 Thienopyridone and iminothienopyridinedione inhibit protein phosphatases through a redox mechanism to oxidise the active site cysteine.
Thienopyridone and iminothienopyridinedione inhibit protein phosphatases through a redox mechanism to oxidise the active site cysteine.
Abstract
Thienopyridone (TP) has been proposed as a selective inhibitor of phosphatases of regenerating liver (PRL or PTP4A). PRLs are dual specificity phosphatases that promote cancer progression and are attractive anticancer targets. TP and iminothienopyridinedione (ITP), a more potent derivative, were shown to be effective inhibitors but the mechanism of inhibition was not established. Here, we perform NMR experiments and in vitro phosphatase assays to show that TP and ITP inhibit protein phosphatases non-specifically through oxidation of the phosphatase catalytic cysteine. We demonstrate that TP and ITP are redox active compounds, inhibiting PRL-3 and multiple other PTPs through oxidation. They also catalyze the oxidation of thioredoxin-1 as well as small molecules, like TCEP, DTT, and glutathione. The reported selectivity of TP and ITP is likely due to the higher susceptibility of PRLs to oxidation. Thus, while TP and ITP effectively inhibit PRLs, their use for studying the cellular function of PRLs is problematic due to the likelihood of off-target effects.
Introduction
PRLs are dual specificity phosphatases frequently overexpressed in malignant cancers.1 PRLs are composed of a single catalytic domain with a conserved farnesylation site.2,3 The three members of the PRL family, PRL-1, PRL-2, and PRL-3, are highly similar with around 80% amino acid sequence identity.4 The phosphatase active sites contain the signature motif (HCxxGxxR) found in all protein tyrosine phosphatases (PTPs) with a catalytic cysteine that serves as a nucleophile for the formation of a thiophosphoryl enzyme intermediate during catalysis.5 The cysteine has a low pKa which results in high nucleophilicity but also renders it susceptible to oxidation.6,7
PRLs are associated with disease progression in many tumour types; however, little is known about their physiological function.8 The most promising hypothesis is a role in regulating intracellular magnesium levels as suggested by the identification of a physical interaction between PRLs and a family of magnesium transporters, CNNMs.9,10 The binding of PRL to CNNM regulates Mg2+ transport and their interaction is regulated by both phosphorylation and oxidation of the catalytic cysteine in PRL.11,12 Magnesium deprivation decreases the phosphorylation of PRLs12 and stimulates PRL mRNA transcription.10,13 PRL mRNA translation is regulated through an upstream open reading frame in the 5′ untranslated region via a magnesium-dependent feedback mechanism.14 Reciprocally, either knockdown or knockout of PRL reduces intracellular Mg2+ levels.13,15 Mg2+, the most abundant divalent cation in cells, plays key roles in cell proliferation and genetic stability.16,17 CNNM-dependent Mg2+ transport has been shown to suppress tumour progression and PRLs promote tumour growth through regulating CNNMs.9,10 Thus, PRL inhibition is an attractive approach for the development of cancer therapeutics.
The first PRL inhibitor identified was pentamidine.18 Subsequently, other small molecular weight inhibitors were reported. Analog-3 is a PRL inhibitor obtained through virtual screenings and is proposed to suppress PRL-3 induced cell-migration.19,20 Cmpd-43 is a small molecule protein–protein interaction inhibitor proposed to disrupt PRL-1 trimerization through binding to the trimer interface of the PRL-1 protein.21–24 The therapeutic potential of Cmpd-43 was demonstrated in its ability to inhibit PRL-1 induced cell proliferation and suppress tumour growth in a murine melanoma xenograft model.21
Thienopyridone (7-amino-2-phenyl-5H-thieno[3,2-c]pyridin-4-one or TP) was identified as a promising small molecule PRL inhibitor.25 It was reported to selectively inhibit PRLs with limited inhibition of 11 other tyrosine and dual specificity phosphatases.25 The inhibitor showed significant inhibition of tumour cell anchorage independent growth and induced p130Cas cleavage and apoptosis through a p53-independent mechanism.25 Other groups showed that TP decreased the interaction between CNNMs and PRLs, and suppressed the proliferation of human breast cancer cells.26
An improved inhibitor, iminothienopyridinedione (7-iminothieno[3,2-c]pyridine-4,6(5H,7H)-dione or ITP), can be produced through photo-oxygenation of TP.27 ITP is 10-fold more potent and substantially more stable in solution than TP.27
However, a major challenge for the development of TP and ITP is the lack of information about their mechanism and their potential for quinone-like redox activity. Redox active compounds can inhibit enzymes indirectly through oxidation of catalytic residues. Here, we investigated the mechanism of inhibition of PRLs by TP and ITP. We found that TP and ITP inhibit PRLs through oxidation of the catalytic cysteine of PRL-3. Testing of six other PTPs showed that TP and ITP are non-specific and could even promote the oxidation of unrelated proteins and small molecules. Consequently, TP and ITP should not be used for the development of PRL-specific inhibitors or cell-based studies of PRL function.
Results
TP and ITP catalyse the oxidation of PRL-3
To understand the mechanism of inhibition, we sought to characterize TP binding to PRL-3 by various biophysical methods. Co-crystallization was conducted with TP and all three members of PRLs. Crystals were only obtained for TP and PRL-1. No inhibitor was observed in the electron density map. Instead, an oxidized form of PRL-1 with a disulphide bond formed between C49 and the catalytic C104 was found in the crystal.
As co-crystallization was unsuccessful, we turned to NMR spectroscopy, which is particularly well-suited for detecting interactions. We previously used NMR to determine the three-dimensional structure of PRL-3.6 The 1H–15N correlation spectrum has a good dispersion of the signals and is ideal for detecting the binding of small molecules.
