Abstract
Modern surgical management of extremity bone sarcomas is governed by limb-sparing surgery combined with adjuvant and neoadjuvant chemotherapy.
All the resection and reconstruction techniques have to achieve oncologic excision margins, with survival rates and functional results superior to amputation.
The main reconstruction techniques of bone defects resulted after resection are: modular endoprosthetic reconstruction; bone graft reconstruction; bone transport; resection arthrodesis; and rotationplasty.
Oncologic resection and modular endoprosthetic reconstruction are the generally approved surgical options adopted for the majority of cases in major specialized bone sarcoma centres.
Good basic principles, efficient multidisciplinary approach and sustained research in the field can provide a better future for the challenge posed by extremity bone sarcoma treatment.
Cite this article: EFORT Open Rev 2019;4:174-182. DOI: 10.1302/2058-5241.4.180048
Keywords: Bone sarcoma, reconstruction, modular endoprostheses
Introduction
The dawn of extremity bone sarcoma management was dominated by amputation as a standard of surgical treatment. Although some attempts were made to perform limb-sparing procedures, by carrying out segmental resection and reconstruction using bone grafts or endoprostheses, the limiting factor for favourable and sustainable results was local tumour recurrence. Everything changed when chemotherapy (neoadjuvant or adjuvant) and radiation therapy were instituted in the treatment protocols for extremity sarcomas during the last quarter of the 20th century, driving down the risk for local recurrence after both ablative or limb-preserving procedures. Furthermore, the development of medical imaging and surgical techniques ensured more efficient, oncologic resections of sarcomas and, with that, limb-sparing procedures became the generally accepted standard of treatment.
Advancements made in recent decades have made limb salvaging a sustainable option in the majority of cases, thus avoiding mutilating procedures like rotationplasty or amputation which bring a big psychological burden to the patients. As the patient survival rates increased dramatically and the limb-salvage procedures became the standard, the attention was focused on developing lasting reconstructions for a patient population comprising mainly young and active individuals.
The main reconstruction options available in the arsenal of musculoskeletal oncology today are: modular endoprosthetic reconstruction; bone graft reconstruction; bone transport; arthrodesis; and rotationplasty. Although each one of these reconstruction possibilities carries its own benefits and disadvantages, they all have to obey a minimum set of requirements and the choice between them has to be made on an individual basis. The main aspects that need to be considered when choosing the suitable procedure for managing an extremity sarcoma are:
the general status of the patient (age, lifestyle, psychological state, local soft tissue conditions, work requirements);
pathological staging of the tumour (grading, histologic type, predictive histological and immunohistochemical markers) after a properly executed biopsy, by an experienced orthopaedic surgeon;
radiological staging (local extension and invasion, location, presence of lung or skip metastases).
The essential requirements for any limb-sparing procedure include: a subsequent tumour recurrence risk lower than after amputation; the possibility of achieving adequate resection margins; a durable reconstruction; low incidence of complications; and no negative effects on the adjuvant therapy protocol. Nowadays, a marginal or wide resection combined with adjuvant chemotherapy and followed by reconstruction is to be preferred over amputation. This makes the resection limit a crucial element in the challenging process of choosing the right surgical technique.
Indication for resection and reconstruction
The indication for reconstruction of a segmental defect after surgical excision of bone sarcoma is valid if the resection performed achieves oncologic safe margins, clear of any tumour tissue, and the functional results and survival rates expected are equal or better than after an amputation performed for the same case (Fig. 1).
Fig. 1.
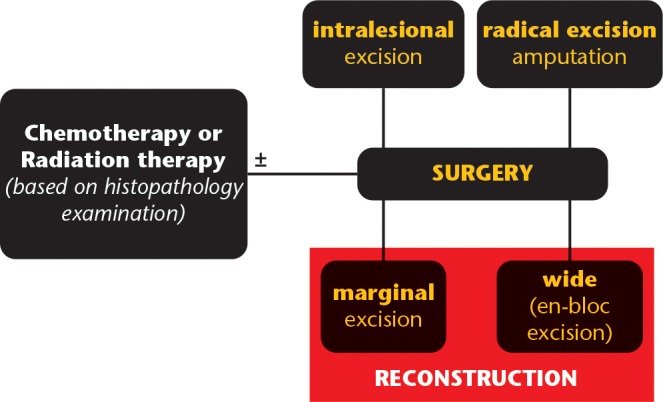
Graphic representation of treatment algorithm for extremity bone sarcomas.
Amputation
Although it was the mainstay of extremity sarcoma treatment in the dawn of musculoskeletal oncology, the advances that favoured limb-sparing procedures have shifted the paradigm away from amputation which nowadays is performed just for selected cases, with a prevalence < 10% in the majority of cancer centres. The indication for limb ablation in the setting of an extremity sarcoma is reserved for those cases that cannot be treated with a limb-sparing procedure due to established loss of limb function or an expected loss of function caused by the tumour invasion or an oncologic resection of the tumour is impossible. Hand or foot tumours are special cases for which the complex anatomy of the involved segment cannot be feasibly reconstructed and commonly require ablative surgery.
