Abstract
Oxidative stress plays a crucial role in the pathogenesis of hyperglycemia mediated complications. Since a great number of researches have reported antioxidant features of saffron, this study investigated the antioxidant effect of saffron stigma extract (SSE) in streptozotocin-induced diabetic rats. Twenty eight diabetic male Wistar rats were divided in four groups containing: two diabetic groups receiving 25 and 100 mg/kg SSE respectively, one diabetic group receiving glibenclamide (0.6 mg/kg) and one diabetic control group receiving normal saline. Seven healthy adult male Wistar rats were also used as normal control group. After treatment (21 days), fasting blood glucose, insulin, oxidative stress markers, and pancreatic regeneration were assessed. The gene expression level of heat shock factor1, heat shock protein 27, and heat shock protein 70, also glucokinase (GK), and glucose 6-phosphatase (G6Pase) were determined using real-time polymerase chain reaction (RT-PCR). SSE in high dose (100 mg/kg) reduced fasting blood glucose (8.3 ± 0.4 mmol/L) compared with diabetic control (24.6 ± 1.2 mmol/L) (P < 0.05). Furthermore, SSE in high dose increased insulin level compared with diabetic control group (12.7 ± 0.6 vs 7.1 ± 0.3 μU/mL). RT-PCR analysis revealed decline in mRNA levels of stress proteins and G6Pase and increase in mRNA level of GK in treatment diabetic groups compared with diabetic control group. Data showed antioxidant and antidiabetic effects of SSE through altering insulin release and glucose metabolism pathways. Hypoglycemic potential of SSE may be due to change in GK and G6Pase enzymes expression. These findings provide a basis for the therapeutic potential of saffron in treatment of diabetes.
Keywords: Antioxidant, Diabetes, Saffron, Stress protein
INTRODUCTION
Diabetes mellitus (DM), most notably abnormal glucose metabolism, is one of the main global causes of mortality and morbidity. It is estimated the prevalence of this disease will have increased to 439 million people worldwide by the year 2030 (1). Previous studies have demonstrated that hyperglycemia-mediated oxidative stress plays a pivotal role in complications of diabetes mellitus (2,3,4,5,6). Since most drugs available for treatment of diabetes are associated with adverse side effects (7), trying to find new hypoglycemic agents with strong antioxidant properties and lower side effects is imperative (2,3). One of the most important natural antioxidant is Crocus sativus L. (saffron) belonging to Iridaceae family, which has been used globally as a spice and a traditional medicine for many centuries. Therapeutic features of saffron including hypoglycemic, anti-inflammatory, anticarcinogenic, antidepressive, and antioxidant activities were previously confirmed in vitro and in vivo (8,9). Heat shock proteins (HSPs), also called stress proteins, are a highly conserved and ubiquitously expressed family of proteins that respond to a wide variety of physical and metabolic stress, including oxidative stress.
Following exposure to various types of stress, the synthesis of HSPs is rapidly initiated (10). Subsequently, HSPs prevent protein denaturation and protect tissues against oxidative stress-induced damage. Hyperglycemia in diabetes stimulates free radical production leading to cell damage (11). Free radicals such as reactive oxygen species play crucial role in the pathophysiology of several diseases such as diabetes (11); therefore, agents with antioxidant properties may have the potential for limiting the diabetes progression (12).
Glucokinase (GK) is one of the most important enzyme in glucose metabolism pathway resulting in lowering plasma glucose. In contrast, glucose 6-phosphatase (G6Pase) is one of the enzymes in gluconeogenesis pathway that causes an increase in blood glucose. Considerable evidence has suggested HSPs (13), GK (14), and G6Pase (15), as associating proteins in glucose homeostasis and consequently diabetes. To date, few studies have investigated to find the molecular mechanism of saffron hypoglycemic action. In addition, the impact of saffron on the expression of HSP-27, HSP-70, heat shock factor 1 (HSF1), GK, and G6Pase, which involve in glucose homeostasis, has not yet been reported. A better insight into the saffron mechanisms of action would enhance the therapeutic potential of this agent either alone or in combination with other antioxidant agents. So, this study was designed to investigate the hypoglycemic effects and antioxidant capacity of saffron stigma extract (SSE) in streptozotocin (STZ)-induced diabetic rats.
