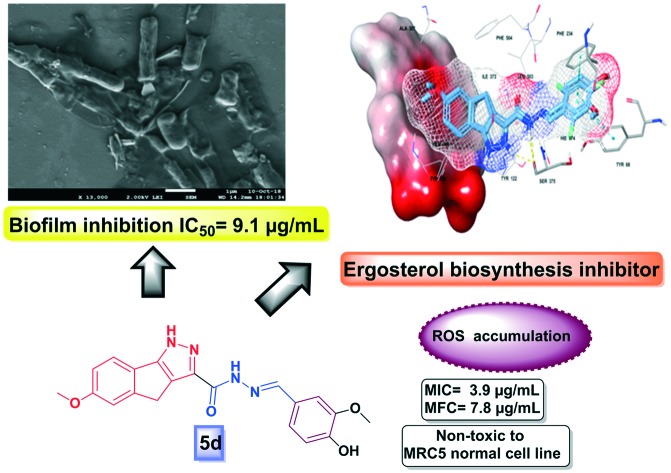
 Non-toxic compounds with antifungal activity, ergosterol inhibition and ROS inducing potential.
Non-toxic compounds with antifungal activity, ergosterol inhibition and ROS inducing potential.
Abstract
A series of new 1,4-dihydroindeno[1,2-c]pyrazole tethered carbohydrazide hybrids (5a–u) were designed, synthesized and evaluated for their antimicrobial activity. Compounds 5d, 5g, 5j, 5k and 5q demonstrated significant activity against the entire panel of test pathogens. Further, compounds 5d and 5g exhibited significant anti-Candida activity. These potential hybrids (5d and 5g) also exhibited promising ergosterol biosynthesis inhibition against Candida albicans, which was further validated through molecular docking studies. Furthermore, compounds 5d and 5g caused intracellular ROS accumulation in C. albicans MTCC 3017 and were non-toxic to normal human lung cell line MRC5. In silico studies revealed that they demonstrated drug likeness and an appreciable pharmacokinetic profile. Overall, the findings demonstrate that 5d and 5g may be considered as promising leads for further development of new antifungal drugs.
Introduction
Infectious diseases in humans are becoming increasingly problematic, predominantly with the rise of multidrug resistance against the existing antimicrobial drugs resulting in reduced efficacy of the available antibiotics.1 Invasive fungal infections pose a continuous and serious risk to human health and life. Clinically, Candidiasis, Aspergillosis and Cryptococcosis are three major fungal infections in immunocompromised patients.2 In recent years, the escalating morbidity and emergence of drug-resistance in life threatening fungal infections pose a significant health problem especially in patients with AIDS and cancer.3 Amongst all the fungal pathogens, Candida albicans is majorly responsible for nosocomial infections and indwelling medical devices (e.g. catheters, ocular lenses, artificial joints, heart valves, vascular bypass grafts, dental implants and central nervous system shunts) serve as substrates for formation of biofilms.4
Currently, an azole class of antifungal agents (fluconazole, itraconazole, ketoconazole and miconazole) are the first line drugs used in antifungal therapy.5 They exhibit their action by inhibiting fungal lanosterol 14α-demethylase (CYP51), which plays a vital role in ergosterol biosynthesis.6 However, it has been observed that biofilm communities of Candida albicans and Candida parapsilosis are relatively resistant to some antifungal drugs like fluconazole, voriconazole, amphotericin B, nystatin, etc.7 Further, these azole-based antifungals are hampered by a narrow antifungal spectrum, drug resistance, liver and reproductive toxicities, drug–drug interactions and low bioavailability which negatively affect their clinical efficacy.8 Therefore, there is still an urgent need to develop novel potent antifungal agents with a broad spectrum of activity, a safe pharmacokinetic profile, low cytotoxicity and high bioavailability.