Amputation procedures can be classified as early amputations when they are performed as a primary means of surgical control or delayed (secondary) amputations after a failed limb-sparing procedure. Another instance when amputation can be performed is when it serves as a palliative measure for non-resectable tumours complicated with pathological fractures, soft-tissue problems, intractable pain or bleeding. The current consensus is that there is no unequivocal superiority of ablative or limb-sparing surgery concerning the long-term survival of the patients with extremity sarcomas. In addition, it is considered that local recurrence, associated with the worst prognosis, is mainly determined by tumour biology. This comes from the hypothesis that the patients with local recurrence are a subset of patients with extremely aggressive and chemoresistant tumours which would have a bad prognosis after any type of surgical treatment.1
Rotationplasty
Named by some a modified amputation procedure, rotationplasty can be employed for those cases when a salvage reconstructive procedure is not feasible and an above-the-knee amputation or a hip disarticulation would be required. The main reason this technique is sometimes preferred over a radical ablative procedure is that it ensures better functional results by providing a longer residual limb with better fitting for an external prosthesis. All rotationplasty procedures require preservation of the blood supply to the distal residual stump and sometimes intact nerve supply.
The most popular rotationplasty is the Van Nes rotationplasty, developed for reconstruction after resection of distal femoral tumours. This technique also requires conservation of the distal blood supply and integrity of the sciatic nerve, the remaining distal part of the extremity being rotated by 180° and reattached to the proximal stump in such way that the ankle joint is set at the contralateral knee joint level. The rotation is required because, for the ankle joint to restore and perform the same function, its extension has to be transformed into a flexion movement with the same direction as the one of the joints it replaces (knee). This biological reconstruction technique is intended for paediatric patients, requiring a complex rehabilitation protocol with external prosthesis to compensate for the shortening of the limb. Although it is considered a limb-sparing procedure and it preserves proprioception, it comes with important aesthetic dilemmas and hesitations, which is why it is very rarely used nowadays.
Another variety is the tibial turnplasty which reconstructs a longer femoral stump using the residual tibia as a vascularized, 180° rotated autograft. Perhaps the most peculiar reconstruction technique is the tibia-hindfoot osteo-musculo-cutaneous rotationplasty which can be used after proximal femoral resections with hip disarticulation. It requires the preservation of the distal tibia, talus and calcaneus which are used as osteoarticular autografts. A proximal femoral stump is reconstructed by rotating the distal segment 180° in the sagittal and 90° in the transversal plane and surgically fusing the calcaneal tuberosity into the acetabulum.2
Segmental bone transport
The technique is based on resection and subsequent reconstruction of the segment using distraction osteogenesis under the stabilization and guidance, most often of an external, Ilizarov-type fixator (ring fixator). Typical segmental defects which can be restored using this technique are metaphyseal or diaphyseal by location, resulted after resections that spare the adjacent joint. Lengths of the defect amendable to this kind of reconstruction have been limited to a maximum of 5–6 cm due to extended periods of external fixation which carry specific risks and the restraints imposed by the soft tissue. The amount of distraction applied per day is constrained to 1 mm for adequate callus formation. In order to achieve maximum lengthening during the shortest period of time, bifocal or trifocal transport techniques, with multiple osteogenesis sites, have been developed. Newer, internal lengthening devices, in the form of special intramedullary nails, have been introduced to prevent the risks associated with the use of external fixation devices.
Specific concerns for using bone transport as a reconstruction procedure after sarcoma resections are: the infection risk augmented by the immunosuppression (chemotherapy and/or radiation therapy); possible tumour cell activation; regenerate fracture; muscle contractures; nerve overstretching; implant failure; nonunion; delayed or premature union; axial or joint alignment alterations; and high psychological burden for the patient. The suggested advantages of this technique are: precise restoration of limb length discrepancies; stimulation effect on soft-tissue healing; easier management of potential infection; and durable biologic reconstruction with good long-term functional results.3
Resection arthrodesis
This reconstruction procedure can be performed after the resection of a tumour neighbouring a joint, with surgical fusion of the joint achieved using bone autografts or allografts and fixation by osteosynthesis implants (long intramedullary nails or plates and screws). The most suitable indication for this procedure is a patient with special, heavy work requirements that include the need for a stable limb with durable reconstruction. Some of the disadvantages of this treatment option are the complete loss of joint motion, lower quality of life and an associated risk for implant failure, fracture or infection.
A special subtype is the temporary resection-arthrodesis of the knee utilizing a long intramedullary rod and acrylic bone cement described by Professor Mario Campanacci (Fig. 2). This technique has been recommended for very young patients with high growing potential as a debulking procedure when metastases are already present or when other reconstruction options are not readily available. In addition, it can be used as a temporary reconstruction until a favourable oncologic prognosis is achieved and a permanent reconstruction can be performed.
