1). THs have been related to several pro-atherogenic condition, but the relationship between thyroid function with atherosclerosis has not been fully determined (2). It could be suggested that, hyperthyroidism is associated with a higher prevalence of arterial abnormalities (3). A low thyroid-stimulating hormone (TSH) level and increased levels of L-thyroxine (T4), triiodothyronine (T3) or both, is recognized as hyperthyroidism. THs activate vascular endothelium and decrease the metabolism of adhesion molecules, which lead to increase of their circulating levels. This theory that vascular endothelium are targets of THs are endorsed by increased endothelial function during hyperthyroidism (4,5). Previous studies revealed that hyperthyroidism elevated the circulating levels of biological mediators, which had major roles in hemostasis, vascular tone regulation, and permeability (3,4). Indeed, vascular endothelial tissues have receptors for THs and respond to changes in the concentrations of circulating THs (6).
In fact, disruption of coronary endothelial function is related to increased risk of cardiovascular disorders (7). Therefore, evaluating the endothelial dysfunction (ED) related biomarkers such as proinflammatory mediators including interleukin-6 (IL-6) and tumor necrosis factor (TNF)-a are notable issues. Studies have shown that the increased circulating levels of IL-6 and TNF-a have main roles in peripheral endothelial dysfunction (7,8,9,10). Definitely, vascular endothelium is responsive in inflammatory reactions, which is engaged in atherosclerosis. In addition, cytokines play an important role in endothelial disruption, which is related to inflammation (11). In this regards, IL-6 as an inflammatory mediator was synthesized by various cells, including endothelial cells (ECs) and also, inflammatory cytokine TNF-a had important function in the impairment of macrovascular and microvascular circulation (11,12). Activation of EC is defined as endothelial expression of cell-surface adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and endothelial leukocyte adhesion molecule E-selectin. This event is characteristically prompted by proinflammatory cytokines such as IL-6 and TNF-a which cause the leukocytes to be attached to the vascular wall (13).
On the other hand, vascular endothelial growth factor (VEGF) is considered as the main mediator in the regulation of vascular growth, vasculature hemostasis (14) and acts as potent mitogen for ECs. In addition, VEGF is related to increased vascular permeability. This angiogenesis related mediator is detectable in many tumors such as thyroid cancerous cells (15). On the other hand, reports have shown the pro-angiogenic role of THs analogues, including T4 (16). However, it is not clear whether endothelial cells are biologically responsive to THs or how THs induce the production of EC-derived proinflammatory mediators. The present study aimed to investigate the impacts of T4 on EDs with insight to atherosclerosis induction related pathways and EC activation.
MATERIALS AND METHODS
Chemicals
L-Thyroxine powder was obtained from Sigma-Aldrich Company (Cat No: T1775, St. Louis, Missouri, USA) and liquidized in ethanol solution. Anti-E-selectin, -ICAM-1, -VEGF, and -β-actin were purchased from Santa Cruz Biotechnology, Inc (California, USA). The RNA Extraction and cDNA synthesis Kits were purchased from GeneAll Company (South Korea). A real time polymerase chain reaction (RT-PCR) master mix was obtained from Ampliqon (Herlev, Denmark). Western blotting materials were purchased from Bio-Rad Laboratories Inc (Hercules, California, USA). TNF-a (BE55181) and IL-6 (BE53061) enzyme linked immunosorbent assay (ELISA) kit was prepared from IBL Company (IBL International GmBH, Hamburg, Germany).
Cell culture and treatments
Human umbilical vein endothelial cells (HUVECs, NCBI code: C554, Pasteur Institute of Iran, Tehran, I.R. Iran) were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in 5% CO2 at 37 °C. For analytical examinations, 70% confluent cells were incubated 24 h before treatment in a serum-free medium, then medium removed and cells washed with PBS. In following, the HUVECs cells were treated with different doses of T4 at 50, 100, and 200 nmol/L for 24 and 48 h, respectively.