MATERIALS AND METHODS
Chemical and reagents
STZ was purchased from Sigma-Aldrich Corp (St. Louis, MO, USA). Fasting blood glucose assay kit was procured from Pars Azmun (I.R. Iran). Insulin ELISA assay kit was supplied by Glory Science (Zhejiang, Thailand). First Strand cDNA synthesis kit was purchased from Fermentas, Massachusetts, USA. Trizol extraction reagent was purchased from Bioneer, Daejeon, Korea.
Study design
In this study 35 adult male Wistar rats (28 diabetic and 7 healthy control), weighing 225 ± 25 g, bred and raised at the university animal quarters were used. The rats were housed in cages and given standard chow diet and water ad libitum.
Type I diabetes was induced by one intraperitoneally injection of STZ (60 mg/kg body mass) before the beginning of treatments. After two weeks, animals with a plasma glucose concentration over 16 mmol/L, were considered diabetic. The 28 diabetic rats were randomly divided into 4 groups (n = 7) as follow, the first two groups were treated orally with 25 and 100 mg of ethanolic extract of SSE per kilogram body weight for 21 days, respectively (16), the third group was diabetic control group and received 1 mL normal saline daily and the forth group of diabetic rats defined as positive control received once daily oral dose of glibenclamide (0.6 mg/kg) (17). Seven healthy rats received 1 mL of normal saline daily during this period and were defined as the healthy control group. At the end of the experiment, blood samples were collected and plasma levels of glucose and other biochemical factors were measured. For gene expression analysis, the liver and pancreas of animals were quickly removed and placed in liquid nitrogen. All animal procedures were approved by the Ethical Committee of Birjand University of Medical Sciences, Birjand, I.R. Iran in accordance with the Institutional Animal Ethics Committee using the ir.bums.REC.1395.60 Code.
Plant material and extraction
Saffron stigma provided from Birjand, South Khorasan, I.R. Iran was air-dried in shadow and grounded into fine powder by a grinder.
Fifty g of saffron powder was extracted by 2 L of ethanol (50% v/v) by refluxing for 48 h. The obtained extract was vacuum-evaporated to obtain the crude extract (6 g, extraction yield 12%). Based on the treatment dose (25 or 100 mg/kg), required quantity of the crude extract was dissolved in distilled water (1 mL) before oral daily administration.
Plasma glucose and insulin assay
At the end of treatment period (21 days), level of glucose and insulin was measured in blood samples. Fasting blood glucose was assayed using commercially available kit based on colorimetric method (sensitivity 0.06 mmol/L). For detection of insulin level, specified ELISA kit was used and followed the manufacturer’s recommendations (sensitivity 0.17 U/mL). For evaluation of pancreatic β-cell function, the homeostatic model assessment (HOMA) β-cell function was also assessed by the following equation (18):
HOMA-β = (20 × I0)/(G0 – 3.5)
in this equation, I0 and G0 represent fasting plasma levels of insulin (μU/mL) and glucose (mmol/L), respectively.
Evaluation of oxidative stress status
Total antioxidant capacity in different groups was measured using ferric reducing antioxidant power method based on Benzie et al. procedure. The method is based on the reduction of Fe3+-tripyridyltriazine complex (colorless complex) to Fe2+-tripyridyltriazine (blue colored complex) formed by the action of electron donating antioxidants at low pH. This reaction is monitored by measuring the change in absorbance at 593 nm (19,20). Plasma level of malondialdehyde (MDA) as a marker of lipid peroxidation was measured using thiobarbituric acid reactive substances (TBARS) method (21,22). TBARS is a well-established assay for screening and monitoring lipid peroxidation. A mixture of plasma samples and TBARS reagent was heated in a boiling water bath and absorbance was finally measured at 532 nm.
Pancreatic islets count assay
For evaluation of saffron potential in regeneration of pancreatic beta cells, number of pancreatic islets was calculated. After fixation of pancreatic tissue in 10% formalin, 5 μm thick sections of blocks were prepared, stained with hematoxylin and eosin and examined under a light microscope and the average number of pancreatic islets per cm2 was calculated based on our previous study (23).