Fig. 2.
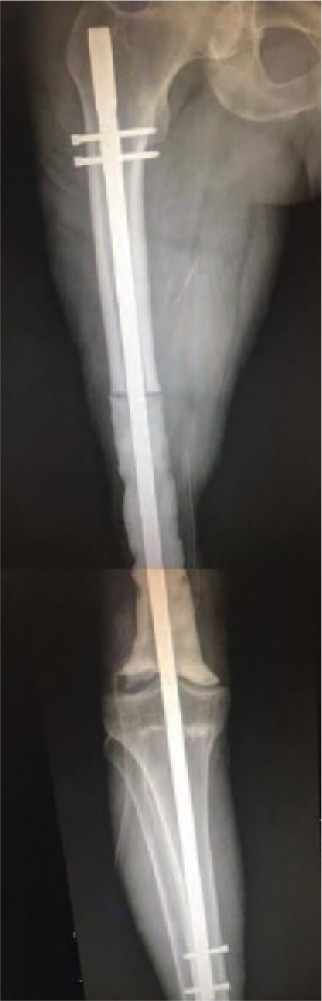
Temporary resection arthrodesis of the knee using a long intramedullary nail and bone cement (Campanacci technique).
Bone graft reconstruction
A very heterogeneous group of reconstruction techniques after bone sarcoma resections use bone grafts in the form of: vascularized or non-vascularized autografts; allografts; osteochondral allografts; allograft and endoprosthetic composite (APC); or synthetic bone grafts.
Bone autografts can be considered the gold standard for bone defects reconstruction because of the following advantages: a biologic reconstruction is granted; highest osteoconductive, osteoinductive and osteogenic potential; and the lowest risk for immune graft rejection or disease transmission. The limitations of this reconstruction material are posed by donor site morbidity and its restricted availability, with the iliac crest and the fibula (vascularized or non-vascularized graft options) as main sources (Fig. 3). The use of a vascularized fibular graft implies a tedious microsurgical reconstruction procedure which is very technically demanding and provides a graft with high healing potential but low biomechanical profile, due to its dimensions, which predisposes it to fracture.4
Fig. 3.
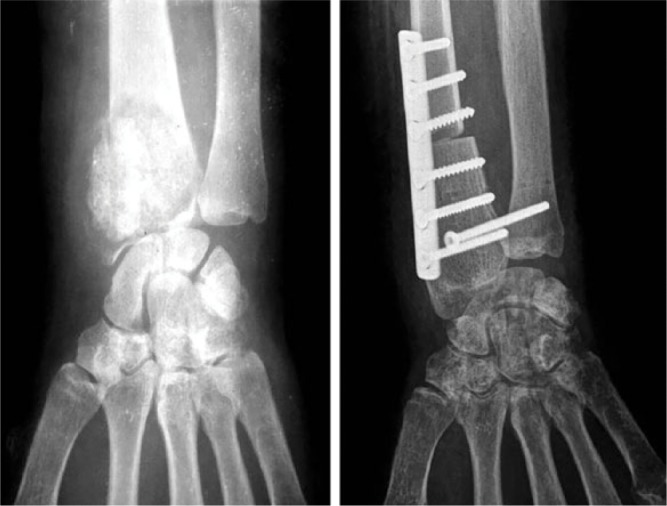
Distal radius reconstruction after giant cell tumour resection using fibular autograft.
Bone allografts can be used for intercalary defects reconstruction or as osteochondral allografts, but often with modest long-term functional results. Despite the fact that they are more accessible and come in larger sizes, there are a few important drawbacks that restrict their use: legal restrains; disease transmission and immune rejection risks; osteoarthritis for osteochondral segments; lower healing potential; the need for prolonged non-weight-bearing; and the infamous bone resorption which is a characteristic for bone allografts (Fig. 4). The same hazards are characteristic for the APC reconstruction technique with some added, implant specific complications such as structural failure, peri-prosthetic fracture, aseptic loosening or infection. The APC usually consists of a revision type implant combined with a bone allograft which is the element that presumably improves stability and soft-tissue coverage by providing more secure, biological tendon and muscle reinsertions.5
Fig. 4.
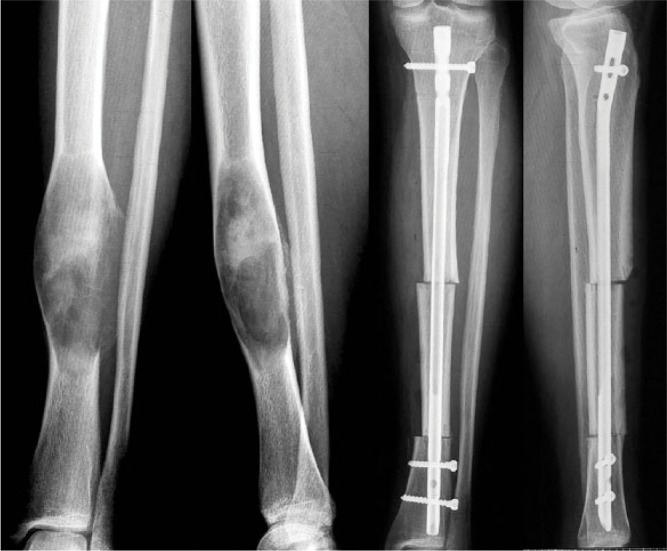
Tibia reconstruction after Parker Jackson lymphosarcoma resection using allografting.