ELISA
The effect of T4 on proinflammatory cytokines was evaluated by determining the IL-6 and TNF-a levels using ELISA method. In this regards, 5 × 103 cells/well of HUVECs cells were seeded overnight and then treated with T4 (50, 100, and 200 nmol/L) for 12, 24, and 48 h, respectively. After treatment times, the cell culture plates were centrifuged in 1500 g for 5 min and then the cell supernatant was collected. This was followed by protein detection based on ELISA kit protocols. The absorbance of wells were read using ELISA reader at 450 nm and then quantified.
Real-time PCR
Cells (106 cells/well) were seeded in culture plates and then treated with T4 (50, 100, 200 nmol/L) (17) for 24 and 48 h respectively. In the following, total RNA was extracted according to the manufacturer’s guidelines. Total RNA was reverse transcribed into cDNA using hyperscript reagent kit and RT-PCR was performed. All the quantitative RT-PCR measurements were carried out using a Mic qPCR (Bio Molecular Systems, Australia) with mastermix. The designed Primer pairs for ICAM-1, E-selectin, VEGF, and β-actin are presented in Table 1. Second-strand synthesis and RT-PCR amplification were done for 40 cycles with denaturation at 95 °C for 15 s, annealing at 62 °C for 30 s, and extension at 72 °C for 30 s, with final extension at 72 °C for 5 min after completion of all cycles. The results were measured by the 2−ΔΔCt method (18).
Table 1.
The designed primer pairs for ICAM-1, E-selectin, VEGF, and β-actin for RT-PCR.
| Target gene | Primer Sequence (5′→3′) | Product Size (bp) | |
|---|---|---|---|
| ICAM-1 | Forward | AGACATAGCCCCACCATGAG | 121 |
| Reverse | CAAGGGTTGGGGTCAGTAGA | ||
| E-selectin | Forward | ATGTGAAGCTGTGAGATGCG | 152 |
| Reverse | CCACTGCAGCTCATGTTGAT | ||
| VEGF | Forward | AGGAGGAGGGCAGAATCATC | 90 |
| Reverse | GGCACACAGGATGGCTTGAA | ||
| β-actin | Forward | CTGGAACGGTGAAGGTGACA | 161 |
| Reverse | TGGGGTGGCTTTTAGGATGG | ||
ICAM-1, intercellular adhesion molecule-1; VEGF, vascular endothelial growth factor; RT-PCR, real time polymerase chain reaction.
Western blotting analysis
HUVECs (106 cells/well) were treated with 50, 100, and 200 nmol/L concentration of T4 for 24 and 48 h, respectively, then trypsinized (19) and collected. Radio immuno-precipitation assay lysis buffer containing complete protease inhibitors was added to cell pellet and maintained on ice for 20 min. The mixture was then centrifuged at 12000 g for 15 min at 4 °C. The collected supernatant was used for protein assay according to Bradford method protocol (20). Proteins were separated on SDS polyacrylamide gel (21) and then transferred onto a polyvinylidene fluoride membrane. The membrane was blocked using 5% skimmed milk solution at room temperature followed by 24 h incubation at 4 °C with primary antibodies (anti-ICAM-1, -E-selectin, -VEGF, and -ß-Actin). The membrane was then washed with TBST and incubated with secondary antibody (Abcam, USA) for 3 h, while the polyvinylidene fluoride was washed with TBST three times. The membrane was incubated with enhanced chemiluminescence western blotting detection reagent (GE Healthcare, Boston, MA, USA) and exposed to X-ray film. Protein bonds were quantified using the image J software and any differences in protein expression levels were identified by normalization to the levels of the ß-actin protein.
Statistical analysis
The data were analyzed using Prism software, version 6.1 and were presented as mean ± standard deviation (SD). For multiple comparisons between groups, one-way analysis of variance (ANOVA) was utilized and P values < 0.05 were considered as significant.