RNA extraction and gene expression analysis
In liver and pancreas tissues, gene expression level of desired genes was studied. For this, the liver and pancreas tissues of rats were removed and quickly placed in liquid nitrogen. Total RNA from the excised tissues of different groups was extracted with the Trizol reagent, in accordance with the manufacturer’s recommendations. The RevertAid H minus first strand cDNA synthesis kit was used to reverse-transcribe 1 μg of RNA in a final volume of 20 μL. Real time polymerase chain reaction (RT-PCR) of β-actin (reference gene), HSP27, HSP70, HSF1, GK, and G6Pase was carried out using specific primers identified in Table 1. The reaction mixture consisted of 2 × ABI SYBR green PCR master mix, 2 μL cDNA and 0.2 μL of each primer. Amplification was performed in the ABI Step One RT-PCR system (Applied Biosystems, Foster City, CA, USA) with 40 cycles of denaturation at 95 °C for 30 s, annealing and extension at 60 °C for 30 s, and data collection at 80 °C for 20 s. The intensities of the mRNA levels were normalized to those of the β-actin product. The relative gene expression between the groups was assayed using 2-ΔΔCT method (24).
Table 1.
The primer sequences used in real time polymerase chain reaction.
| Genes | Sequences |
|---|---|
| HSPA4 (HSP70) | Forward: 5’-TGGCATTTTCAGTGTGTCCAG-3’ Reverse: 5’-CACCTGCATCTTCTCTTCTTCCT-3’ |
| HSF1 | Forward: 5’-CTGGCCAGCATTCAGGAACTT-3’ Reverse: 5’-GTAGTGCACCAGCTGCTTTC-3’ |
| HSPB1 (HSP27) | Forward: 5’-CCCTGGACGTCAACCACTTC-3’ Reverse: 5’-AGCCATGTTCATCCTGCCTT-3’ |
| GK | Forward: 5’-TGGTGCTTTTGAGACCCGTT-3’ Reverse: 5’-GAAGCCCCAGAGTGCTTAGG-3’ |
| G6Pase | Forward: 5’-CGTCACCTGTGAGACTGGAC-3’ Reverse: 5’-ACGACATTCAAGCACCGGAA-3’ |
| β-actin | Forward: 5’-GTCCACCCGCGAGTACAAC-3’ Reverse: 5’-GACGACGAGCGCAGCGATA-3’ |
HSP, heat shock protein; GK, glucokinase; G6Pase, glucose-6-phosphatase
Statistical analysis
The data are expressed as mean ± SD. Statistical analyses of the results were performed using oneway ANOVA (Graph Pad Prism 6 for windows) followed by Tukey’s post hoc test, and the level of P < 0.05 was considered significant between the treated and untreated groups. In gene expression analysis, differences in relative expression were analyzed using Student’s t test.
RESULTS
Effect of saffron ethanolic extract on plasma biochemical factors
The alteration of fasting blood glucose and insulin levels in the normal and diabetic rats is depicted in Table 2. High plasma level of glucose and low level of insulin was measured in diabetic rats as compared with healthy control rats. Treatment with SSE especially in high dose significantly reduced the plasma glucose concentration (8.3 ± 0.4 mmol/L) close to the records in the normoglycemic rats (7.4 ± 0.4 mmol/L). Moreover this treatment significantly increased insulin levels in the blood of diabetic rats (12.7 ± 0.6 mmol/L) compared with diabetic control group (7.1 ± 0.3 mmol/L) (P < 0.05). This effect of SSE notably in high dose was similar to glibenclamide (Table 2). HOMA-β cell function represented reduced beta cell function in diabetic control group, which markedly increased to normal value after treatment with different doses of SSE (Table 2).
Table 2.