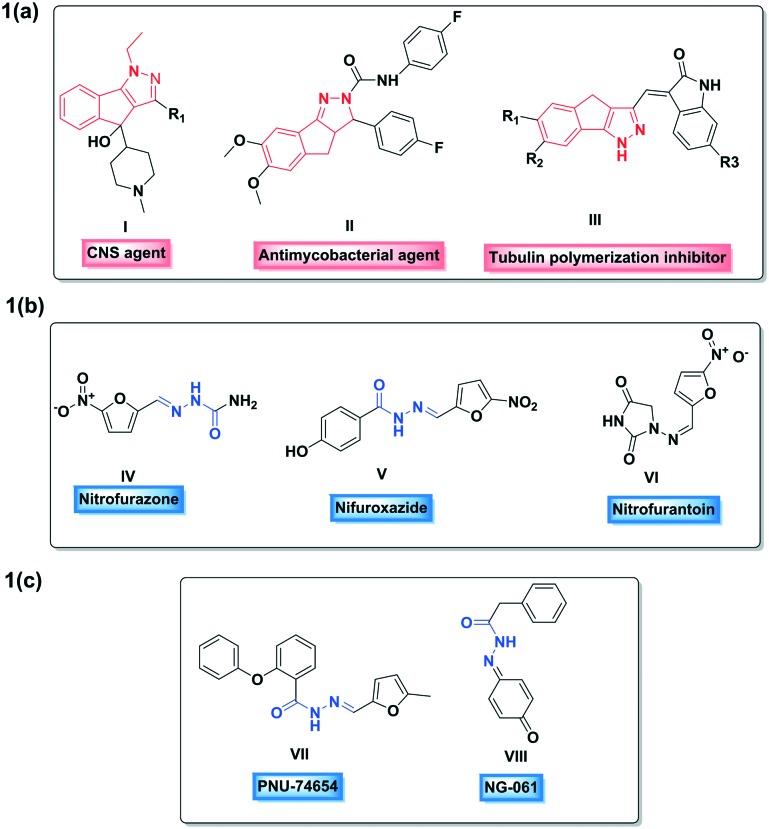
1,4-Dihydroindeno[1,2-c]pyrazole is a novel pseudoazulenic framework containing two fused five-membered rings, which was first synthesized by Boyd in 1965.9 The indenopyrazole pharmacophore has gained appreciable interest in recent years owing to its diverse biological activities10 (I, II and III; Fig. 1a) such as anti-psychotic, anti-mycobacterial, anticancer, antimicrobial and antihypertensive activities. Similarly, hydrazide–hydrazone which constitutes an azomethine group (–NH–N CH–) linked with a carbonyl group is a key bioactive framework in medicinal chemistry exhibiting remarkable versatile biological activities11 including antimicrobial, anticonvulsant, anti-inflammatory, anti-malarial, anti-platelet, anticancer, anti-tubercular activities, etc. Some widely used chemotherapeutic drugs such as nitrofurazone (IV), nitrofuroxazide (V) and nitrofurantoin (VI) (Fig. 1b) and some biologically active natural products such as PNU-74654 (VII) and NG-061(VIII) (Fig. 1c) are known to possess this hydrazide–hydrazone motif.12
Fig. 1. (a) Structures of some indenopyrazole derivatives; (b) structures of a few chemotherapeutic agents bearing a hydrazide–hydrazone core; (c) structures of a few natural products containing a hydrazide–hydrazone motif.