RESULTS
Effect of thyroxine on interleukin-6 and tumor necrosis factor
Significant elevated IL-6 levels in T4 treated cases (50, 100, and 200 nmol/L after 12, 24, and 48 h) were obtained when compared with the untreated cells (P < 0.05, 0.01, and 0.001, respectively; Fig. 1). Further analysis showed that there were no significant differences between various T4 treated concentrations in all examined treatment times (P > 0.05).
Fig. 1.
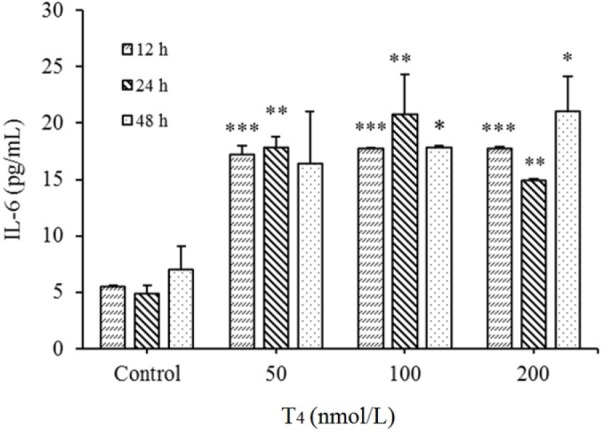
Up regulation of interleukin-6 (IL-6) levels in HUVECs by T4 exposure. After treatments with T4 (50, 100 and 200 nmol/L) for 12, 24, and 48 h, protein expression was evaluated by ELISA method. *P < 0.05, ** P < 0.01, and *** P < 0.001, indicate significant differences in comparison with control group. Data are presented as mean ± SD. Values derived from three independent experiments. T4, thyroxin.
TNF-α levels of T4 treatments (50, 100, and 200 nmol/L) were increased in all studied times compared to the controls (P < 0.001, Fig. 2) whereas no significant differences in the levels of TNF-α between different concentrations of T4 were found (in all examined treatment times) (P > 0.05).
Fig. 2.
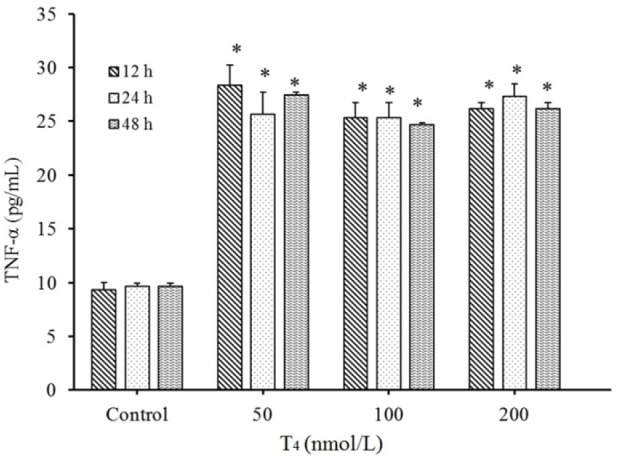
Up regulation of tumor necrosis factor-α (TNF-α) level in HUVECs by T4 exposure. After treatments with T4 (50, 100, and 200 nmol/L) for 12, 24, and 48 h, protein expression evaluated by ELISA method. *P < 0.001 indicates significant differences compared with control group. Data are presented as mean ± SD. Values derived from three independent experiments.
On the other hand, serum IL-6 and TNF-α level increased after T4 treatments in all tested concentrations, but there were no significant differences between various treatment times (P > 0.05).