Effects of saffron stigma extract on biochemical parameters of streptozotocin-induced diabetic rats. Data are expressed as mean ± SD of seven animals in each group. * Indicates significant differences compared to the diabetic control group (P < 0.05).
| Groups | Healthy control | Diabetic control | Diabetic + SSE (25 mg/kg) | Diabetic + SSE (100 mg/kg) | Diabetic + glibenclamide |
|---|---|---|---|---|---|
| FBS (mmol/L) | 7.4 ± 0.4* | 24.6 ± 1.2 | 8.9 ± 0.7* | 8.3 ± 0.4* | 7.9 ± 0.8* |
| Insulin (μU/mL) | 15.2 ± 0.7* | 7.1 ± 0.3 | 10.2 ± 0.5* | 12.7 ± 0.6* | 13.9 ± 0.4* |
| HOMA-β cell function | 77.9* | 6.7 | 37.7* | 52.9* | 63.1* |
| MDA (μmol/L) | 1.47 ± 0.5* | 4.3 ± 0.7 | 2.39 ± 0.3* | 1.82 ± 0.2* | 1.65 ± 0.1* |
| Total antioxidant (μmol/L) | 780 ± 14* | 550 ± 21 | 625 ± 18* | 690 ± 16* | 710 ± 15* |
| Number of pancreatic islets/cm2 | 43 ± 3.5* | 13 ± 1.8 | 21 ± 1.9* | 29 ± 2.6* | 34 ± 0.4* |
SSE, Saffron stigma extract; FBS, fasting blood glucose; MDA, malondialdehyde.
Effect of saffron ethanolic extract on pancreatic islets count
The effects of SSE on pancreatic islets count are summarized in Table 2. The data showed a significant decrease in number of pancreatic islets in diabetic control group compared with normoglycemic group (P < 0.05). SSE in specified doses increased β cells count. This effect was notable for high dose of SSE (100 mg/kg) and was similar to glibenclamide (Table 2).
Effect of saffron ethanolic extract on oxidative stress status
The diabetic rats manifested significantly increased MDA levels and also significantly decreased total antioxidant power (P < 0.05) compared to healthy control group. Treatment with saffron ethanolic extract significantly reduced the MDA level and also increased the total antioxidant power (P < 0.05) (Table 2). Amelioration of lipid peroxidation and increase in antioxidant capacity was seen also in glibenclamide treated group. Consumption of the drug improved oxidative stress status to normoglycemic group (Table 2). Similarly SSE in high dose improved oxidative stress status.
Effect of saffron stigma extract on transcriptional levels of HSF1, HSP27, and HSP70
As indicated in Fig. 1, comparing with the normal control, diabetic control illustrated a significant increase in pancreatic expression of mRNA levels of stress proteins up to three times for HSF1, HSP27, and HSP70 (P < 0.05). The results demonstrated a significant reduction in mRNA levels of the mentioned genes in diabetic rats treated with SSE compared to the diabetic control (P < 0.05). This downregulation showed a dose-dependent manner.
Fig. 1.
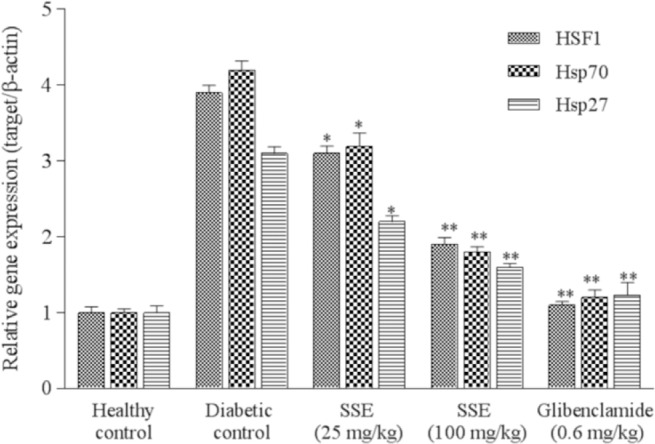
Effects of saffron stigma extract (SSE) on the expression of HSF1, HSP27, and HSP70 in mRNA levels. Values are expressed as mean ± SD of three experiments and represented relative gene expression (target gene/β-actin). Significant differences are indicated in comparison with diabetic control group (*P < 0.05 and **P < 0.01). SSE, saffron stigma extract; HSF, heat shock factor; HSP, heat shock proteins.