A special reconstruction technique that uses extracorporeal irradiation (ECI) of autografts or allografts has been developed, with promising oncologic results especially for children that need surgical resection of primary bone sarcomas.6
Synthetic bone grafts can be used as a substitute for the biologic bone grafts. As the bone defects resulted after extremity sarcoma resections are rather large, the main problem for these substitutes is to provide adequate structural support along with good osteoconductive and osteoinductive properties. The main groups of bioceramics used as bone graft substitutes are based on: calcium sulphate; calcium phosphate (CaP ceramics); hidroxyapatite (HA); and bioactive glass. There is continuous research aimed at improving the biology of these synthetic grafts (addition of bone morphogenic proteins (BMPs), tissue engineering and stem cell technology) or their biomechanical proprieties (incorporation of carbon nanotubes (CNTs), nanostructural design).7
Modular endoprosthetic reconstruction
The principle of limb-sparing surgery dominates the modern management of primary malignant bone tumours. For the reconstruction of massive bone defects resulted after the resection of bone sarcoma standard nowadays is the use of modular endoprosthetic systems, performed usually in major cancer centres, by experienced surgeons. The origins of these modular reconstruction systems can be traced to the first resection and reconstruction procedures performed for malignant bone tumours, which required custom-made implants for each case. All the progress made in medical imaging, chemotherapy or surgical techniques has increased the survival of the patients; thus, the demand for custom implants has also increased exponentially. This raised some new challenges for the implant manufacturers concerning material engineering, design, intra-operative versatility and nonetheless long manufacturing times.
The solution came with the concept of modular reconstruction systems which allowed a great deal of flexibility and variety in use, a big help for surgeons who can now adapt better to the intra-operative scenario and change the reconstruction plan accordingly. Component standardization allowed for better quality control of implants and drove down the costs and production times. Despite the fact that the complication rate is higher compared with primary joint replacement surgery, the overall survival rate for the megaprosthetic systems can reach up to 91%.8 Although modularity has solved many of the problems, there are still some difficult cases which require custom-made implants for complex reconstructions, especially after pelvic resections.
Technical considerations and reconstruction principles
The most commonly reconstructed segments are the proximal tibia, proximal humerus, distal and proximal femur, and the adjacent joints. The design of the modular systems allows not only segmental bone reconstruction but also total bone replacement of humerus or femur (Fig. 5). The reconstructed segment can incorporate one or more joints, or it can be an intercalary, diaphyseal segment which is being replaced (Fig. 6). There are some basic guidelines that have to be obeyed when performing a modular endoprosthetic reconstruction: careful preoperative planning; adequate resection margins; restoration of limb length and normal axis of motion; proper selection of components and fixation technique; good bone preparation; and appropriate soft-tissue reconstruction (tendon reattachment, muscle flaps, skin flaps).
Fig. 5.
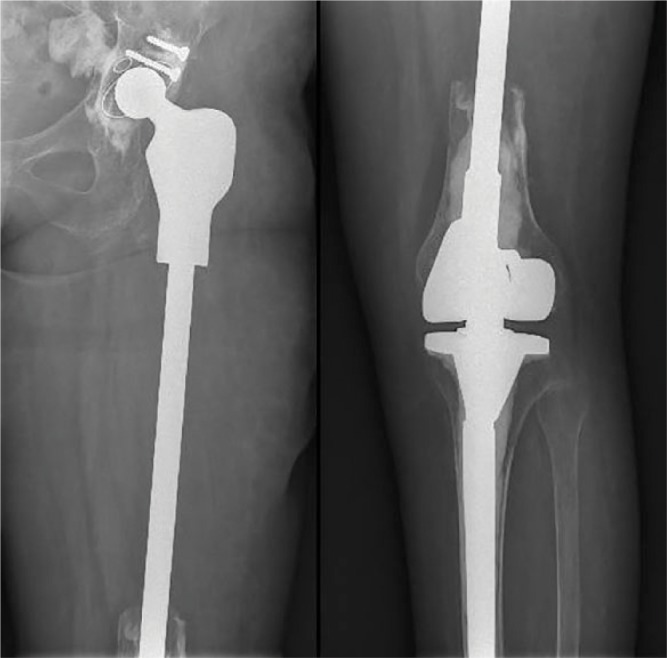
Total femoral reconstruction with ‘push through’ diaphyseal module.
Fig. 6.
