Abstract
Background:
Deep venous thrombosis (DVT) is associated with significant morbidity and mortality. Thus, there is a great need to demonstrate a more efficient biomarker that would confirm the diagnosis of DVT. Our work aimed to evaluate the role of platelet-derived growth factor-beta (PDGF-B) as a new marker of DVT and its correlation with other radiological and laboratory tools used for the diagnosis.
Materials and Methods:
A case–control study enrolled forty patients selected from our university hospital between April 2018 and August 2018, who divided into two groups: Group I (n = 20) consisted of patients diagnosed with acute venous thrombosis and Group II (n = 20) consisted of patients diagnosed with chronic venous thrombosis. Twenty samples were collected from age- and gender-matched apparently healthy controls to be used as a control. Venous duplex ultrasonography, routine laboratory investigations, D-dimer (DD), and protein expression of PDGF-B were performed on all patients.
Results:
There was a highly significant increase in a protein expression of PDFG-B in all cases of acute and chronic venous thrombosis compared to the control group with P < 0.001; furthermore, it was more specific than DD for the detection of DVT (specificity 95% and 90%, respectively).
Conclusion:
Our study submits a novel association of PDGF-B plasma levels with DVT, and PDGF-B is considered to be a more specific indicator for DVT than is DD.
Keywords: D-dimer, deep vein thrombosis, platelet-derived growth factor-beta, venous duplex ultrasonography
INTRODUCTION
Venous thromboembolism (VTE) is the most prevalent vascular disease after acute myocardial infarction and stroke. It is appeared by two main clinical events: deep venous thrombosis (DVT) and pulmonary embolism.[1]
DVT and its sequel are associated with significant morbidity and mortality. Clinical diagnosis is insensitive and inaccurate because the common signs and symptoms of the disease overlap considerably with other conditions.[2] It is unacceptable to diagnose DVT clinically and commit patients to the risks associated with anticoagulation without confirmatory objective testing.[3]
The presence of a residual thrombus after the first episode of DVT is a separate risk factor for recurrence. After the first episode of VTE, patients are forty times more likely to develop a recurrent event compared to previously unaffected individuals.[4]
Venous duplex ultrasonography (US) is thefirst-line investigation for the vast majority of patients with suspected DVT; however, it is difficult and less sensitive in patients with obesity, edema, tenderness, recent hip or knee arthroplasty, cast, overlying bandages, and immobilization devices.[5]
D-dimer (DD) is a marker of endogenous fibrinolysis and should, therefore, be detectable in patients with DVT. Several studies have shown that the DD assay has a high negative predictive value and is a sensitive but not specific marker of DVT because elevated DD may be present due to various causes such as liver disease, high rheumatoid factor, and many other conditions.[6] However, the safety and added value of relying on a negative DD test to exclude a diagnosis of DVT remain controversial. Thus, the discovery of a single laboratory marker that would confirm the diagnosis of the disease could be considered the holy grail of clinical medicine.[7]
Platelet-derived growth factor (PDGF) is composed of two homologous polypeptide chains (A and B), both chains can be produced by platelets, macrophages, and endothelial cells, whereas vascular smooth muscle cell produces only PDFG-A chains.[8]
PDGFB was expressed in endothelium and platelets, so it is a key cell types in thrombosis development.[9] PDGFs have been reported to induce pathological mesenchymal responses in a variety of vascular disorders, including atherosclerosis, pulmonary hypertension, and restenosis, but it has not been linked to VTE previously.[10]
Virchow's triad involves three contributing risk factors for DVT favoring clot formation of venous stasis, vascular injury, and hypercoagulability. A venous thrombus consists of two components, an outer red cell dense fibrin clot and an inner platelet-rich white thrombus called lines of Zahn.[11]
At the site of vascular injury, platelets are activated by adhesion to the subendothelium to form the thrombus, leading to the release of some factors stored in platelets as cytokines and growth factors.[12] Among these factors, PDGF-B is stored in the α-granules of the platelets and is released in the platelet release reaction; therefore, an increase in plasma PDGF will reflect a state of platelet activation.[13]
If a PDGF-B concentration was superior to a standard value in healthy or normal controls, this indicates a presence or risk of developing thrombosis.[14] The levels of PDGF-B are a significantly higher concentration of more than 10%, preferably more than 20%, 30%, 40%, or 50%, in patients with VTE.[15]
Results from the VEREMA affinity proteomics study were identified PDGF as a novel VTE-associated plasma protein and need other studies of further investigation.[16]
Hence, in our work, we aimed to evaluate the role of PDGF-B as a new marker of DVT and its correlation with other radiological and laboratory tools used for the diagnosis.