Effect of saffron stigma extract on transcriptional levels of glucokinase and glucose 6-phosphatase
As shown in Fig. 2, there was an upregulation of G6Pase in liver tissue of diabetic rats compared with the healthy control. Treatment with SSA especially in high dose reduced (up to 3 times) G6Pase expression of the diabetic rats compared with diabetic control group (P < 0.05). This downregulation had a dose-dependent fashion. Also, there was a significant decrease of GK gene expression in the liver tissue of diabetic rats compared with normal group. Treatment with SSE in high dose showed a significant increase (up to 2 times) in the expression of GK compared to the diabetic control group (P < 0.05) (Fig. 2).
Fig. 2.
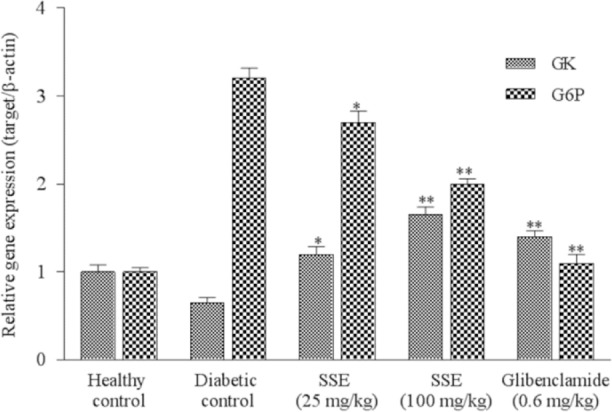
Effects of SSE on the gene expression of GK and G6Pase. Values are expressed as mean ± SD of three experiments and represented relative gene expression. Significant differences are indicated in comparison with diabetic control group (*P < 0.05 and **P < 0.01). SSE, saffron stigma extract; GK, glucokinase; G6Pase, glucose-6-phosphatase.
DISCUSSION
In this study, we showed that SSE reduced transcription levels of stress proteins in pancreas tissue of diabetic rats. Also ethanolic SSE was able to adjust key enzymes of glucose metabolism. The results of the present study revealed that alcoholic extract of saffron ameliorates hyperglycemia and related oxidative stress status. Several studies have demonstrated that diabetes is associated with oxidative stress; therefore, agents with high antioxidant properties may have beneficial potential for diabetes therapy (2,5,6). Numerous studies on medicinal properties of saffron have proved its potent antioxidant properties (25,26,27,28). The present study in line with the others also suggests that saffron can significantly increase the total antioxidant capacity and reduce peroxidation of lipids (29,30,31). Pancreatic islets assay revealed significant decrease of β cell count in diabetic control group that had been improved by SSE treatment. HOMA-β showed decline in β cell function in diabetic control group resulting in high glucose level and related oxidative stress. The plasma glucose lowering effect of SSE could be a result of regulation of pancreatic islets in these groups. Saffron stigma may be helpful in regeneration of β cells in pancreas following improvement of β cells function. Reduced expression of pancreatic stress proteins in SSE treated groups, revealed amelioration of oxidative stress in pancreas tissue resulting in regeneration of pancreas islets. In this era, our results in line with kang et al., study (25) suggest that saffron can strongly increase insulin secretion, sensitivity, and glucose uptake.
Considerable evidence suggested that HSPs play crucial roles in maintaining cellular homeostasis and protecting the cells against stress (23,24,25,26,27,28,29,30,31,32,33,34). In response to the stressful conditions such as heat shock, hypoxia, hydrogen peroxide, uncoupled oxidative phosphorylation, reactive oxygen species, infection (endotoxin), and inflammation, these proteins are upregulated and act as chaperones to prevent protein misfolding (34,35,36,37). Our results are compatible with our previous studies demonstrated that the expression of HSF1, HSP27, and HSP70 increased significantly in the pancreas of diabetic rats (11,12). Based on literature, regulation and expression of HSPs in diabetes have a tissue-specific manner (38). Increased expression of HSPs in pancreas tissue may be attributed to the need for protection from the exocrine pancreatic enzyme, trypsin. HSP70 prevents the activation of trypsinogen, and thus cellular auto digestion by trypsin (39).
The data obtained here, supported antioxidant potential of saffron extract which reduced HSPs expression in pancreas of treated diabetic groups that could be the result of improvement of oxidative stress status in response to saffron. We showed this effect was dose-dependent and in high dose, saffron ethanolic extract had a similar behavior to glibenclamide.