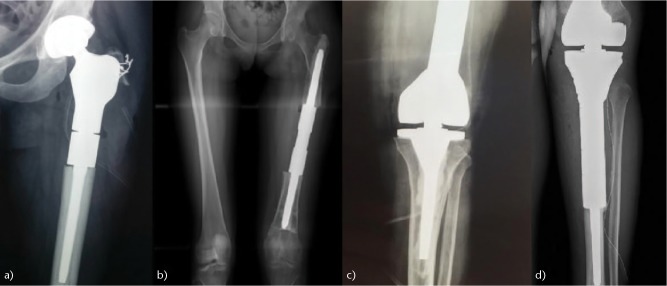
(a) Proximal femur, (b) diaphyseal, (c) distal femur and (d) proximal tibia modular endoprosthetic reconstructions.
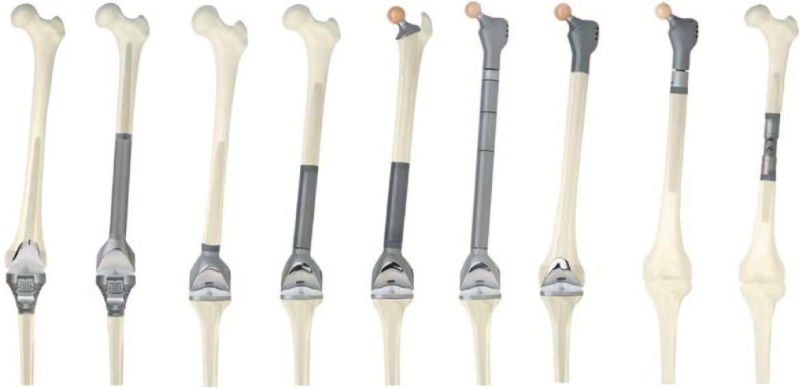
Different design variations of the systems used for the same segment replacement allow a variable level of bone sparing. For example, the femoral greater trochanter or a big part of the condyles can be spared in some instances. Diaphyseal segments of variable lengths can be either totally replaced or the cortical bone can be spared using ‘push through’ modules (Fig. 7). By using lower profile implants and thus preserving more bone material, the reconstruction is more anatomical and also provides better biomechanics by sparing muscle and tendon insertions.
Fig. 7.
Modular endoprosthetic reconstruction options for proximal tibia or proximal, diaphyseal, distal or total femur. Reprinted with permission from MEGASYSTEM-C® Tumor and Revision Surgery – Implants & Instruments, Waldemar Link GmbH & Co. KG Hamburg, Germany.
Some other technical advantage offered by the reconstruction systems is the possibility to adjust, intra-operatively, the torsional position between modules and the total length of the construct. This represents a crucial advantage during the very complex and challenging resection and reconstruction procedures performed in musculoskeletal oncology. The stem designs support cemented or cementless fixation by taking advantage of different stem shapes and hydroxyapatite or hydroxyapatite tricalcium phosphate coating used to promote induction of osteogenesis. The preferred method of stem fixation is usually without using acrylic cement, with the exception of the cases where a stable, cementless fixation cannot be achieved. Poor bone quality or inadequate size, residual bone segment are the main reasons for choosing a cemented stem fixation.
Another recognized feature is the use of circumferential porous metal or hydroxyapatite covered collars at the contact point between the stem shoulder and the bone resection margin in order to stimulate bone ingrowth. This extracortical bone bridging is believed to increase the mechanical stability and seal the intramedullary canal, shielding the bone–stem interface from any kind of external debris that can cause osteolysis.9 Another approach to obtaining good bone ingrowth is by applying high pressures at the level of bone–implant interface (compressive osseointegration). This is made possible by using special technology with shorter prestressed stems which allows fixation into short residual bone segments.
A challenging problem is the reattachment of tendons and muscles, which is crucial to post-operative functional recovery and overall stability of the system. For this purpose, the design of the components has been adapted to allow and promote fixation of the soft tissue. Reattachment options include plate and screws systems (patellar tendon reattachment) or direct suturing to the metallic components or to specially designed, flexible, synthetic mesh (polyethylene terephthalate (PET), polypropylene, polytetrafluoroethylene or carbon fibre) attached to the metallic part. The ingrowth potential of the soft tissue into the metallic components has been investigated with promising results offered by a porous tantalum and titanium.
Concerning the joint replacement, knee reconstruction is achieved using a rotating hinge system as a standard for maximum stability and mobility. For the hip joint, use of a bipolar acetabular component is recommended, which, in combination with careful restoration of the muscle insertions and augmented by capsular reconstruction using synthetic grafts, gives the lowest risk of dislocation. By using lower constraint implants, especially for the knee (rotational hinge), the mechanical stress transmitted to the adjoining structures and to the implant components is reduced; hence, the risk for mechanical complications is minimized.10
Contraindication and failure classification
An absolute contraindication for limb salvage and reconstruction with modular systems is represented by one of the following situations: 1) a massive tumour which encloses major neurovascular structures; 2) an improper biopsy procedure and/or subsequent site complications; 3) inadequate soft-tissue coverage; or 4) a pathological fracture which usually implies considerable contamination. Some relative contra-indications for choosing a limb-sparing procedure are: an extremely young patient with a high risk for limb length inequality; a mediocre response to neoadjuvant therapy (radiation therapy or chemotherapy); and exceptional work requirements.