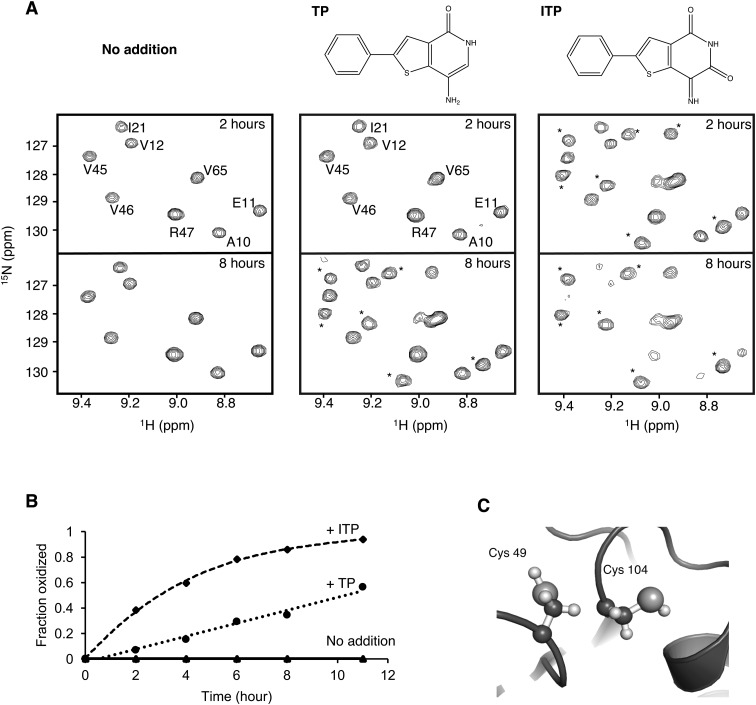
Unexpectedly, the addition of TP or ITP led to a time-dependent change in the PRL-3 spectrum with the appearance of a second set of signals (Fig. 1A). The specific changes in the NMR spectrum after incubation with TP or ITP correspond to the previously reported spectrum of oxidized PRL-3. ITP had a stronger effect than TP and, after 11 hours, all of the PRL-3 signals had converted to the second form (Fig. 1B). Like other PTP-family phosphatases, PRLs contain a second cysteine residue adjacent to the active site (Fig. 1C). This cysteine readily forms a disulphide bond with the catalytic cysteine to inactivate the enzyme.
Fig. 1. TP and ITP promote PRL-3 oxidation. (A) 15N–1H correlation spectra of 0.25 mM PRL-3 incubated without (left), with 0.25 mM TP (middle) and 0.25 mM ITP (right) for various durations. Peaks corresponding to oxidized PRL-3 are marked with asterisks. (B) Growth of the signal from oxidized PRL-3 valine 45 as a function of time. ITP was approximately four times more efficient than TP in facilitating the conversion of PRL-3 from the reduced to the oxidized state. (C) The structure of the PRL catalytic site (PDB ; 5k24) showing the cysteines that oxidize to form a disulphide bond. The thiol of cysteine 104 is essential for catalysis.
Higher concentrations of TP without pre-incubation also led to conversion to the oxidized form. No other changes were detected, suggesting no binding of TP to PRL-3 (Fig. S1†).6
We next used phosphatase assays to characterize TP and ITP inhibition of PRL activity. PRLs show low enzymatic activity due to the long lifetime of the thiophosphoryl enzyme intermediate.6,12 This leads to burst kinetics which is characterized by two rates of enzyme activity. Upon the addition of a substrate, there is an initial, rapid turnover of one mole of the product per mole of the enzyme. This is followed by a much slower steady-state rate of activity that is limited by the lifetime of the thiophosphoryl intermediate. Here, we used a fluorogenic substrate to measure the initial rate of catalysis (Fig. 2A).11
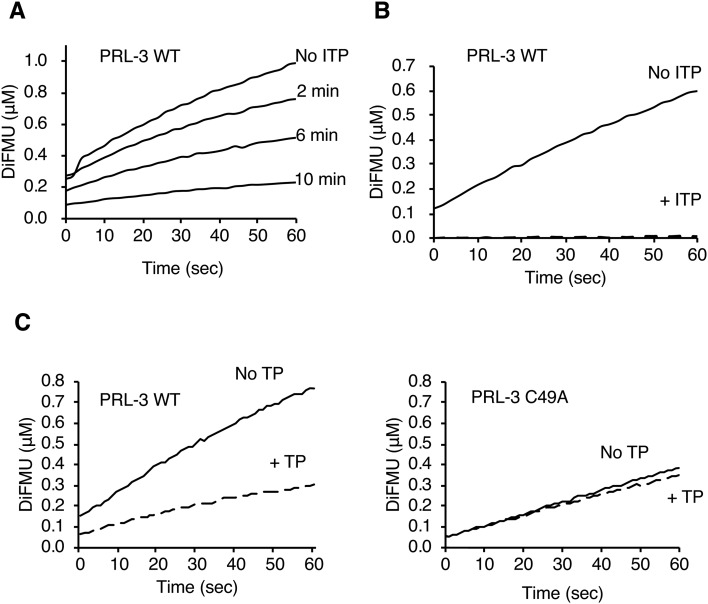
Fig. 2. ITP inhibits PRL-3 by catalyzing the formation of a disulfide bond with the catalytic cysteine. (A) In vitro phosphatase assay of PRL-3 (3 μM) with the fluorogenic substrate DiFMUP (25 μM). Pre-incubation with ITP (0.6 μM before addition of DiFMUP) showed a time-dependent inhibition of phosphatase activity. (B) PRL-3 was inhibited by a sub-stoichiometric amount of ITP. Concentrated PRL-3 (60 μM) was pre-treated with ITP (0.6 μM) for 3 hours before dilution and assaying. (C) Treatment with TP (0.6 μM) for 10 min inhibited wild-type PRL-3 (WT) but not the C49A mutant, which is unable to form a disulphide bond.
As expected, ITP was an effective inhibitor of PRL-3 activity with less than 20% of activity remaining after 10 minutes of incubation (Fig. 2A). In addition, the effect of ITP addition was time-dependent with only partial inhibition at 2 min and full inhibition after 10 min (Fig. 2A). The inhibition could even be achieved with a sub-stoichiometric 1-to-100 ratio of inhibitor to enzyme (Fig. 2B). Sub-stoichiometric amounts of TP similarly lead to a strong inhibition of PRL-3 phosphatase activity (Fig. 2C). These results suggested that TP and ITP were acting through oxidation of the catalytic cysteine and not as inhibitors (competitive or allosteric) that directly bind the phosphatase. To test this, we turned to mutagenesis to generate a form of PRL-3 resistant to TP and ITP inhibition.