GK and G6Pase are important enzymes in regulation of glucose metabolism. We suggested that SSE may reduce blood glucose by increasing the expression of GK and decreasing that of G6Pase. The gene expression results obtained from current study is in agreement with our previous studies (11,12) that showed significant decrease in the GK expression and a significant increase in G6Pase expression of diabetic group. The results of the current study demonstrated a significant reduction in G6Pase expression among diabetic rats treated with SSE compared with control group in a dose-dependent manner. Our results also revealed treatment of diabetic rats with SSE significantly increase the expression level of GK that could be specific target for more investigation. According to these results, it seems gluconeogenesis pathway (G6Pase) most affected by diabetes than glycolysis pathway (GK) and treatment procedure could improve gluconeogenesis better than glycolysis. Consumption of SSE at 100 mg/kg for 21 days had a similar effect to glibenclamide that could be mentioned in pharmacotherapy of diabetes.
CONCLUSION
To put it in a nutshell, the result of the current study illustrated that ethanolic SSE could ameliorate oxidative stress in STZ-diabetic rats resulting improvement of pancreatic islets regeneration and function. Reduced levels of stress proteins and improvement of glycolysis pathway in treated diabetic rats confirmed antioxidant potential and hypoglycemic effect of saffron. It seems that evaluating the stress protein and key enzymes at the protein level using western blotting assay, provide us more accurate evaluation at molecular level.
ACKNOWLEDGEMENTS
This investigation was financially supported (Grant No. 711) by the Vice Chancellery of Research of Birjand University of Medical Sciences, Birjand, I.R. Iran.
REFERENCES
- 1.Ogurtsova K, da Fernandes RJD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 3.Pari L, Latha M. Antidiabetic effect of Scoparia dulcis: effect on lipid peroxidation in streptozotocin diabetes. Gen Physiol Biophys. 2005;24(1):13–26. [PubMed] [Google Scholar]
- 4.Davi G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7(1-2):256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 5.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4(1):5–15. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenercioglu AK, Saler T, Genc E, Sabuncu H, Altuntas Y. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33(2):118–124. doi: 10.1007/BF03346565. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Eur J Med. 2009;122(6 Suppl):S309–S318. doi: 10.1016/j.amjmed.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Moshiri E, Basti AA, Noorbala AA, Jamshidi AH, Hesameddin Abbasi S, Akhondzadeh S, Crocus sativus L. (petal) in the treatment of mild-tomoderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13(9-10):607–611. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Jafarova AF, Caballero-Ortega H, Riveron-Negrete L, Pereda-Miranda R, Rivera-Luna R, Hernandez MJ, et al. In vitro evaluation of the chemopreventive potential of saffron. Rev Invest Clin. 2002;54(5):430–436. [PubMed] [Google Scholar]
- 10.Oksala NKJ, Ekmekçi FG, Özsoy E, Kirankaya Ş, Kokkola T, Emecen G, et al. Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Biol. 2014;3:25–28. doi: 10.1016/j.redox.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmati M, Serki E, Gholami M, Hoshyar R. Effects of an ethanolic extract of Berberis vulgaris fruits on hyperglycemia and related gene expression in streptozotocin-induced diabetic rats. Clinical Phytoscience. 2016;2:3–9. [Google Scholar]
- 12.Gholami M, Hemmati M, Taheri-Ghahfarokhi A, Hoshyar R, Moossavi M. Expression of glucokinase, glucose 6-phosphatase, and stress protein in streptozotocin-induced diabetic rats treated with natural honey. Int J Diabetes Dev Ctries. 2016;36(1):125–131. [Google Scholar]
- 13.Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14(2):113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matschinsky FM, Magnuson MA, Zelent D, Jetton TL, Doliba N, Han Y, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55(1):1–12. [PubMed] [Google Scholar]
- 15.Haber BA, Chin S, Chuang E, Buikhuisen W, Naji A, Taub R. High levels of glucose-6- phosphatase gene and protein expression reflect an adaptive response in proliferating liver and diabetes. J Clin Invest. 1995;95(2):832–841. doi: 10.1172/JCI117733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmati M, Asghari S, Zohoori E. Effects of alcoholic and aqueous extract of barberry, jujube and saffron petals on serum level of adiponectin and lipid profile in diabetic rats. Iran J Endocrinol Metab. 2015;16(5):329–337. [Google Scholar]