PATIENTS AND METHODS
Study design
It is a case–control study.
Study participants
This study enrolled forty patients selected from our university hospital between April 2018 and August 2018, who suffered from venous thrombosis of the lower limbs and twenty control samples.
Inclusion criteria
The participants were categorized into the following two groups: Group I (n = 20) consisted of patients diagnosed with acute venous thrombosis and Group II (n = 20) consisted of patients diagnosed with chronic venous thrombosis.
Exclusion criteria
Patients with congenital thrombophilia (antithrombin, protein S and protein C deficiencies, homozygosity V Leiden or the G20210A polymorphism in prothrombin gene or heterozygosity) were excluded. In addition, patients with active cancer within the last 5 years, liver failure, kidney failure, and antiphospholipid syndrome and patients with menopausal replacement therapy or estrogen-containing hormonal contraceptive pills were excluded from the study.
The matching criteria
The controls were age and sex matched, free of personal history of venous and arterial thrombotic disease.
Ethical considerations
The study was conducted according to the World Medical Association Declaration of Helsinki for studies on human subjects. It was approved by the Institutional Review Board of our institution, and a written informed consent was obtained from all patients.
Procedures and variables assessment
All patients were subjected to a detailed medical history, clinical examination, venous duplex US, and laboratory investigations.
Clinical examination
DVT may be asymptomatic or cause pain and swelling, tenderness, discoloration, or redness of the affected area in an extremity, and skin that is warm to the touch specifically in acute type.
Diagnosis of DVT either acute or chronic clinically is hard distinguished between them or even between DVT and other DD with the same symptoms but less serious like cellulitis or a pulled muscle. But may reach to diagnosis by history, duration of insult, and physical examination and is confirmed by objective testing, typically with duplex US.
Radiological examination by venous duplex ultrasonography
In acute DVT, the diagnostic US criteria remain noncompressibility of the vein with being hypoechoic thrombus within the vein lumen, venous distention, complete absence of spectral or color Doppler signal within the vein lumen, loss of flow phasicity, and loss of response to Valsalva or augmentation. In chronic DVT, the vein is incompressible, narrow, and irregular and shows an echogenic thrombus attached to the venous walls with the development of collaterals.
All patients with chronic DVT in our research receive warfarin treatment. Warfarin inhibits the Vitamin K epoxide reductase complex 1, which is an essential enzyme for activating the Vitamin K in the body. Through this mechanism, warfarin can deplete functional Vitamin K reserves and therefore reduce the synthesis of active clotting factors II, VII, IX, and X in the liver, so warfarin does not suppress platelet aggregation. And therefore, warfarin has no effect on PDGF-B.
Laboratory investigations
Laboratory investigations including complete blood count using SYSMEX KX21N (Japan), biochemical analysis using Cobas c311 (Germany) for kidney function, liver function, and fasting blood glucose, coagulation profile using Stago STA (France), and DD using Immulite 1000 (USA).
Then, protein expression of PDGF-B is analyzed using the Western blotting technique as illustrated in Figure 1.
Figure 1.
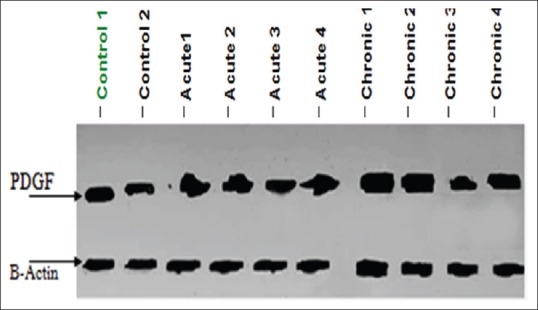
Western blotting analysis of platelet-derived growth factor-beta antibody for control (1 and 2), Group I (acute cases 1, 2, 3, and 4), and Group II (chronic 1, 2, 3, and 4) samples
Plasma samples were fractionated through 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Equal amounts of protein (30 μg) were separated and transferred onto Hybond™ nylon membranes (GE Healthcare, Buckinghamshire, UK). Immunoblots were probed with the primary antibody; anti-PDGF-B (ab23914 Abcam, Cambridge, UK) at 4°C overnight. The membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized using a gel documentation system (GelDoc-it, UVP, England) and Total lab analysis software (CLIQS, liverpool, UK), www.totallab.com (Ver. 1.0.1).