PRL-3 C49A mutant is resistant to inhibition by TP
Protein phosphatases are subject to regulation through redox reactions of the catalytic cysteine.7 These serve to reversibly inactivate the proteins and are thought to be important in potentiating or modulating the signalling pathways involving protein phosphorylation.28 While a large number of chemical forms are possible, disulphide formation is the major form observed with PRL-3 in the absence of reducing agents.6
To generate an oxidation resistant form of PRL-3, we mutated the cysteine adjacent to the catalytic cysteine to alanine.29 The C49A mutant showed roughly 50% of wild-type activity but was otherwise identical to the wild-type enzyme (Fig. 2C). The mutant was markedly resistant to TP treatment and showed a 10% decrease in activity compared to a 55% decrease for the wild-type enzyme under identical conditions. This suggests that the formation of the C49–C104 disulphide bond is the main mechanism of TP inhibition. Consistent with the existence of other oxidized states, inhibition of phosphatase activity was observed with treatment with the more potent inhibitor ITP. In addition to the disulphide, the cysteine thiol of PRL phosphatases can be potentially oxidized to sulphenyl (–SOH), sulphinyl (–SO2H), sulphonyl (–SO3H) or sulphenilamide (–SNH–) moieties, all of which would inactivate the enzyme.7
TP and ITP oxidise other proteins
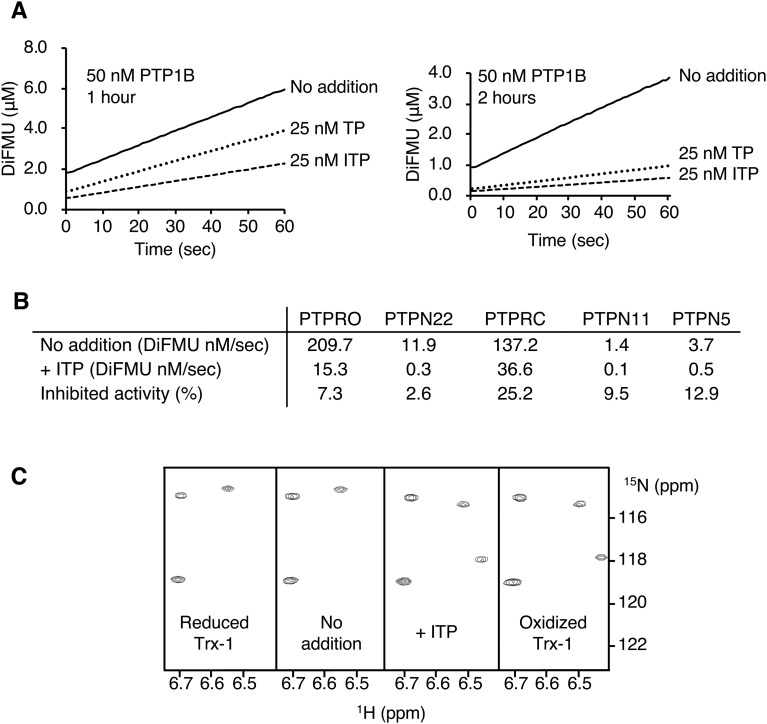
To address the specificity of TP and ITP, we examined their effects on one of the best-characterized protein phosphatases. PTP1B is a regulator of glucose homeostasis and energy metabolism and undergoes redox regulation through oxidation of its catalytic cysteine.28 PTP1B pre-incubated with either TP or ITP showed substantially decreased activity and this inhibition effect is time-dependent (Fig. 3A). The inhibition of PTP1B required a longer inhibition period than that required for PRL-3, which we attribute to the greater susceptibility of PRL-3 to oxidation. As observed with PRL-3, nearly complete inhibition was observed with a sub-stoichiometric amount of the inhibitors. ITP at half the PTP1B concentration was sufficient to inhibit 85% of its activity.
Fig. 3. TP and ITP inhibit PTPs and promote Trx-1 oxidation. (A) In vitro phosphatase assay of PTP1B incubated with TP or ITP shows inhibition of phosphatase activity. Concentrations are after addition of DiFMUP. (B) Other phosphatases are inhibited by ITP. Phosphatases (50 nM) were treated with 2 μM ITP for 2.5 hours prior to dilution with DiFMUP and measurement of phosphatase activity for 1 min. (C) 15N–1H correlation spectra of Trx-1 (0.2 mM) incubated with ITP (50 μM) for two hours at 30 °C show the conversion of Trx-1 from the reduced to oxidized form.
Five phosphatases were then used to examine the specificity of ITP. The phosphatase activities of PTPRO, PTPN22, PTPRC, PTPN11, and PTPN5 were inhibited by more than 70% after 2.5 hours of incubation with ITP, indicating that ITP lacks specificity (Fig. 3B).
We asked next if TP or ITP could catalyse the oxidation of a completely unrelated protein, thioredoxin-1 (Trx-1). Thioredoxins facilitate the cysteine thiol–disulphide exchange through a signature catalytic motif CXXC.30 We used NMR spectra to monitor the oxidation state of Trx-1 as the residues in the catalytic site are well resolved and show large redox-dependent chemical shifts (Fig. 3C). Comparison of the 1H–15N correlation spectra for reduced and oxidized Trx-1 showed that a 2 hour treatment with ITP was sufficient to completely convert Trx-1 to the oxidized form even in the presence of 2 mM TCEP. The same result was obtained with TP, whereas Trx-1 incubated without ITP remained reduced (Fig. 3C).