- 17.Lal VK, Gupta PP, Awanish P. Hypoglycemic effect of Kyllinga triceps in STZ induced diabetic rats. J Diabetes Metab. 2012;3:6–8. [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 20.Nili-Ahmadabadi A, Ali-Heidar F, Ranjbar A, Mousavi L, Ahmadimoghaddam D, Larki-Harchegani A, et al. Protective effect of amlodipine on diazinon-induced changes on oxidative/antioxidant balance in rat hippocampus. Res Pharm Sci. 2018;13(4):368–376. doi: 10.4103/1735-5362.235164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nili-Ahmadabadi A, Alibolandi P, Ranjbar A, Mousavi L, Nili-Ahmadabadi H, Larki-Harchegani A, et al. Thymoquinone attenuates hepatotoxicity and oxidative damage caused by diazinon: an in vivo study. Res Pharm Sci. 2018;13(6):500–508. doi: 10.4103/1735-5362.245962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of oxidative stress during Diabetes mellitus. J Biomark. 2013. 2013 doi: 10.1155/2013/378790. Article ID: 378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C(3):357–364. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 24.Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583(4):759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Kang C, Lee H, Jung ES, Seyedian R, Jo M, Kim J, et al. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. 2012;135(4):2350–2358. doi: 10.1016/j.foodchem.2012.06.092. [DOI] [PubMed] [Google Scholar]
- 26.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212(2):167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Samarghandian S, Azimi-Nezhad M, Farkhondeh T. Immunomodulatory and antioxidant effects of saffron aqueous extract (Crocus sativus L.) on streptozotocin-induced diabetes in rats. Indian Heart J. 2017;69(2):151–159. doi: 10.1016/j.ihj.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaee Khorasany A, Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: a review. Iran J Basic Med Sci. 2016;19(5):455–469. [PMC free article] [PubMed] [Google Scholar]
- 29.Kanakis CD, Tarantilis PA, Tajmir-Riahi HA, Polissiou MG. Crocetin, dimethylcrocetin, and safranal bind human serum albumin: stability and antioxidative properties. J Agric Food Chem. 2007;55(3):970–977. doi: 10.1021/jf062638l. [DOI] [PubMed] [Google Scholar]
- 30.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54(23):8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 31.Asdaq SM, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. 2010;162(2):358–372. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Yang S, Vlantis AC, Liu SY, Ng EK, Chan AB, et al. Expression of antioxidant molecules and heat shock protein 27 in thyroid tumors. J Cell Biochem. 2016;117(11):2473–2481. doi: 10.1002/jcb.25539. [DOI] [PubMed] [Google Scholar]
- 33.Altinoz E, Oner Z, Elbe H, Cigremis Y, Turkoz Y. Protective effects of saffron (its active constituent, crocin) on nephropathy in streptozotocin-induced diabetic rats. Hum Exp Toxicol. 2015;34(2):127–134. doi: 10.1177/0960327114538989. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal A, Ashutosh, Chandra G, Singh AK. Heat shock protein 70, oxidative stress, and antioxidant status in periparturient crossbred cows supplemented with α-tocopherol acetate. Trop Anim Health Prod. 2013;45(1):239–245. doi: 10.1007/s11250-012-0196-z. [DOI] [PubMed] [Google Scholar]
- 35.Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, et al. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2013;304(3):289–299. doi: 10.1152/ajprenal.00517.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy VS, Raghu G, Reddy SS, Pasupulati AK, Suryanarayana P, Reddy GB. Response of small heat shock proteins in diabetic rat retina. Invest Ophthalmol Vis Sci. 2013;54(12):7674–782. doi: 10.1167/iovs.13-12715. [DOI] [PubMed] [Google Scholar]
- 37.Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1(2):97–98. doi: 10.1379/1466-1268(1996)001<0097:dothsr>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14(3):291–299. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burkart V, Liu H, Bellmann K, Wissing D, Jaattela M, Cavallo MG, et al. Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem. 2000;275(26):19521–19528. doi: 10.1074/jbc.M002265200. [DOI] [PubMed] [Google Scholar]