Statistical methods
Data were coded and entered using the Statistical Package for the Social Sciences version 25 (IBM Corp., Armonk, NY, USA). Data were summarized as the mean, standard deviation (SD), and median for the quantitative data and as the frequency (count) and relative frequency (percentage) for the categorical data. Comparisons between groups were assessed using the analysis of variance with the multiple comparisons post hoc test when comparing more than two groups or the unpaired t-test when comparing two groups in normally distributed quantitative variables. The nonparametric Kruskal–Wallis and Mann–Whitney tests were used for nonnormally distributed quantitative variables. Normality of data was double checked by normality plot and Shapiro Wilk test some of the data was not normally distributed so it was presented by median and range beside mean and SD. in case of non-normally distributed data, posthoc test was done by Mann Whitney test corrected by Bonferroni correction of multiple comparisons. For comparing categorical data, the Chi-square (χ2) test was performed. An exact test was used when the expected frequency was <5. Correlations between quantitative variables were performed using Spearman's correlation coefficient. P < 0.05 was considered statistically significant and P < 0.01 was considered highly statistically significant.
RESULTS
Forty patients (19 males and 21 females) aged from 33 to 65 years with a mean of 49 ± 7 years and a median of 49 years. A total of 20 samples were collected from age- and gender (8 males and 12 females)-matched apparently healthy controls, to be used as a control group. Ages of the control group ranged from 33 to 60 years with a mean of 43.3 ± 8.25 years and a median of 41 years.
Different demographic and laboratory parameters for case and control groups are shown in Table 1. There was a highly significant increase in DD and PDFG-B in all cases (3.26 ± 4.42 and 23.42 ± 2.87, respectively) compared to that in the control group (0.26 ± 0.09 and 18.95 ± 1.54, respectively) with P < 0.001 for both.
Table 1.
Comparison of different demographic and laboratory parameters in the studied groups
| Cases (n=40) | Controls (n=20) | P | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 19 (47.5) | 8 (40) | 0.582 |
| Female | 21 (52.5) | 12 (60) | |
| Diabetes mellitus, n (%) | |||
| Yes | 5 (12.5) | 0 | 0.159 |
| No | 35 (87.5) | 20 (100.0) | |
| Hypertension, n (%) | |||
| Yes | 8 (20) | 0 | 0.043 |
| No | 32 (80) | 20 (100) | |
| Age (years), mean±SD | 49±7 | 43.3±8.25 | 0.011 |
| WBCs (×109/L), mean±SD (median) | 8.57±5.65 (7.00) | 7.08±2.35 (6.80) | 0.447 |
| RBCs (×1012/L), mean±SD (median) | 4.06±0.88 (4.20) | 4.68±0.49 (4.70) | 0.001 |
| Hb (gm/dL), mean±SD (median) | 10.97±2.29 (10.90) | 13.3±1.32 (13.05) | <0.001 |
| Platelet (×109/L), mean±SD and (median) | 265.53±114.54 (251.00) | 238.8±47.66 (242.00) | 0.616 |
| PT (s), mean±SD (median) | 13.98±2.82 (12.85) | 12.9±0.61 (13.00) | 0.025 |
| PC (%), mean±SD (median) | 84.24±19.61 (90.00) | 98.45±4.24 (100.00) | <0.001 |
| INR, mean±SD (median) | 1.25±0.43 (1.09) | 1.01±0.03 (1.00) | 0.001 |
| APTT (s), mean±SD (median) | 60.98±22.15 (59.50) | 31.68±4.64 (31.00) | <0.001 |
| D-dimer (ng/ml), mean±SD (median) | 3.26±4.42 (1.35) | 0.26±0.09 (0.25) | <0.001 |
| Percentage of protein expression of PDGF-B, mean±SD (median) | 23.42±2.87 (23.00) | 18.95±1.54 (19.00) | <0.001 |
P<0.05 was considered statistically significant and P<0.01 was considered highly significant. SD=Standard deviation; WBCs=White blood cells; RBCs=Red blood cells; Hb=Hemoglobin; PT=Prothrombin time; PC=Prothrombin concentration; INR=International normalization ratio; APTT=Activated partial thromboplastin time; PDGF-B=Platelet-derived growth factor-beta
The comparison between DD and PDGF-B in Group I, Group II, and the control group is shown in Table 2. There was a highly significant increase in PDGF-B in chronic venous thrombosis cases compared to that in the acute venous thrombosis cases and the control group (P = 0.001 and < 0.001, respectively), and in acute venous thrombosis, PDGF-B was increased compared to that in the control group (P < 0.001).