Effects of thyroxine on the VEGF, ICAM-1, and E-selectin gene expression
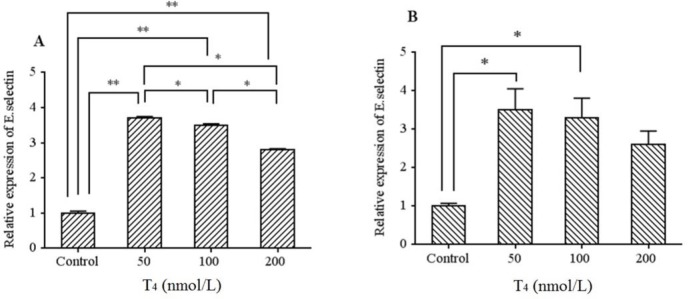
mRNA levels of ICAM-1, E-selectin, and VEGF were detected by RT-PCR and the Ct values of each samples were converted to fold changes using 2-ΔΔCt formula and then compared with the fold changes of the control group. Based on our results, E-selectin mRNA was significantly increased in all the examined concentrations after 24 and 48 h compared to the control cells (except 200 nmol/L). However, as presented in Fig. 3A, the gene expression levels of E-selectin were increased more significantly at lower T4 concentrations. Indeed higher E-selectin levels were observed at lower T4 concentrations in both times.
Fig. 3.
mRNA levels of E-selectin in HUVECs cells treated by T4 (50, 100, and 200 nmol/L) for (A) 24 h and (B) 48 h exposure times. Data presented as mean fold change ± SD. Values derived from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences in comparison with control group.
After 24 and 48 h, ICAM-1 gene expression levels increased in all examined T4 concentrations compared to the control (P< 0.05 and 0.01, respectively) (Fig. 4A and 4B). In addition, the gene expression levels of ICAM-1 had highest levels at 100 nmol/L of T4 compared with other T4 concentrations in both times.
Fig. 4.
mRNA levels of ICAM-1 in HUVECs cells treated by T4 (50, 100, and 200 nmol/L) for (A) 24 h and (B) 48 h exposure times. Data presented as mean fold change ± SD. Values derived from three independent experiments. *P < 0.05, **P < 0.01, and indicate significant differences in comparison with control group. ICAM-1, intercellular adhesion molecule 1.
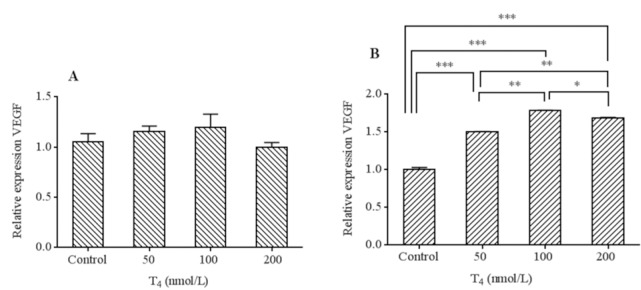
Based on our findings VEGF mRNA levels elevated in all T4 treatment compared to the control group (Fig. 5) which in the cases of 48 h incubation time, the extent of increase was more noticeable and reached significant levels (P < 0.001, Fig. 5B).
Fig. 5.
mRNA levels of VEGF in HUVECs cells treated by T4 (50, 100 and 200 nmol/L) for (A) 24 h and (B) 48 h exposure times. Data presented as mean fold change ± SD. Values derived from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences in comparison with control group. VEGF, Vascular endothelial growth factor.
No significant differences in the levels of VEGF mRNA between different concentrations of T4 after 24 h were found (Fig. 5A). There were significant differences in the levels of VEGF between different concentrations of T4 after 48 h (P < 0.05). Additionally, higher VEGF levels were presented at 100 nmol/L in all exposure times.
Effects of thyroxine on the E-selectin, ICAM-1, and VEGF protein expression
To identify, E-selectin, ICAM-1, and VEGF protein expression levels in HUVECs cells, protein bindings were assessed by western blotting and band intensities were quantified using the Image J software. As presented in Fig. 6A and 6B, E-selectin protein levels increased after 24 and 48 h of treatments (50, 100, and 200 nmol/L of T4) compared to untreated cells. The extent of increase after 24 h was more noticeable than 48 h treatment times. The protein expression level of E-selectin was in accordance with the RT-PCR gene expression results in all selected doses.
Fig. 6.