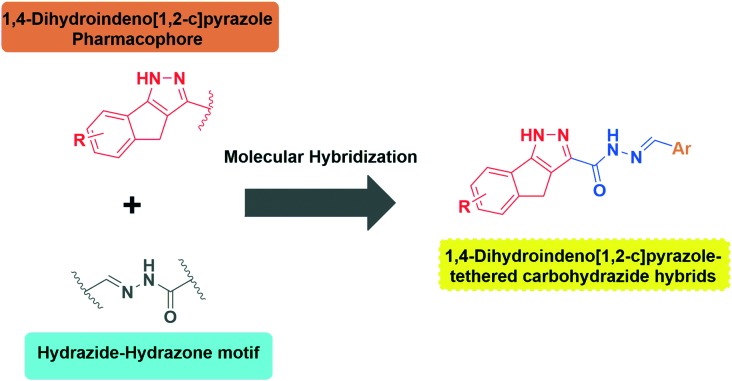
Although 1,4-dihydroindeno[1,2-c]pyrazole derivatives have gained immense interest as potential anticancer agents, as per a literature survey, their antimicrobial activity is relatively unexplored. Furthermore, inspired by the biological significance of 1,4-dihydroindeno[1,2-c]pyrazoles and hydrazide–hydrazone derivatives it was presumed that it would be interesting to hybridize (Fig. 2) these two vital scaffolds through molecular hybridisation into a single molecular motif in order to synergize the efficacy and to explore their antimicrobial activity. Consequently, encouraged from the findings described above and in continuation of our previous work on the synthesis of biologically active heterocycles,13 we herein report the synthesis, antimicrobial evaluation, in silico drug likeness and ADMET prediction of new 1,4-dihydroindeno[1,2-c]pyrazole tethered carbohydrazide hybrids (5a–u).
Fig. 2. Design of new 1,4-dihydroindeno[1,2-c]pyrazole-tethered carbohydrazide hybrids.
Results and discussion
Chemistry
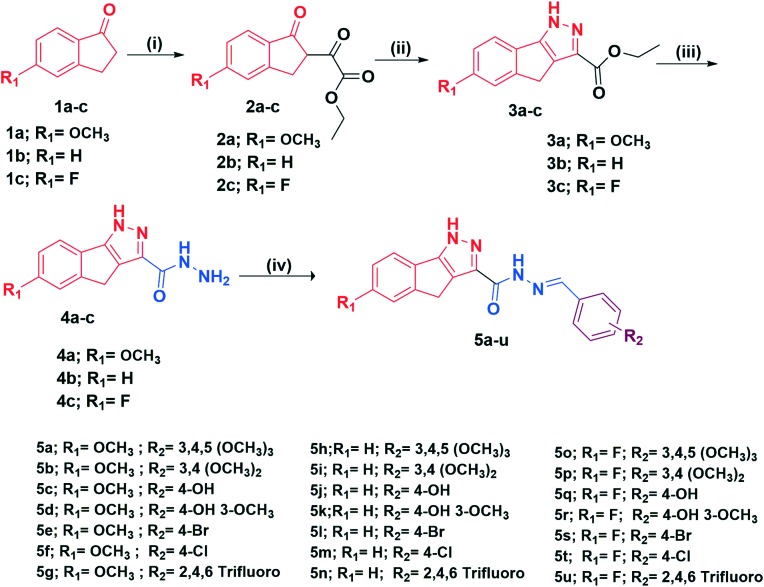
The synthesis of 1,4-dihydroindeno[1,2-c]pyrazole-tethered carbohydrazide hybrids (5a–u) is outlined in Scheme 1. The target compounds (5a–u) were straightforwardly obtained by condensation of equimolar amounts of substituted aromatic benzaldehydes with substituted 1,4-dihydroindeno[1,2-c]pyrazole-3-carbohydrazides (4a–c) in the presence of a catalytic amount of glacial acetic acid in refluxing ethanol. The crucial intermediates, substituted 1,4-dihydroindeno[1,2-c]pyrazole-3-carbohydrazides (4a–c) were synthesized conveniently in three steps. Initially, the substituted indanones (1a–c) were reacted with diethyl oxalate in the presence of freshly prepared sodium ethanolate in ethanol to afford diketo esters (2a–c) in good yields which subsequently on further dehydrative cyclization with hydrazine dihydrochloride in ethanol yielded the corresponding indenopyrazole carboxylates (3a–c) in good yields. In the next step, these esters were then reacted with NH2–NH2·H2O in ethanol to furnish substituted 1,4-dihydroindeno[1,2-c]pyrazole-3-carbohydrazides (4a–c) in excellent yields. All the final compounds were characterized by 1H NMR, 13C NMR, mass and HRMS spectral data.