Table 2.
Comparison of previous highly significant parameters in acute venous thrombosis cases (Group I), chronic venous thrombosis cases (Group II), and control group
| Mean±SD (median) | P1 | P2 | P3 | |||
|---|---|---|---|---|---|---|
| Group I (n=20) | Group II (n=20) | Controls (n=20) | ||||
| D-dimer (ng/L) | 4.4±4.84 (2.45) | 2.12±3.75 (0.40) | 0.26±0.09 (25) | 0.003 | <0.001 | <0.001 |
| Percentage PDGF-B | 21.95±1.73 (22.00) | 24.9±3.06 (25.50) | 18.95±1.54 (19.00) | 0.001 | <0.001 | <0.001 |
| Hb (gm/dL) | 10.96±2.34 (10.90) | 10.98±2.30 (10.95) | 13.3±1.32 (13.05) | 0.979 | <0.001 | <0.001 |
| PC (%) | 92.05±5.02 (93.00) | 76.43±25.21 (90.00) | 98.45±4.24 (100.00) | 0.013 | <0.001 | 0.001 |
| APTT (s) | 82.45±5.47 (82.50) | 39.50±2.52 (39.50) | 31.68±4.64 (31.00) | <0.001 | <0.001 | <0.001 |
P1=P-value between Group I and Group II; P2=P-value between Group I and the controls group; P3=P-value between Group II and the control group; SD=Standard deviation; Hb=Hemoglobin; PC=Prothrombin concentration; APTT=Activated partial thromboplastin time
There was a highly significant correlation between DD and PDGF-B in all cases and the control group (P < 0.001) as illustrated in Figure 2.
Figure 2.
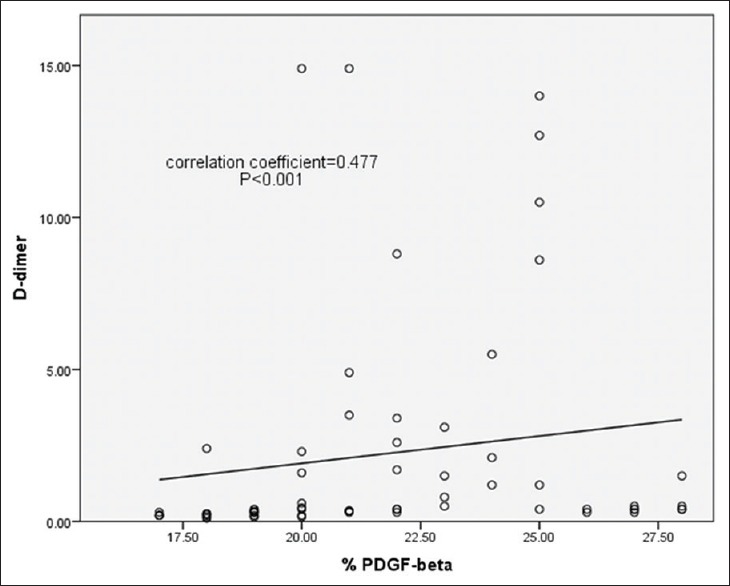
The correlation coefficient between D-dimer and platelet-derived growth factor-beta in all cases and the control group (P < 0.001)
The output data of the receiver operator characteristic curve for discrimination power of PDGF-B and DD to differentiate between cases and control is shown in Table 3. The cutoff value was 21.5 and 0.375 for PDGF-B and DD, respectively. The specificity and sensitivity of PDGF-B were 95% and 72.5%, respectively, and that of DD 90% and 90%, respectively.
Table 3.