TP and ITP catalyse the oxidation of small molecules
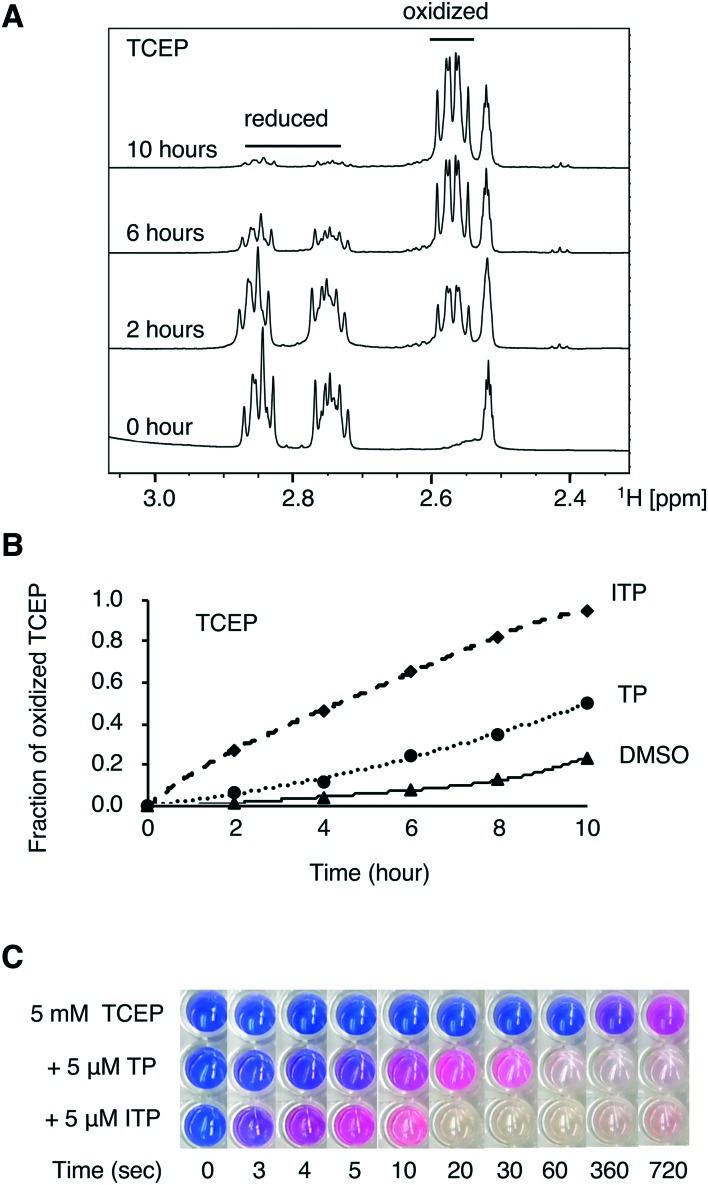
We next tested the ability of TP and ITP to catalyse the oxidation of small molecules. The spectra of a freshly prepared solution of tris(2-carboxyethyl)phosphine (TCEP) and glutathione were acquired in the absence and presence of TP and ITP (Fig. 4A). The fraction of reduced and oxidized molecules was measured by integrating the NMR signals. The addition of TP or ITP led to a rapid increase in the rate of oxidation by atmospheric oxygen. After 2 hours, the fraction of oxidized TCEP was five-fold with TP and 20-fold with ITP (Fig. 4B). We also measured the TP and ITP oxidation of glutathione, a more physiologically relevant reducing agent (Fig. S2†). The NMR spectra showed that the initial rate of oxidation of glutathione was increased by 70% with TP and 3-fold with ITP, confirming that TP and ITP catalyse the oxidation of small molecules.
Fig. 4. TP and ITP facilitate the oxidation of small molecules. (A) 1H NMR of TCEP (10 mM) incubated with ITP (1 mM) in 80% d6-acetone and 20% d6-DMSO. (B) Time course of oxidation of TCEP in the presence of TP or ITP. The fraction of oxidized TCEP was obtained by integration of the NMR signals. (C) Redox activity assay showing TP and ITP increase the rate of reduction of resazurin by TCEP.
TP and ITP are redox active
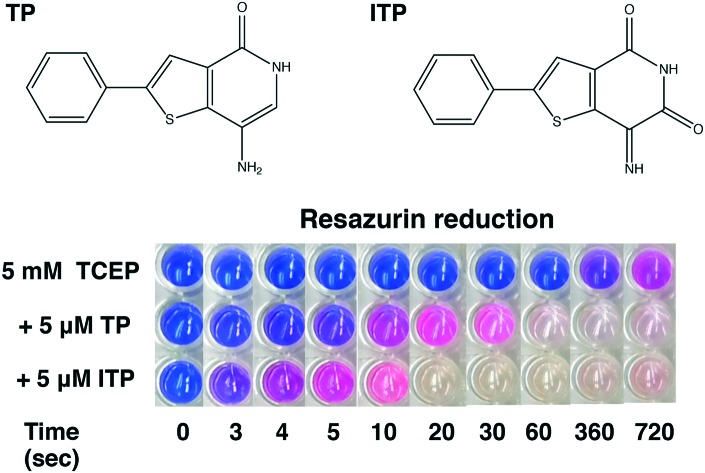
We further verified the general redox activity of TP and ITP through an assay with resazurin as a redox indicator. Resazurin is a blue dye, which can be reduced to a pink dye, resorufin, which can be further reduced to hydroresorufin, which is colourless.31 Resazurin is frequently used to measure cellular respiration32 but also functions as a redox indicator, turning pink under partially anaerobic conditions and colourless under fully anaerobic conditions.
The reduction of resazurin by dithiothreitol (DTT) is the basis for a counter screen developed to identify redox active small molecules coming from high throughput drug screening studies.33 We performed the experiment by incubating TCEP and the redox indicator with TP or ITP. Alone, TCEP took 5 min to reduce resazurin and induce a detectable colour change (Fig. 4C). In contrast, the addition of TP or ITP induced comparable colour changes after 5 and 3 s. At longer incubation times, the mixture rapidly turned colourless, indicating the complete reduction of resazurin to hydroresorufin. After 2 hours of incubation, all three solutions turned pink, due to the exhaustion of TCEP and re-oxidation of hydroresorufin by atmospheric oxygen. This resazurin conversion experiment demonstrates that TP and ITP are redox active.
Catalase suppresses PTP inhibition
To further elucidate the inhibition mechanism, we analysed the effect of catalase on PTP inhibition by ITP. Catalase is an enzyme that catalyses the decomposition of hydrogen peroxide.34 Previously, catalase has been used to identify redox cycling compounds that appear as false positives in screens for caspase-8 and CDC25B inhibitors.35–37 We used catalase in an analogous fashion to determine whether ITP indirectly inhibits PTPs through the generation of hydrogen peroxide.