Scheme 1. Synthesis of new 1,4-dihydroindeno[1,2-c]pyrazole-tethered carbohydrazide hybrids (5a–u). Reagents and conditions: (i) NaOEt/EtOH, diethyl oxalate 5 h, 0 °C–rt, 70–81%; (ii) NH2NH2·2HCl, EtOH, 3 h, reflux, 75–80%; (iii) hydrazine hydrate, EtOH 3 h, reflux, 85–90%; (iv) substituted benzaldehydes, a few drops of glacial acetic acid, EtOH 3–4 h, reflux, 67–88%.
Biological evaluation
The synthesized 1,4-dihydroindeno[1,2-c]pyrazole-tethered carbohydrazide hybrids (5a–u) were tested against various pathogenic microbes in vitro. In addition, the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) were evaluated for the most promising hybrids that inhibited the test pathogens. Efforts were made to elucidate the mechanism of action of the promising derivatives by investigating ergosterol inhibition and intracellular ROS levels generated.
Antimicrobial activity
All the synthesized derivatives (5a–u) were evaluated for their in vitro antimicrobial activity by the agar well diffusion method14 with standard ciprofloxacin and miconazole as controls against both Gram-positive and Gram-negative bacteria along with a fungal strain, respectively. All the test microbial pathogens were procured from Microbial Type Culture Collection and Gene Bank, CSIR-Institute of Microbial Technology, Chandigarh, India. The bacterial panel employed was Gram positive bacterial strains such as Micrococcus luteus MTCC 2470, Staphylococcus aureus MTCC 96, Staphylococcus aureus MLS-16 MTCC 2940, and Bacillus subtilis MTCC 121, while Gram negative bacterial strains included Escherichia coli MTCC 739, Pseudomonas aeruginosa MTCC 2453 and Klebsiella planticola MTCC 530.
Among all the tested derivatives, 5a, 5b, 5c, 5d, 5g, 5h, 5l and 5m–5u exhibited good to moderate activity against different test pathogens. Moreover, compounds 5d, 5g, 5j, 5k and 5q exhibited promising antimicrobial activity against the entire panel of test pathogens including Gram-positive and Gram-negative bacteria and a Candida strain with MIC values ranging from 3.9 to 7.8 μg mL–1 while compounds 5a, 5b and 5c were found to exhibit antibacterial activity against Gram-positive bacterial strains and be inactive against Gram-negative strains. Moreover, 5e and 5i were found to be inactive against all the test pathogens. In addition, compound 5o was found to be active against all the bacterial strains and inactive against the fungal strain. Furthermore, compounds 5d and 5g exhibited a MIC value of 3.9 μg mL–1, equipotent to standard miconazole against Candida albicans MTCC 3017. The MIC values of all the derivatives corresponding to their antibacterial activity are tabulated in Table 1.
Table 1. Antimicrobial activity of 1,4-dihydroindeno[1,2-c]pyrazole-tethered carbohydrazide hybrids (5a–u).