The output data of the receiver operating characteristic curve for discriminative power of platelet-derived growth factor-beta and D-dimer to differentiate between cases and control
| Area under curve | 95% CI | P | Cutoff value | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Percentage PDGF-B | 0.913 | 0.844 | 0.982 | <0.001 | 21.5 | 72.5 | 95 |
| D-dimer | 0.940 | 0.883 | 0.997 | <0.001 | 0.375 | 90 | 90 |
CI=Confidence interval; PDGF-B=Platelet-derived growth factor-beta
DISCUSSION
DVT is a common condition that cannot be diagnosed solely on the basis of clinical presentation due to the lack of sensitivity and specificity of signs and symptoms,[17] and a missed diagnosis of DVT can lead to sudden death, chronic cardiopulmonary dysfunction, and impaired quality of life.[18]
PDGF-B is one of the most potent mitogens and chemoattractants for vascular smooth muscle cells as it contains a retention motif (cluster of positively charged amino acids) proposed to bind to negatively charged proteoglycans on the cell surface. Thus, they have an important role in the coagulation process.[12]
The expression of PDFG-B was found to be higher following the vascular injury to promote vascular smooth muscle cell proliferation and migration in the subendothelial region.[19]
Venous thromboembolism biomarker (VEBIOS) study was the first large-scale comprehensive screening for plasma proteomic biomarkers for VTE risk and was proofed that PDGF-B is a novel plasma biomarker for VTE, the inventors aimed to identify novel biomarkers that may improve current clinical diagnosis and prediction tools for VTE risk. They conducted a high-throughput affinity plasma proteomic screening targeting about 400 proteins in relation to VTE risk in a case–control study composed of 88 VTE patients and 85 controls from Sweden. Main findings were replicated in an independent French sample collection (FARIVE) enrolling 580 cases and 589 controls.[20]
Plasma levels of PDGF-B were found associated with VTE in VEBIOS and FARIVE. The significance for the association of PDGF-B protein with VTE risk was P = 0.002.[16]
As a continuation in researches in this subject, we aimed in this study to demonstrate the role of PDGF-B as a specific indicator for DVT.
The present study showed highly significant increase in PDFG-B in all cases compared to control group (23.42 and 18.95, respectively) with P < 0.001. In addition, there was highly significant increase in PDFG-B in both acute and chronic cases of deep venous thrombosis compared to control group (21.95, 24.9, and 18.95, respectively). These results are in accordance with Bruzelius et al. which identified PDGF-B as a novel VTE-associated plasma protein and demonstrated the feasibility of proteome-wide screenings for novel biomarkers for DVT.[16]
The present study found that DD shows a highly significant increase in cases compared to the control group (3.26 and 0.26, respectively) with P < 0.001; furthermore, there was highly significant increase in DD in both acute and chronic cases compared to the control group (4.4 ± 2.12 and 0.26, respectively) with P value of 0.003, <0.001, and < 0.001, respectively.
Regarding the discriminative power of PDGF-B and DD to differentiate between cases and controls, the present study stated that the specificity of PDGF-B was greater than that of DD (95% and 90%, respectively). Thus, we found that PDGF-B was more specific than DD in cases of DVT and was an important tool for the accurate diagnosis of DVT. Therefore, DD can be used as a screening test followed by the estimation of PDGF-B expression in positive cases to confirm the diagnosis because elevated DD may be present in various conditions such as inflammation, pregnancy, liver disease, and cancer. This is in agreement with the study by Owaidah et al., which found that a negative DD result can rule out thrombosis in patients with a low pretest probability score; however, the pretest probability score is less sensitive for DVT and cannot rule out patients with thrombosis.[7]
The study by Heim et al. found that there was a wide variation in the sensitivities of DD for the diagnosis of DVT and an even wider variation in specificities; thus, they did not support the general use of the DD assay alone as a standard test to rule out DVT.[21]
The study by Wells et al. also demonstrated that DVT could be ruled out in patients judged clinically unlikely to have DVT, and a negative DD test and US could be safely omitted in such patients.[22]
In addition, PDGF-B was found to be higher in chronic DVT cases versus acute cases; therefore, it can be used for the diagnosis of chronic cases that to be confused radiologically by venous duplex US.