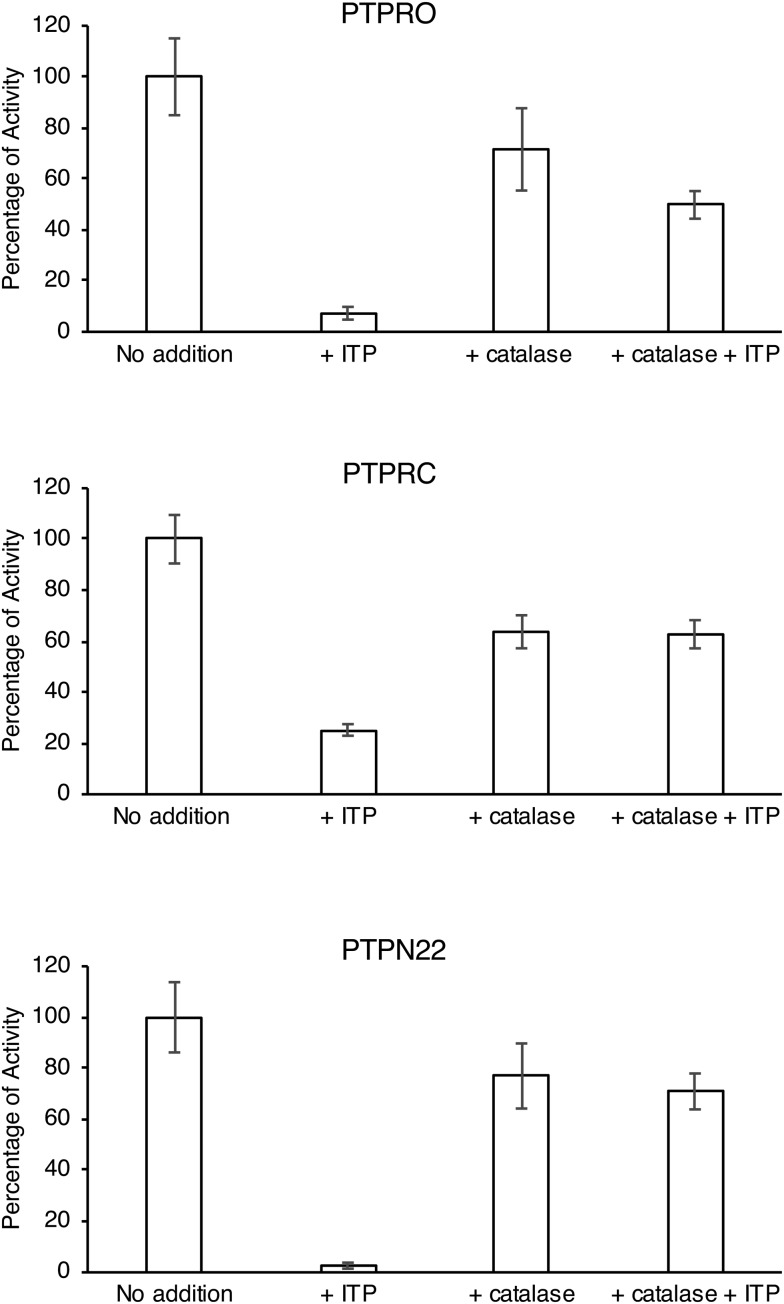
After 2.5 hours of incubation with ITP, PTP activity was substantially inhibited, while in the presence of catalase, the inhibition of PTP by ITP was suppressed. This experiment indicated that the redox cycling reaction of ITP and DTT produced hydrogen peroxide, which oxidized the catalytic cysteine and inactivated the PTPs (Fig. 5).35–38 In the presence of catalase, the hydrogen peroxide was efficiently decomposed and PTP inhibition by ITP was substantially suppressed.34
Fig. 5. Catalase blocks the ITP inhibition of phosphatase activity. Uninhibited phosphatase activities (no addition) were 209.7 ± 15.8 nM s–1 for PTPRO, 137.2 ± 6.5 nM s–1 for PTPRC, and 11.9 ± 0.8 nM s–1 for PTPN22. Pre-incubation with ITP for 2.5 hours inhibited all three phosphatases; co-incubation with catalase completely suppressed the inhibition. Experiments were performed in triplicate with error bars indicating the standard deviation.
Reactivation of PRL-3
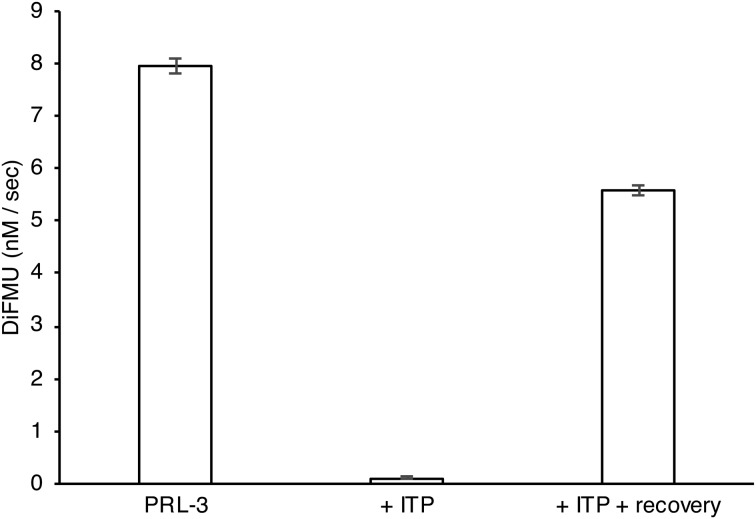
To confirm that PRL-3 was inhibited by ITP through oxidation of the catalytic cysteine, we sought to recover the PRL-3 activity with reducing agents.39,40 PRL-3 was first treated with ITP to inhibit all of the phosphatase activity. Then, the inhibited PRL-3 was diluted into a buffer containing DTT and catalase. This resulted in around 80% recovery of the PRL-3 phosphatase activity (Fig. 6). In contrast, PRL-3 diluted into a buffer without reducing agents remained fully inhibited. This experiment proves that the inhibition of PRL-3 by ITP occurs through oxidation and not through reversible binding.