| Test comp. | Minimum inhibitory concentration (μg mL–1) |
|||||||
| Gram positive bacteria |
Gram negative bacteria |
Fungus | ||||||
| M. l | S. a | S. a | B. s | E. c | P. a | K. p | C. a | |
| 5a | 3.9 | 15.6 | 7.8 | 3.9 | >125 | >125 | >125 | >125 |
| 5b | 3.9 | 3.9 | 7.8 | 7.8 | >125 | >125 | >125 | >125 |
| 5c | 3.9 | 7.8 | 7.8 | 7.8 | >125 | >125 | >125 | >125 |
| 5d | 3.9 | 3.9 | 3.9 | 7.8 | 3.9 | 7.8 | 3.9 | 3.9 |
| 5e | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 5f | 3.9 | 3.9 | 7.8 | 7.8 | 3.9 | >125 | >125 | >125 |
| 5g | 7.8 | 3.9 | 3.9 | 7.8 | 3.9 | 7.8 | 7.8 | 3.9 |
| 5h | >125 | 15.6 | 15.6 | 7.8 | >125 | >125 | >125 | >125 |
| 5i | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 5j | 7.8 | 3.9 | 3.9 | 7.8 | 3.9 | 7.8 | 7.8 | 7.8 |
| 5k | 7.8 | 3.9 | 7.8 | 3.9 | 7.8 | 7.8 | 3.9 | 7.8 |
| 5l | >125 | 15.6 | 15.6 | 15.6 | >125 | >125 | >125 | >125 |
| 5m | >125 | 3.9 | 3.9 | 7.8 | 7.8 | >125 | >125 | >125 |
| 5n | >125 | >125 | >125 | 15.6 | >125 | >125 | >125 | >125 |
| 5o | 7.8 | 3.9 | 7.8 | 3.9 | 3.9 | 7.8 | 7.8 | >125 |
| 5p | >125 | >125 | 3.9 | >125 | >125 | >125 | >125 | >125 |
| 5q | 7.8 | 3.9 | 3.9 | 7.8 | 3.9 | 7.8 | 7.8 | 7.8 |
| 5r | >125 | >125 | 3.9 | >125 | >125 | >125 | >125 | 31.2 |
| 5s | >125 | 7.8 | 15.6 | 7.8 | >125 | >125 | >125 | >125 |
| 5t | >125 | 15.6 | 15.6 | 7.8 | >125 | >125 | >125 | >125 |
| 5u | >125 | 7.8 | 3.9 | 7.8 | >125 | >125 | >125 | 31.2 |
| MCZ | — a | — | — | — | — | — | — | 3.9 |
| CPF | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | — |
aNo activity; MCZ – miconazole; CPF – ciprofloxacin; bold MIC values signify promising activity; M. l – Micrococcus luteus MTCC 2470; S. a – Staphylococcus aureus MTCC 96; S. a Staphylococcus aureus MLS-16 MTCC 2940; B. c – Bacillus subtilis MTCC 121; E. c – Escherichia coli MTCC 739; P. a – Pseudomonas aeruginosa MTCC 2453; K. a – Klebsiella planticola MTCC 530; C. a – Candida albicans MTCC 3017.
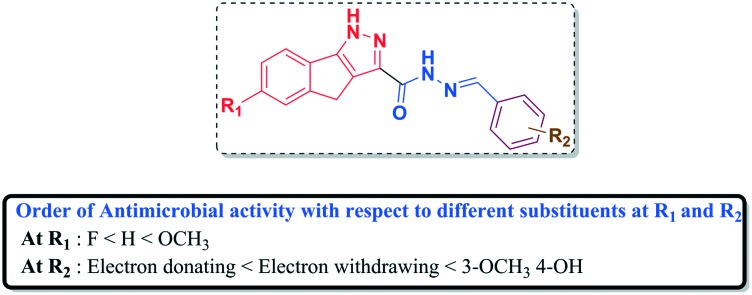
A structure–activity relationship (SAR) interpreted based on the above antimicrobial results is presented in Fig. 3. The presence of an electron donating group (–OCH3) at R1 significantly increased the antimicrobial activity as observed in the case of compounds 5d and 5g. Promising antibacterial and antifungal activities were noticed when both electron donating and withdrawing groups were present at R2 (5d and 5k). The presence of a methoxy substituent at R1 and fluoro substituents at R2 increased the antimicrobial activity (5g). Moreover, the presence of an electron donating group at R1 and electron withdrawing groups (–OH and –fluoro) were found to be crucial for antibacterial and antifungal activities, respectively. Overall, the order of antimicrobial activity with respect to different substituents at R1 and R2 is presented in Fig. 3.