This is in agreement with Karande et al., who demonstrated that the diagnosis of recurrent DVT is difficult and requires the knowledge of the extent of a residual thrombus that is present at the completion of anticoagulant therapy and depends on the skill of the operator.[5]
CONCLUSION
Every effort should be made to implement the diagnostic strategies for DVT due to the major limitation of DD testing. In our study, we submit a novel association of PDGF-B plasma levels with DVT considering it a more specific marker and indicator for acute venous thrombosis and chronic venous thrombosis cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Previtali E, Bucciarelli P, Passamonti SM, Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011;9:120–38. doi: 10.2450/2010.0066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JS, Moon T, Kim TH, Kim SY, Choi JY, Lee KB, et al. Deep vein thrombosis in patients with pulmonary embolism: Prevalance, clinical significance and outcome. Vasc Specialist Int. 2016;32:166–74. doi: 10.5758/vsi.2016.32.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sathe PM, Patwa UD. D dimer in acute care. Int J Crit Illn Inj Sci. 2014;4:229–32. doi: 10.4103/2229-5151.141435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asim M, Al-Thani H, El-Menyar A. Recurrent deep vein thrombosis after the first venous thromboembolism event: A single-institution experience. Med Sci Monit. 2017;23:2391–9. doi: 10.12659/MSM.901924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karande GY, Hedgire SS, Sanchez Y, Baliyan V, Mishra V, Ganguli S, et al. Advanced imaging in acute and chronic deep vein thrombosis. Cardiovasc Diagn Ther. 2016;6:493–507. doi: 10.21037/cdt.2016.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youssf AI, Ismail MF, ElGhamry R, Reyad MR. Diagnostic accuracy of D-dimer assay in suspected pulmonary embolism patients. Egypt J Chest Dis Tuberc. 2014;63:411–7. [Google Scholar]
- 7.Owaidah T, AlGhasham N, AlGhamdi S, AlKhafaji D, ALAmro B, Zeitouni M, et al. Evaluation of the usefulness of a D dimer test in combination with clinical pretest probability score in the prediction and exclusion of venous thromboembolism by medical residents. Thromb J. 2014;12:28. doi: 10.1186/s12959-014-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim AH, Liu H, Focia PJ, Chen X, Lin PC, He X, et al. Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proc Natl Acad Sci U S A. 2010;107:11307–12. doi: 10.1073/pnas.1000806107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam FW, Vijayan KV, Rumbaut RE. Platelets and their interactions with other immune cells. Compr Physiol. 2015;5:1265–80. doi: 10.1002/cphy.c140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone J, Hangge P, Albadawi H, Wallace A, Shamoun F, Knuttien MG, et al. Deep vein thrombosis: Pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7:S276–84. doi: 10.21037/cdt.2017.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tannenberg P, Chang YT, Muhl L, Laviña B, Gladh H, Genové G, et al. Extracellular retention of PDGF-B directs vascular remodeling in mouse hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2018;314:L593–605. doi: 10.1152/ajplung.00054.2017. [DOI] [PubMed] [Google Scholar]
- 13.Rezayani S, Farazmandfar T, Shahbazi M. Association assessment of platelet derived growth factor B gene polymorphism and its expression status with susceptibility to coronary artery disease. Egypt J Med Hum Genetics. 2017;18:359–63. [Google Scholar]
- 14.Vedantham S, Piazza G, Sista AK, Goldenberg NA. Guidance for the use of thrombolytic therapy for the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:68–80. doi: 10.1007/s11239-015-1318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Xie L, Guo W. PDGF/PDGFR effects in osteosarcoma and the “add-on” strategy. Clin Sarcoma Res. 2018;8:15. doi: 10.1186/s13569-018-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruzelius M, Iglesias MJ, Hong MG, Sanchez-Rivera L, Gyorgy B, Souto JC, et al. PDGFB, a new candidate plasma biomarker for venous thromboembolism: Results from the VEREMA affinity proteomics study. Blood. 2016;128:e59–66. doi: 10.1182/blood-2016-05-711846. [DOI] [PubMed] [Google Scholar]
- 17.Pulivarthi S, Gurram MK. Effectiveness of d-dimer as a screening test for venous thromboembolism: An update. N Am J Med Sci. 2014;6:491–9. doi: 10.4103/1947-2714.143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goubran HA, Sholkamy S, El-Haddad A, Mahmoud A, Rizkallah MA, Sobhy G, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting: Report from the ENDORSE study in Egypt. Thromb J. 2012;10:20. doi: 10.1186/1477-9560-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen SB, Hindberg K, Solomon T, Smith EN, Lapek JD, Jr, Gonzalez DJ, et al. Discovery of novel plasma biomarkers for future incident venous thromboembolism by untargeted synchronous precursor selection mass spectrometry proteomics. J Thromb Haemost. 2018;16:1763–74. doi: 10.1111/jth.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heim SW, Schectman JM, Siadaty MS, Philbrick JT. D-dimer testing for deep venous thrombosis: A metaanalysis. Clin Chem. 2004;50:1136–47. doi: 10.1373/clinchem.2004.031765. [DOI] [PubMed] [Google Scholar]
- 22.Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–35. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]