For improving evaluation of the results, a systematization of failure possibilities has been developed for the reconstructions achieved with modular megaprostheses.11 The classification resulted includes two main groups of failure modes, mechanical and non-mechanical, each of them incorporating further subcategories (Table 1). Another advantage suggested by this categorization is an easier identification of risk factors and elements which can be addressed for improvement of the overall results.
Table 1.
Modular endoprothetic failure classification
| Main category | Failure mode | Classification |
|---|---|---|
| Mechanical failure | Soft-tissue failure | Type I |
| Aseptic loosening | Type II | |
| Structural failure | Type III | |
| Non-mechanical failure | Infection | Type IV |
| Tumour progression | Type V |
Henderson E, Groundland J, Marulanda GA, et al. Peri-operative expectations with revision of lower extremity segmental megaprosthesis for tumor. Podium presented at the American Academy of Orthopedic Surgeons 2010 Annual Meeting; March 9-13, 2010; New Orleans, LA. Podium No. 409
Expandable endoprostheses
A large patient population that requires surgery for bone sarcomas is represented by skeletally immature children, for whom reconstruction with modular endoprosthetic systems is usually not a feasible option. The main challenge comes from the limb length differences resulted from affected growth plates and the difficulty of following recovery protocols. The alternative represented by amputation or rotationplasty comes with a high psychological burden.
In order to address these problems, expandable endoprostheses with different lengthening mechanisms were conceived and developed over the last decade. A minimum limb length inequality of 3 to 4 cm is to be expected in order to consider using a growing prosthesis.12 First, adjustments for elongation used to require open surgery, which carried a high risk for infection and associated multiple anaesthesia. The modern expandable modular systems use non-invasive lengthening procedures, with the internal expansion mechanism activated and controlled by external electromagnetic sources.13
Discussion
Although the survival rates of patients treated for extremity sarcomas have improved significantly and the reconstructive procedures are now the rule for surgical treatment, the matter of these surviving patients is much more complex and requires greater efforts for improving their post-operative quality of life. Overall, the musculoskeletal complications rate for long-term survivors of extremity sarcomas is very high, up to 80% in some reports, and the functional outcomes are still limited.14 Although the complication rates for the modular megaprostheses reconstructions are consistently greater compared with regular endoprosthetic techniques, their use has become the standard of care for > 90% of the extremity sarcoma cases, in the hands of experienced musculoskeletal oncology surgeons.
The overall survival periods for modular megaprostheses reach up to 80% at ten years and consistently over this percent at five years.15 The most feared complication, with the highest incidence reported in literature (5% to 12%) is infection (type IV failure), which has a different risk distribution for specific anatomic locations of the resection. This disparate risk association comes from the variable soft-tissue coverage for individual segments charging the proximal tibial reconstructions with the highest risk for infection and making the proximal femur least hazardous.16 The contrary can be said about proximal femoral reconstructions when we refer to the dislocation risk which is the greatest for the hip joint replacement.
A great deal of progress has been made in the surgical reconstruction of bone defects resulted after resection of not only extremity bone sarcoma but also of metastatic lesions or other non-neoplastic conditions like severe bone loss due to trauma, infection and revision surgery, bone defects caused by nonunion, malunion, implant failure or peri-prosthetic fractures.17,18 Every specific type of complication is under scrutiny and continuous research is aimed at developing new technologies and ways to prevent or treat this onerous situations.