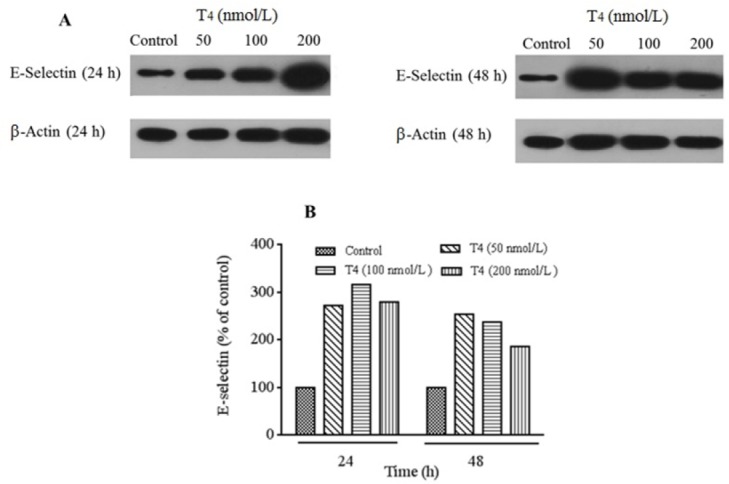
Protein detection for evaluation of E-selectin expression performed using western blotting technique. (A) Protein expression levels of E-selectin in HUVECs cells treated by T4 (50, 100, and 200 nmol/L) for 24 and 48 h and (B) T4 treatment significantly increased relative expression of E-selectin compared with control group. For analysis protein's bands densities, Image J software was used to analyze the densities. Each protein bands normalized to β-actin bands.
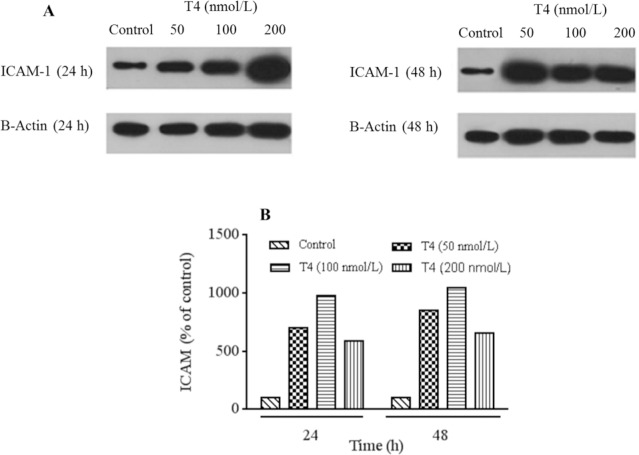
ICAM-1 protein band assessment showed that, this protein levels was increased after 24 and 48 h of treatments in all examined T4 concentrations (Fig. 7A and 7B). Furthermore, T4 at 100 nmol/L exhibited highest impact compared to other tested concentrations. The results of protein expression assay of ICAM-1 were parallel to RT-PCR gene expressions.
Fig. 7.
Protein detection for evaluation of ICAM-1 expression performed using western blotting technique. (A) Protein expression levels of ICAM-1 in HUVECs cells treated by T4 (50, 100, and 200 nmol/L) for 24 and 48 h and (B) T4 treatment significantly increased relative expression of ICAM-1 compared with control group. For analysis protein's bands densities, Image J software was used to analyze the densities. Each protein bands normalized to β-actin bands. ICAM-1, Intercellular adhesion molecule 1.
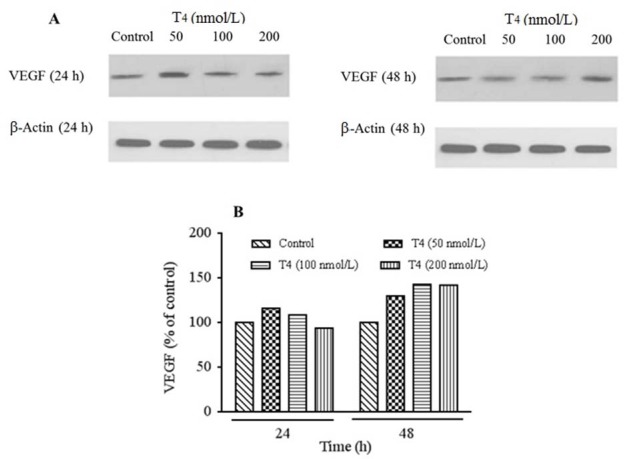
Analysis of western blotting protein bands showed that, VEGF protein levels were elevated over 24 and 48 h of treatment with 50, 100, and 200 nmol/L of T4 compared to the untreated cells (Fig. 8A and 8B). According to our findings, VEGF protein expression at 100 nmol/L had the highest effect compared to the other examined T4 concentrations after 48 h treatment whereas after 24 h exposure, higher protein expression was present in 50 nmol/L of T4. VEGF protein expression was in agreement with RT-PCR gene expressions.