Fig. 6. Recovery of PRL-3 activity with reducing agents. ITP inhibition of PRL-3 was reversed by incubation in 5 mM TCEP, 10 mM DTT and 0.4 μM catalase (recovery). The experiment was performed in triplicate. The error bars represent standard deviation.
Discussion
After almost 25 years of study, PRLs remain as enigmatic and unusual enzymes. A number of different cellular substrates have been proposed but there is still no consensus about their targets. PRLs are weakly active. In in vitro experiments, the formation of a stable phosphocysteine intermediate leads to burst kinetics in which the enzyme manifests a rapid initial burst of activity followed by a much slower steady-state rate.6 In cells, the half-life of the intermediate could be shorter in theory but magnesium deprivation experiments show only a slow accumulation of the unphosphorylated enzyme suggesting that PRL activity is similar in vitro and in vivo.12 PRLs are also unusually sensitive to inactivation through oxidation of the catalytic cysteine. Only half of the protein is reduced in the presence of 0.5 mM DTT.6 Not surprisingly, PRLs are difficult targets for drug development.
TP and ITP were proposed as specific inhibitors of PRLs based on a number of criteria, which can be reinterpreted in terms of the properties of PRLs. Previous studies showed that 1 μM ITP had around 50% inhibition for CDC25B and p38α.39 CDC25B has redox regulation that is similar to that of PRLs, with an active site cysteine prone to oxidation.41 Similarly, the formation of an intermolecular disulphide bond has been proposed as a regulatory mechanism for p38α.42 Thus, ITP inhibition of CDC25B and p38α likely resulted from the oxidation of their cysteines as is the case for PRLs. Previous studies25,39 did not observe significant inhibition of PTP1B, which likely reflects the greater stability of the PTP1B active site. With prolonged incubation time, we found that even a sub-stoichiometric amount of TP and ITP can fully inhibit the PTP1B phosphatase activity. We also showed that other PTPs can be inhibited by ITP and this inhibition can be suppressed by catalase, indicating that the inhibition by ITP is through the generation of hydrogen peroxide.
Superficially, our results agree with the comprehensive study of PRL inhibition by TP and ITP by McQueeney et al.39 Lineweaver–Burk analysis showed that TP and ITP are noncompetitive inhibitors of PRLs,39 which is consistent with the oxidation of PRL active site and a decrease in enzyme Vmax. Furthermore, we confirmed that the dilution of the PRL-3 pre-incubated with ITP in a buffer containing reducing agents leads to partial recovery of the PRL enzymatic activity.39 However, we showed that the recovery of phosphatase activity was not observed when dilution was done with a buffer that does not contain reducing agents. This rules out the reversible binding of a non-competitive inhibitor.
The key results that demonstrate that TP and ITP inhibit through oxidation of the PRL active site are: 1) the binding of TP to PRL-3 was not detected in NMR titrations, 2) direct observation of the oxidized state by NMR spectroscopy, 3) full inhibition by sub-stoichiometric amounts of the inhibitor, 4) time dependence of inhibition, 5) resistance of the C49A PRL-3 mutant, 6) lack of specificity, 7) phosphatase reactivation by reducing agents, and 8) the prevention of inhibition by co-incubation with catalase. TP and ITP are clearly redox cycling compounds that produce hydrogen peroxide in the presence of TCEP or DTT.35–37
Conclusions
We have determined that TP and ITP are non-specific protein phosphatase inhibitors that act through oxidation of the catalytic cysteine. Considering their lack of specificity and mechanism of action, TP and ITP are inappropriate compounds for therapeutic development or studies of PRL function. Future drug screening for PRL inhibitors needs to be carefully designed to account for the unique sensitivity of PRLs to oxidation.
Experimental
Protein expression and purification
PRL-3 wild-type (1–169) and C49A mutant were expressed and purified as His-tag fusion proteins as described previously.12 The PRL-3 C49A mutant was generated with the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies) and confirmed by DNA sequencing. PTP1B (1–298) was sub-cloned and expressed as described previously.43 Phosphatases PTPN22 G126V, V147G (Addgene plasmid # 38933), PTPN11 (Addgene plasmid # 38965), PTPRO (Addgene plasmid # 38969), and PTPN5 (Addgene plasmid # 39169) were gifts from Nicola Burgess-Brown. PTPN22, PTPN11, and PTPRO were expressed and purified as His-tag fusion proteins as described previously.12
PTPN5 was expressed and purified as a GST-tag fusion protein. The plasmids were extracted from the cells and transformed into E. coli strain BL21 (DE3). PTPN5 was expressed at 37 °C in Luria broth (LB). 10 ml of overnight culture was used and it was grown to an optical density of 0.8 and induced with 1 mM IPTG for 4 hours at 30 °C. The bacteria were harvested and pellets were re-suspended in 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 1 mM PMSF, 15 mM β-mercaptoethanol, and 0.1 mg ml–1 lysozyme. Glutathione Sepharose 4B (GE Healthcare Life Science) was used for GST-tagged protein purification and 20 mM glutathione was used for elution. PTPN5 was further purified with size exclusion chromatography using a HiLoad Superdex 200 pg prepacked column (GE Healthcare Life Science) in 20 mM HEPES, 100 mM NaCl, and 5 mM TCEP, at pH 7.0.
PTPRC (627-1228) codon-optimized for E. coli (Bio Basic Inc., Markham, Canada) was sub-cloned into NdeI and XhoI sites of the pET29a vector with a C-terminal His6-tag. The construct was verified by DNA sequencing and transformed into E. coli strain BL21 (DE3). It was expressed in cells grown at 37 °C in LB to an optical density of 0.6 and induced with 1 mM IPTG overnight at 18 °C. PTPRC was purified as a His-tag fusion protein as described previously.12
Synthesis of TP and ITP
TP was obtained from Zamboni Chem Solutions and its identity was confirmed by NMR spectroscopy (Fig. S3†). ITP was prepared by placing TP dissolved in d4-methanol by the window for 2 days as described.27 The precipitate was washed with d4-methanol and dissolved in d6-DMSO. NMR spectroscopy was used to monitor the conversion and confirm the identity of the final product (Fig. S3†).