In the treatment of extremity bone sarcoma, predictors of local recurrence have a great importance because this complication has the greatest impact on patient survival. A study conducted by Jay et al found that a chemotherapy response of < 90% necrosis and < 2 mm resection margins are the best predictors of local recurrence when compared with narrower and wider margins as well as classical margin description (intralesional, marginal, wide and radical).19 A new method for predicting metastatic occurrence in osteosarcoma cases has been suggested, based on age, sex, tumour size, anatomic site, tumour grade, histologic classification, monocyte ratio and neutrophil-to-lymphocyte ratio.20 The importance of immunohistochemistry (IHC) in bone sarcomas has proven its diagnostic and prognostic role. Due to novel IHC procedures and molecular studies, new more-targeted therapies will be available in the near future.21
The risk for early infection after modular megaprosthetic reconstructions has been addressed by modifying the implants surface through coating with various bacteriostatic or bactericidal agents like silver, iodine, nitric oxide, antibiotics and antimicrobial peptides with new research being directed at inhibiting bacterial colonization of modified implants by way of biomimetic surface functionalization (lotus effect, dragonfly wings).22,23 Computer-assisted surgery in the form of custom, individual patient instruments or computerized intra-operative guidance has been proposed as an effective way of ensuring better resection margins and a more precise matching of allografts.24 Three-dimensional (3D) printing, also called additive layer manufacturing (ALM), nowadays allows the fabrication of more durable, lighter, custom implants or personalized instruments.25
There are some other modern topics of interest in bone sarcoma surgery worth mentioning. Robotic surgery in conjunction with computer guidance could also help obtain improved resection margins in the future.26 A FDG-PET (fludeoxyglucose positron emission tomography) tracer can be administered pre-operatively for certain types of tumours and the radioactive uptake can be measured with a probe during surgery, providing a better assessment of tumour margins.27,28 The use of nanotechnology in orthopaedic surgery is a concept that is being currently explored. In order to improve local control after resection, bioactive orthopaedic implants can be used for local delivery of treatment to the tumour bed.29 In the future, a reliable biomarker of response to chemotherapy could help manage bone sarcoma more efficiently along with new imaging techniques such as spectroscopy in functional MRI (fMRI) or blood markers for circulating tumour cells.30,31
Conclusion
Contemporary surgical management of extremity bone sarcomas is based mainly on endoprosthetic reconstructive procedures that take advantage of the modular systems, offering immediate mechanical support and functional recovery. Despite this trend, there is still an important role for all the other reconstruction options and ablative procedures to be played in musculoskeletal oncology. The treatment pillars for obtaining good oncological results in treating extremity bone sarcomas are precise diagnosis combined with excellent surgical technique and adjuvant chemotherapy. Proper resection followed by reconstruction is to be preferred over amputation nowadays and numerous innovations are helping the surgeons obtain better margins, and to provide solid reconstructions with acceptable patient morbidity. We always have to keep in mind that the management of extremity bone sarcomas is a multidisciplinary teamwork and specialized centres with experienced musculoskeletal surgeons represent the best future for approaching this challenging pathology.
Footnotes
ICMJE Conflict of interest statement: The author declares no conflict of interest relevant to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Erstad DJ, Ready J, Abraham J, et al. Amputation for extremity sarcoma: contemporary indications and outcomes. Ann Surg Oncol 2018;25(2):394–403. doi: 10.1245/s10434-017-6240-5 [DOI] [PubMed] [Google Scholar]
- 2. Most MJ, Sim FH. Limb Salvage in Skeletally Immature Patients with Extremity Sarcoma. InCañadell’s Pediatric Bone Sarcomas. Cham: Springer; 2016:75–101. [Google Scholar]
- 3. Lesensky J, Prince DE. Distraction osteogenesis reconstruction of large segmental bone defects after primary tumor resection: pitfalls and benefits. Eur J Orthop Surg Traumatol 2017;27(6):715–727. doi: 10.1007/s00590-017-1998-5 [DOI] [PubMed] [Google Scholar]
- 4. Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J 2016;98(1_Supple_A):6–9. doi: 10.1302/0301-620X.98B.36350 [DOI] [PubMed] [Google Scholar]
- 5. Gautam D, Malhotra R. Megaprosthesis versus allograft prosthesis composite for massive skeletal defects. J Clin Orthop Trauma 2018;9(1):63–80. doi: 10.1016/j.jcot.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong AM, Millington S, Ahern V, et al. Limb preservation surgery with extracorporeal irradiation in the management of malignant bone tumor: the oncological outcomes of 101 patients. Ann Oncol 2013;24(10):2676–2680. doi: 10.1093/annonc/mdt252 [DOI] [PubMed] [Google Scholar]
- 7. Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2017;2(4):224–247. doi: 10.1016/j.bioactmat.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palumbo BT, Henderson ER, Groundland JS, et al. Advances in segmental endoprosthetic reconstruction for extremity tumors: a review of contemporary designs and techniques. Cancer Contr 2011;18(3):160–170. [DOI] [PubMed] [Google Scholar]