Fig. 8.
Protein detection for evaluation of VEGF expression performed using western blotting technique. (A) Protein expression levels of VEGF in HUVECs cells treated by T4 (50, 100, and 200 nmol/L) for 24 and 48 hand (B) T4 treatment significantly increased relative expression of VEGF compared with control group. For analysis of protein's band densities, Image J software was used to analyze the densities. Each protein bands normalized to β-actin bands. VEGF, Vascular endothelial growth factor
DISCUSSION
It has been reported that THs have noticeable effects on cardiovascular system, so thyroid abnormalities have led to abnormal cardiovascular functions. Indeed, endothelial cells are the possible targets for THs (22,23,24). In the present study we evaluated the possible effects of THs on ED related mediators.
Our findings confirmed the association between T4 treatments and ED in HUVEC. Furthermore, our findings may suggest that T4 administration is associated with ED, which is reflected by increased proinflammatory cytokines (IL-6 and TNF-α) and higher levels of ED markers including VEGF, ICAM-1, and E-selectin. Moreover, we found that T4 treatment could enhance ED in these cellular models, at different treatment times.
However, the impairment of endothelial function could be explained by the increased level of potential ED markers as cardiovascular risk factors, thus it could be suggested that T4 exerted atherogenic activity or acted as a major determinant in vascular homeostasis regulation. In agreement with our results, Popławska-Kita et al. indicated that both subclinical hyperthyroidism and overt hyperthyroidism may be accompanied with ED, including elevated levels of IL-6 (4). In another survey, Burggraaf et al. reported that increased THs was related to increased levels of several endothelium-associated proteins. They did not find any relationships between hyperthyroidism and levels of the inflammation markers IL-6, TNF-a. This discordance in comparison with our results may be related to the fact that Burggraf et al. evaluated the circulating levels of these parameters, while we detected ED mediators in endothelial cell levels (3). In another survey, hyperthyroid rat had elevated circulating levels of TNF-a. These researchers suggested that altered levels of TNF-a resulted from thyroid effects on oxidative stress in rat liver (25). Overall, it could be suggested that inflammatory response against ED is dependent on the functions of IL-6 and enormous proinflammatory cytokines including TNF-α (11). Previous studies reported that ICAM-1 not only acts as leukocyte adhesion molecule, but also participates in inflammatory responses in vascular wall by induction of ECs activation, which result in atherosclerotic plaque developments (26). In this regards, Hara et al. reported that THs increases the expression of E-Selectin from EC in patients with Graves’ disorder, which is comparable with our results (27). Other reports proposed that, ICAM-1 and IL-6 might be considered as major element in the assessment of the inflammatory events in the thyroid gland throughout radioiodine treatments, particularly Graves’ disease (28). Recently, numerous studies evaluated the T4-induced angiogenesis (17). In these ways, VEGF as a noticeable inducer of angiogenesis and important mitogen for EC, activates specific signal transduction pathways, which causes EC proliferation and angiogenesis (29). In addition, in our study, THs administration stimulated the expression of VEGF in gene and protein levels, which shows the antigenic effects of T4.
In agreement with our findings, Liu et al. demonstrated that T4 induced the migration and formation of tube-like structures in HUVECs. In addition, they indicated that T4 up regulated the expression of basic fibroblast growth factor mRNA via effects on integrin avb3/PKD/HDAC5 signaling pathway, which had a main role in angiogenesis (17).