Fig. 3. Structure–activity relationship of 1,4-dihydroindeno[1,2-c]pyrazole-tethered carbohydrazide hybrids (5a–u).
Antifungal activity
Members of the genus Candida are commensals that exist on the skin and human gastrointestinal and genitourinary tract causing no harm until there is an alteration in the host microenvironment. An alteration in the host immune system might result in over growth of Candida and Candida-biofilms, which cause infections that can range from simple fungal thrush to life threatening complications. Immunodeficient individuals like diabetic patients, patients undergoing treatments for cancer and HIV-AIDS and children are more prone to Candida infections. In 75% of women, Candida infections may affect them at least once in their lifetime.15 In recent years, biofilms formed by members of Candida species caused serious complications in various medical prosthetics.16 In view of all these serious clinical impediments caused by Candida members, the antifungal potential of the most promising derivatives namely 5d, 5g, 5j, 5k and 5q was evaluated17 against a panel of pathogenic Candida strains including Candida albicans MTCC 183, C. albicans MTCC 227, C. albicans MTCC 1637, C. albicans MTCC 3017, C. albicans MTCC 3018, C. albicans MTCC 4748, C. albicans MTCC 7315, C. parapsilosis MTCC 1744, C. glabrata MTCC 3019, C. krusei MTCC 3020 and Issatchenkia hanoiensis MTCC 4755. The active hybrids such as 5d, 5g, 5j, 5k and 5q exhibited good to moderate antifungal activity with MIC values ranging from 3.9–31.2 μg mL–1. The MFC values were found to be 2-fold the antifungal activity values ranging from 7.8 to 125 μg mL–1. The MIC and MFC values are tabulated in Table 2.
Table 2. Minimum inhibitory concentration/minimum fungicidal concentration of the most active derivatives (5d, 5g, 5j, 5k and 5q).
| Fungal strain | Minimum inhibitory concentration (minimum fungicidal concentration, μg mL–1) |
|||||
| 5d | 5g | 5j | 5k | 5q | MCZ | |
| C. a 183 | 15.6 (31.2) | — | — | — | — | 3.9 (7.8) |
| C. a 227 | 3.9 (7.8) | 7.8 (15.6) | 7.8 (15.6) | 7.8 (15.6) | 7.8 (15.6) | 3.9 (7.8) |
| C. a 1637 | 7.8 (15.6) | 15.6 (31.2) | 15.6 (31.2) | 15.6 (31.2) | 15.6 (31.2) | 3.9 (7.8) |
| C. a 3018 | 7.8 (15.6) | 7.8 (15.6) | 7.8 (15.6) | 15.6 (31.2) | 15.6 (31.2) | 3.9 (7.8) |
| C. a 4748 | 7.8 (15.6) | 15.6 (31.2) | >31.2 (>62.5) | 3.9 (7.8) | 15.6 (31.2) | 3.9 (7.8) |
| C. a 7315 | 7.8 (15.6) | 15.6 (31.2) | 15.6 (31.2) | 7.8 (15.6) | 15.6 (31.2) | 3.9 (7.8) |
| C. p 1744 | 7.8 (15.6) | 15.6 (31.2)2 | 15.6 (31.2) | 15.6 (31.2) | 15.6 (31.2) | 3.9 (7.8) |
| C. g 3019 | 7.8 (15.6) | 15.6 (31.2) | >31.2 (>62.5) | 3.9 (7.8) | 15.6 (31.2) | 3.9 (7.8) |
| C. k 3020 | 7.8 (15.6) | 15.6 (31.2) | 15.6 (31.2) | 15.6 (31.2) | 15.6 (31.2) | 3.9 (7.8) |
| I. h 4755 | 15.6 (31.2) | 15.6 (31.2) | 31.2 (62.4) | 15.6 (31.2) | 31.2 (62.4) | 3.9 (7.8) |
Minimum bactericidal concentration (MBC)
Further, the most active hybrids (5d, 5g, 5j, 5k and 5q) were evaluated for their minimum bactericidal concentration against all the bacterial strains with ciprofloxacin as a standard.18 Interestingly, compounds 5d, 5g, 5j, 5k and 5q were found to be promising against all the test pathogens. The MBC values were found to be 2-fold the antibacterial activity values ranging from 7.8 to 15.6 μg mL–1. The MBC results are tabulated in Table 3.