- 9. Goulding KA, Gaston CL, Grimer RJ. Outcomes and options for prosthetic reconstruction after tumour resection about the knee. Curr Surg Rep 2014;2(2):42. doi: 10.1007/s40137-013-0042-x [DOI] [Google Scholar]
- 10. Rod Fleury T, Miozzari HH, Hoffmeyer PJ. Management of malignant bone tumors around the knee. Rev Med Suisse 2014;10(455):2403–2406, 2408. [PubMed] [Google Scholar]
- 11. Henderson ER, Groundland JS, Pala E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg [Am] 2011;93-A(5):418–429. doi: 10.2106/JBJS.J.00834 [DOI] [PubMed] [Google Scholar]
- 12. Gilg MM, Wibmer C, Bergovec M, Grimer RJ, Leithner A. When do orthopaedic oncologists consider the implantation of expandable prostheses in bone sarcoma patients? Sarcoma 2018;2018:3504075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruggieri P, Mavrogenis AF, Pala E, et al. Outcome of expandable prostheses in children. J Pediatr Orthop 2013;33(3):244–253. doi: 10.1097/BPO.0b013e318286c178 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Pineda I, Hudson MM, Pappo AS, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: a report from the St. Jude Lifetime Cohort Study. J Cancer Surviv 2017;11(1):1–12. doi: 10.1007/s11764-016-0556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Capanna R, Scoccianti G, Frenos F, et al. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin Orthop Relat Res 2015;473(3):820–830. doi: 10.1007/s11999-014-3736-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pala E, Henderson ER, Calabrò T, et al. Survival of current production tumor endoprostheses: complications, functional results, and a comparative statistical analysis. J Surg Oncol 2013;108(6):403–408. doi: 10.1002/jso.23414 [DOI] [PubMed] [Google Scholar]
- 17. Szendrői M, Antal I, Szendrői A, Lazáry Á, Varga PP. Diagnostic algorithm, prognostic factors and surgical treatment of metastatic cancer diseases of the long bones and spine. EFORT Open Rev 2017;2(9):372–381. doi: 10.1302/2058-5241.2.170006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Gori M, Scoccianti G, Frenos F, et al. Modular endoprostheses for nonneoplastic conditions: midterm complications and survival. Biomed Res Int 2016;2016:2606521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeys LM, Thorne CJ, Parry M, et al. A novel system for the surgical staging of primary high-grade osteosarcoma: the Birmingham Classification. Clin Orthop Relat Res 2017;475:842–850. doi: 10.1007/s11999-016-4851-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang S, Zheng S, Hu K, et al. A predictive model to estimate the pretest probability of metastasis in patients with osteosarcoma. Medicine (Baltimore) 2017;96(3):e5909. doi: 10.1097/MD.0000000000005909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang P, Li J, Song Y, Wang X. MiR-129-5p inhibits proliferation and invasion of chondrosarcoma cells by regulating SOX4/Wnt/β-Catenin signaling pathway. Cell Physiol Biochem 2017;42(1):242–253. [DOI] [PubMed] [Google Scholar]
- 22. Schmolders J, Koob S, Schepers P, et al. Lower limb reconstruction in tumor patients using modular silver-coated megaprostheses with regard to perimegaprosthetic joint infection: a case series, including 100 patients and review of the literature. Arch Orthop Trauma Surg 2017;137(2):149–153. doi: 10.1007/s00402-016-2584-8 [DOI] [PubMed] [Google Scholar]
- 23. Shirai T, Tsuchiya H, Nishida H, et al. Antimicrobial megaprostheses supported with iodine. J Biomater Appl 2014;29(4):617–623. doi: 10.1177/0885328214539365 [DOI] [PubMed] [Google Scholar]
- 24. Jeys L, Morris G, Evans S, et al. Surgical innovation in sarcoma surgery. Clin Oncol (R Coll Radiol) 2017;29(8):489–499. doi: 10.1016/j.clon.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 25. Murr LE, Gaytan SM, Martinez E, Medina F, Wicker RB. Next generation orthopaedic implants by additive manufacturing using electron beam melting. Int J Biomater 2012;2012:245727. doi: 10.1155/2012/245727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lang JE, Mannava S, Floyd AJ, et al. Robotic systems in orthopaedic surgery. J Bone Joint Surg [Br] 2011;93-B(10):1296–1299. doi: 10.1302/0301-620X.93B10.27418 [DOI] [PubMed] [Google Scholar]
- 27. Iwamoto S, Burrows RC, Born DE, Piepkorn M, Bothwell M. The application of direct immunofluorescence to intraoperative neurosurgical diagnosis. Biomol Eng 2000;17(1):17–22. [DOI] [PubMed] [Google Scholar]
- 28. Mondal SB, Gao S, Zhu N, et al. Real time fluorescence image-guided oncologic surgery. Adv Cancer Res 2014;124:171–211. doi: 10.1016/B978-0-12-411638-2.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savvidou OD, Bolia IK, Chloros GD, et al. Applied nanotechnology and nanoscience in orthopedic oncology. Orthopedics 2016;39(5):280–286. doi: 10.3928/01477447-20160823-03 [DOI] [PubMed] [Google Scholar]
- 30. Kubo T, Furuta T, Johan MP, Adachi N, Ochi M. Percent slope analysis of dynamic magnetic resonance imaging for assessment of chemotherapy response of osteosarcoma or Ewing sarcoma: systematic review and meta-analysis. Skeletal Radiol 2016;45(9):1235–1242. doi: 10.1007/s00256-016-2410-y [DOI] [PubMed] [Google Scholar]
- 31. Amit P, Malhotra A, Kumar R, et al. Evaluation of static and dynamic MRI for assessing response of bone sarcomas to preoperative chemotherapy: correlation with histological necrosis. Indian J Radiol Imaging 2015;25(3):269–275. doi: 10.4103/0971-3026.161452 [DOI] [PMC free article] [PubMed] [Google Scholar]