On the other hand, we confirmed the atherogenic role of T4 on HUVECs cells. Indeed T4-induced inflammation and atherogenesis may be due to these facts, which have been described by Szmitko et al. After ECs activation due to inflammatory events, the elevated levels of selectins, VCAM-1, and ICAM-1 stimulate the monocytes adherence (30) and also adhesive molecules are regulated by proinflammatory cytokines including IL-1 and TNF-α, which consequently lead to monocytes migration and related stigma (31). To the best of our knowledge, this is the first survey that compares the effects of T4 on proinflammatory mediators (IL-6 and TNF-α) and ED related factors. In summary, our results confirmed the relationship between T4 and endothelium function in human cellular model, which was found to be able to predict the atherosclerosis risk factors.
CONCLUSIONS
Overall, our study suggests the atherosclerotic effects of THs and the relationships of hyperthyroidism with atherogenesis. According to the present study, T4 had strong effects on the gene and protein expression levels of ICAM-1 and E-selectin as leukocyte adhesion molecules and VEGF as angiogenesis main mediator. In this regards, increased expression of proinflammatory and EC related mediators were associated with augmentation of atherosclerosis risks due to THs. Further studies should be conducted to confirm the impacts of T4 on the function of endothelial cells and inflammatory mediators in order to obtain more reliable data.
ACKNOWLEDGMENT
This research was financially supported (Grant No. 95-01-43-2787) by The Urmia University of Medical Sciences, Urmia, I.R. Iran. We are thankful to the technicians of Cellular and Molecular Research Center, Urmia University of Medical Sciences, Urmia, I.R. Iran for their technical helps.
REFERENCES
- 1.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 2.Bano A, Chaker L, Mattace-Raso FU, van der Lugt A, Ikram MA, Franco OH, et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the Rotterdam Study. Circ Res. 2017:CIRCRESAHA. 117.311603. doi: 10.1161/CIRCRESAHA.117.311603. [DOI] [PubMed] [Google Scholar]
- 3.Burggraaf J, Lalezari S, Emeis J, Vischer U, De Meyer P, Pijl H, et al. Endothelial function in patients with hyperthyroidism before and after treatment with propranolol and thiamazol. Thyroid. 2001;11(2):153–160. doi: 10.1089/105072501300042820. [DOI] [PubMed] [Google Scholar]
- 4.Popławska-Kita A, Siewko K, Telejko B, Modzelewska A, Myśliwiec J, Milewski R, et al. The changes in the endothelial function and haemostatic and inflammatory parameters in subclinical and overt hyperthyroidism. Int J Endocrinol. 2013. 2013 doi: 10.1155/2013/981638. DOI: 10.1155/2013/981638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coban E, Aydemir M, Yazicioglu G, Ozdogan M. Endothelial dysfunction in subjects with subclinical hyperthyroidism. J Endocrinol Invest. 2006;29(3):197–200. doi: 10.1007/BF03345539. [DOI] [PubMed] [Google Scholar]
- 6.Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. 2017;14(1):39–55. doi: 10.1038/nrcardio.2016.174. [DOI] [PubMed] [Google Scholar]
- 7.Naya M, Tsukamoto T, Morita K, Katoh C, Furumoto T, Fujii S, et al. Plasma interleukin-6 and tumor necrosis factor-α can predict coronary endothelial dysfunction in hypertensive patients. Hypertens Res. 2007;30(6):541–548. doi: 10.1291/hypres.30.541. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 9.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-α and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 11.Esteve E, Castro A, López-Bermejo A, Vendrell J, Ricart W, Fernández-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30(4):939–945. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Park Y, Wu J, ping Chen X, Lee S, Yang J, et al. Role of TNF-a in vascular dysfunction. Clin Sci. 2009;116(3):219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123(2):540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan J, Guo Q, Qureshi AR, Anderstam B, Eriksson M, Heimbürger O, et al. Circulating vascular endothelial growth factor (VEGF) and its soluble receptor 1 (sVEGFR-1) are associated with inflammation and mortality in incident dialysis patients. Nephrol Dial Transplant. 2013;28(9):2356–2363. doi: 10.1093/ndt/gft256. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Milosveski V, Schramek C, Fong G, Becks G, Hill D. Presence and possible role of vascular endothelial growth factor in thyroid cell growth and function. J Endocrinol. 1998;157(1):5–12. doi: 10.1677/joe.0.1570005. [DOI] [PubMed] [Google Scholar]
- 16.Davis PJ, Davis FB, Mousa SA. Thyroid hormone- induced angiogenesis. Curr Cardiol Rev. 2009;5(1):12–16. doi: 10.2174/157340309787048158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Zheng N, Shi Y-N, Yuan J, Li L. Thyroid hormone induced angiogenesis through the integrin avß3/protein kinase D/histone deacetylase 5 signaling pathway. J Mol Endocrinol. 2014;52(3):245–254. doi: 10.1530/JME-13-0252. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2”^CT method. methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Clynes M, editor. Animal Cell Culture Techniques. Springer Berlin Heidelberg. 2012:54–67. [Google Scholar]
- 20.Kruger NJ. The Bradford Method for Protein Quantitation. In: Walker JM, editor. The Protein Protocols Handbook. Humana Press; 2002. pp. 15–21. [Google Scholar]
- 21.Schägger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 22.Cabral MD, Teixeira P, Soares D, Leite S, Salles E, Waisman M. Effects of thyroxine replacement on endothelial function and carotid artery intima-media thickness in female patients with mild subclinical hypothyroidism. Clinics. 2011;66(8):1321–1328. doi: 10.1590/S1807-59322011000800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich J, Kuchler-Bopp S, Boutillier S, Ittel M, Reeber A, Zaepfel M, et al. Expression of thyroid hormone receptors alpha and beta-1 messenger RNAs in human endothelial cells. The T3 hormone stimulates the synthesis of the messenger RNA of the intercellular adhesion molecule-1. Cell Mol Biol (Noisy-le-grand) 1997;43(8):1205–1212. [PubMed] [Google Scholar]
- 24.Baumgartner-Parzer S, Wagner L, Reining G, Sexl V, Nowotny P, Müller M, et al. Increase by tri-iodothyronine of endothelin-1, fibronectin and von Willebrand factor in cultured endothelial cells. J Endocrinol. 1997;154(2):231–239. doi: 10.1677/joe.0.1540231. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez V, Videla LA, Tapia G, Israel Y. Increases in tumor necrosis factor-a in response to thyroid hormone-induced liver oxidative stress in the rat. Free Radic Res. 2002;36(7):719–725. doi: 10.1080/10715760290032566. [DOI] [PubMed] [Google Scholar]
- 26.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61(1):22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 27.HARA H, SUGITA E, SATO R, BAN Y. Plasma selectin levels in patients with Graves’ disease. Endocr J. 1996;43(6):709–713. doi: 10.1507/endocrj.43.709. [DOI] [PubMed] [Google Scholar]
- 28.Jurgilewicz DH, Rogowski F, Łebkowska U, Citko A, Jaroszewicz E, Parfieńczyk A. E-selectin, L-selectin, ICAM-1 and IL-6 concentrations changes in the serum of patients with hyperthyroidism in the early period of radioiodine I-131 therapy. Nucl Med Rev. 2002;5(1):39–42. [PubMed] [Google Scholar]
- 29.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 30.Milani AT, Khadem-Ansari MH, Rasmi Y. Effects of thyroid-stimulating hormone on adhesion molecules and pro-inflammatory cytokines secretion in human umbilical vein endothelial cells. Res Pharm Sci. 2018;13(6):546–556. doi: 10.4103/1735-5362.245966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szmitko PE, Wang C-H, Weisel RD, de Almeida JR, Anderson T J, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108(16):1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]