NMR spectroscopy
NMR spectra were acquired at 30 °C at 600 MHz (14 T) on a Bruker Avance NMR spectrometer with a TXI cryoprobe. 15N-labeled PRL-3 samples were prepared for 15N–1H correlation spectroscopy in 50 mM phosphate buffer, 100 mM NaCl, 5 mM TCEP, and 10% D2O, at pH 6.8. For time course experiments, 0.25 mM TP or ITP was incubated with 0.25 mM 15N-labeled PRL-3 and 15N–1H correlation NMR spectra (40 min) were acquired every 2 hours.
The 15N-labeled reduced Trx-1 at 0.2 mM was prepared by incubation with 10 mM DTT, and buffer exchanged into 50 mM phosphate, 100 mM NaCl, 2 mM TCEP, and 10% D2O, at pH 6.8. The spectra of oxidized Trx-1 were obtained by incubating Trx-1 with 5 mM oxidized glutathione. For the Trx-1 oxidation experiment, 50 μM TP or ITP was incubated with reduced Trx-1 and NMR spectra were taken every 2 hours.
NMR spectra for monitoring TCEP oxidation were obtained in a mixture of 80% d6-acetone and 20% d6-DMSO with 10 mM TCEP and 1 mM TP or ITP. Spectra for monitoring glutathione oxidation were obtained in a mixture of 80% d6-DMSO and 20% D2O with 10 mM reduced glutathione and 1 mM TP or ITP.
Phosphatase assays
Phosphatase assays were conducted at room temperature with the fluorescent substrate, 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP), from the EnzChek Phosphatase Assay Kit (Life Technologies). The assays (50 μL volume) were performed with 25 μM DiFMUP in the assay buffer (20 mM HEPES, 100 mM NaCl, 5 mM TCEP, pH 7.0). The change in fluorescence at 455 nm was monitored for 60 s on a SpectraMax M5e plate reader (Molecular Devices) and the concentration of the reaction product, 6,8-difluoro-7-hydroxy-4-methylcoumarin (DiFMU), was calibrated with an external standard. The enzyme concentrations were 3 μM for PRL-3, 50 nM for PTP1B, and 25 nM for PTPRO, PTPN22, PTPRC, PTPN11, and PTPN5. For assays with inhibitors, the enzyme was incubated with the inhibitor at twice the final concentration for 2.5 hours and then diluted into an equal volume of the buffer containing DiFMUP for measurement of phosphatase activity.
Reactivation of phosphatase activity
PRL-3 (60 μM) was incubated with ITP (6 μM) for 3 hours in 20 mM HEPES, 100 mM NaCl, and 5 mM TCEP, at pH 7.0. To reactivate the PRL activity, treated PRL-3 was diluted 10-fold and incubated for 2 hours in a buffer containing 0.4 μM Aspergillus niger catalase (>4000 unit per mg, Sigma-Aldrich), 20 mM HEPES, 100 mM NaCl, 5 mM TCEP, and 10 mM DTT, at pH 7.0. The control was diluted into a buffer without catalase, TCEP, or DTT. For measurements of phosphatase activity, the samples were diluted into an equal volume of the DiFMUP assay buffer and assayed as described previously.
Oxygen measurements with resazurin
100 μM resazurin (Sigma-Aldrich) was dissolved in a buffer containing 20 mM HEPES, 100 mM NaCl, and 5 mM TCEP, at pH 7. TP or ITP (5 μM) was added and the change in colour was recorded with a video camera.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank Dr. Robert Zamboni for the gift of TP. This work was made possible by a research grant from the Natural Sciences and Engineering Research Council of Canada, RGPIN-2014-04686.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9md00175a
References
- Saha S., Bardelli A., Buckhaults P., Velculescu V. E., Rago C., St Croix B., Romans K. E., Choti M. A., Lengauer C., Kinzler K. W., Vogelstein B. Science. 2001;294:1343–1346. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- Rios P., Li X., Kohn M. Rev. Geophys. 2013;280:505–524. doi: 10.1111/j.1742-4658.2012.08565.x. [DOI] [PubMed] [Google Scholar]
- Zeng Q., Hong W., Tan Y. H. Biochem. Biophys. Res. Commun. 1998;244:421–427. doi: 10.1006/bbrc.1998.8291. [DOI] [PubMed] [Google Scholar]
- Al-Aidaroos A. Q., Zeng Q. J. Cell. Biochem. 2010;111:1087–1098. doi: 10.1002/jcb.22913. [DOI] [PubMed] [Google Scholar]
- Denu J. M., Dixon J. E. Curr. Opin. Chem. Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- Kozlov G., Cheng J., Ziomek E., Banville D., Gehring K., Ekiel I. J. Biol. Chem. 2004;279:11882–11889. doi: 10.1074/jbc.M312905200. [DOI] [PubMed] [Google Scholar]
- Funato Y., Miki H. Methods. 2014;65:184–189. doi: 10.1016/j.ymeth.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Hardy S., Kostantin E., Hatzihristidis T., Zolotarov Y., Uetani N., Tremblay M. L. Rev. Geophys. 2018;285:3886–3908. doi: 10.1111/febs.14503. [DOI] [PubMed] [Google Scholar]
- Funato Y., Yamazaki D., Mizukami S., Du L., Kikuchi K., Miki H. J. Clin. Invest. 2014;124:5398–5410. doi: 10.1172/JCI76614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Uetani N., Wong N., Kostantin E., Labbe D. P., Begin L. R., Mes-Masson A., Miranda-Saavedra D., Tremblay M. L. Oncogene. 2015;34:986–995. doi: 10.1038/onc.2014.33. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kozlov G., Li X., Wu H., Gulerez I., Gehring K. Sci. Rep. 2017;7:48. doi: 10.1038/s41598-017-00147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulerez I., Funato Y., Wu H., Yang M., Kozlov G., Miki H., Gehring K. EMBO Rep. 2016;17:1890–1900. doi: 10.15252/embr.201643393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Funato Y., Miki H. Biochem. J. 2018;475:1129–1139. doi: 10.1042/BCJ20170756. [DOI] [PubMed] [Google Scholar]
- Hardy S., Kostantin E., Wang S. J., Hristova T., Galicia-Vazquez G., Baranov P. V., Pelletier J., Tremblay M. L. Proc. Natl. Acad. Sci. U. S. A. 2019;116:2925–2934. doi: 10.1073/pnas.1815361116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetani N., Hardy S., Gravel S. P., Kiessling S., Pietrobon A., Wong N. N., Chenard V., Cermakian N., St-Pierre J., Tremblay M. L. JCI Insight. 2017;2(13):91722. doi: 10.1172/jci.insight.91722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Baaij J. H., Hoenderop J. G., Bindels R. J. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- Funato Y., Miki H. J. Biochem. 2019;165:219–225. doi: 10.1093/jb/mvy095. [DOI] [PubMed] [Google Scholar]
- Pathak M. K., Dhawan D., Lindner D. J., Borden E. C., Farver C., Yi T. Mol. Cancer Ther. 2002;1:1255–1264. [PubMed] [Google Scholar]
- Hoeger B., Diether M., Ballester P. J., Kohn M. Eur. J. Med. Chem. 2014;88:89–100. doi: 10.1016/j.ejmech.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan P., Varsano G., Rubio T., Hennrich M. L., Sachsenheimer T., Galvez-Santisteban M., Martin-Belmonte F., Gavin A. C., Brugger B., Kohn M. J. Cell Sci. 2016;129:4130–4142. doi: 10.1242/jcs.190215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yu Z. H., Liu S., Zhang L., Zhang R. Y., Zeng L. F., Zhang S., Zhang Z. Y. Cancer Res. 2016;76:4805–4815. doi: 10.1158/0008-5472.CAN-15-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z. H., Zhang Z. Y. Chem. Rev. 2018;118:1069–1091. doi: 10.1021/acs.chemrev.7b00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yu Z.-H. and Zhang Z.-Y., in Protein Tyrosine Phosphatases, Springer, 2016, pp. 121–138. [Google Scholar]
- Jeong D. G., Kim S. J., Kim J. H., Son J. H., Park M. R., Lim S. M., Yoon T. S., Ryu S. E. J. Mol. Biol. 2005;345:401–413. doi: 10.1016/j.jmb.2004.10.061. [DOI] [PubMed] [Google Scholar]
- Daouti S., Li W. H., Qian H., Huang K. S., Holmgren J., Levin W., Reik L., McGady D. L., Gillespie P., Perrotta A., Bian H., Reidhaar-Olson J. F., Bliss S. A., Olivier A. R., Sergi J. A., Fry D., Danho W., Ritland S., Fotouhi N., Heimbrook D., Niu H. Cancer Res. 2008;68:1162–1169. doi: 10.1158/0008-5472.CAN-07-2349. [DOI] [PubMed] [Google Scholar]
- Kostantin E., Hardy S., Valinsky W. C., Kompatscher A., de Baaij J. H., Zolotarov Y., Landry M., Uetani N., Martinez-Cruz L. A., Hoenderop J. G., Shrier A., Tremblay M. L. J. Biol. Chem. 2016;291:10716–10725. doi: 10.1074/jbc.M115.705863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamoun J. M., McQueeney K. E., Patil K., Geib S. J., Sharlow E. R., Lazo J. S., Wipf P. Org. Biomol. Chem. 2016;14:6398–6402. doi: 10.1039/c6ob00946h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K. Rev. Geophys. 2013;280:346–378. doi: 10.1111/febs.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger B., Rios P., Berteotti A., Hoermann B., Duan G., Köhn M. ACS Omega. 2017;2:9171–9180. doi: 10.1021/acsomega.7b01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt C., Lillig C. H., Holmgren A. Biochim. Biophys. Acta. 2008;1783:641–650. doi: 10.1016/j.bbamcr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Twigg R. S. Nature. 1945;155:401–402. [Google Scholar]
- O'Brien J., Wilson I., Orton T., Pognan F. Eur. J. Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Lor L. A., Schneck J., McNulty D. E., Diaz E., Brandt M., Thrall S. H., Schwartz B. J. Biomol. Screening. 2007;12:881–890. doi: 10.1177/1087057107304113. [DOI] [PubMed] [Google Scholar]
- Jones P., Suggett A. Biochem. J. 1968;110:621–629. doi: 10.1042/bj1100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. K., Barrett D. G., Blackburn K., Cory M., Dallas W. S., Davis R., Hassler D., McConnell R., Moyer M., Weaver K. Arch. Biochem. Biophys. 2002;399:195–205. doi: 10.1006/abbi.2002.2757. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Foster C. A., Tierno M. B., Shun T. Y., Shinde S. N., Paquette W. D., Brummond K. M., Wipf P., Lazo J. S. Assay Drug Dev. Technol. 2009;7:250–265. doi: 10.1089/adt.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Pecci L., Pensa B., Cannella C. Biochem. Biophys. Res. Commun. 1977;78:596–603. doi: 10.1016/0006-291x(77)90221-2. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Soares K. M., Shinde S. N., Foster C. A., Shun T. Y., Takyi H. K., Wipf P., Lazo J. S. Assay Drug Dev. Technol. 2008;6:505–518. doi: 10.1089/adt.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeney K. E., Salamoun J. M., Burnett J. C., Barabutis N., Pekic P., Lewandowski S. L., Llaneza D. C., Cornelison R., Bai Y., Zhang Z. Y., Catravas J. D., Landen C. N., Wipf P., Lazo J. S., Sharlow E. R. Oncotarget. 2018;9:8223–8240. doi: 10.18632/oncotarget.23787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. R., Kwon K. S., Kim S. R., Rhee S. G. J. Biol. Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- Buhrman G., Parker B., Sohn J., Rudolph J., Mattos C. Biochemistry. 2005;44:5307–5316. doi: 10.1021/bi047449f. [DOI] [PubMed] [Google Scholar]
- Bassi R., Burgoyne J. R., DeNicola G. F., Rudyk O., DeSantis V., Charles R. L., Eaton P., Marber M. S. J. Biol. Chem. 2017;292:16161–16173. doi: 10.1074/jbc.M117.785410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend B., Aubry I., Marcellus R. C., Gehring K., Tremblay M. L. ChemBioChem. 2010;11:1583–1593. doi: 10.1002/cbic.201000208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.