Table 3. Minimum bactericidal (MBC) of the most active derivatives (5d, 5g, 5j, 5k and 5q).
| Test compound | Minimum bactericidal (MBC) μg mL–1 |
||||||
| Gram positive bacteria |
Gram negative bacteria |
||||||
| M. l | S. a | S. a | B. s | E. c | P. a | K. p | |
| 5d | 7.8 | 7.8 | 7.8 | 15.6 | 7.8 | 15.6 | 7.8 |
| 5g | 15.6 | 7.8 | 7.8 | 15.6 | 7.8 | 15.6 | 15.6 |
| 5j | 15.6 | 7.8 | 7.8 | 15.6 | 7.8 | 15.6 | 15.6 |
| 5k | 15.6 | 7.8 | 15.6 | 7.8 | 15.6 | 15.6 | 7.8 |
| 5q | 15.6 | 7.8 | 7.8 | 15.6 | 7.8 | 15.6 | 15.6 |
| CPF | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 |
Inhibition of ergosterol biosynthesis
It is a well-known fact that most of the antifungal agents inhibit fungal cell growth by targeting the ergosterol biosynthesis pathway.19 Ergosterol plays a pivotal role in maintaining the cell integrity and function of different Candida strains. Therefore, the disruption of this pathway (lanosterol 14α-demethylase enzyme) by antifungal agents causes fungistasis.
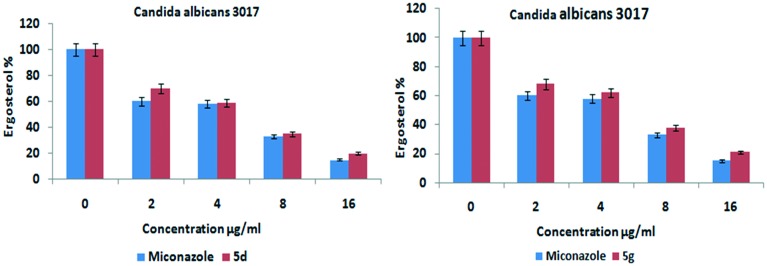
Considering these facts, the most promising compounds were evaluated for ergosterol inhibition activity by using the modified sterol extraction method,20 wherein the steady state amounts of ergosterol formed in C. albicans were quantified and UV-spectral scans were evaluated to understand the sterol profiles of C. albicans. The control group showed 100% ergosterol, while there was a dose dependent decrease in the ergosterol content with regard to the compound (5d and 5g) treated Candida strain and standard miconazole drug. As the dose of the tested compounds increased from 0 to 16 μg mL–1, the concentration of ergosterol content decreased. The UV-visible scans revealed a flat line at the effective doses of compounds 5d and 5g, signifying that no detectable levels of ergosterol formed. These results reveal that the tested compounds inhibited the ergosterol biosynthesis which is in correlation with the antifungal activity results. The results to this regard are presented in Fig. 4.
Fig. 4. Ergosterol inhibition assay for the most promising derivatives (5d and 5g).
ROS quantification in Candida albicans
According to the recent literature, some of the available antifungals (miconazole, amphotericin B and nystatin) also act by inducing the accumulation of ROS (reactive oxygen species) in fungal pathogens such as C. albicans, A. fumigatus and Cryptococcus spp. subsequently leading to fungal cell death.21
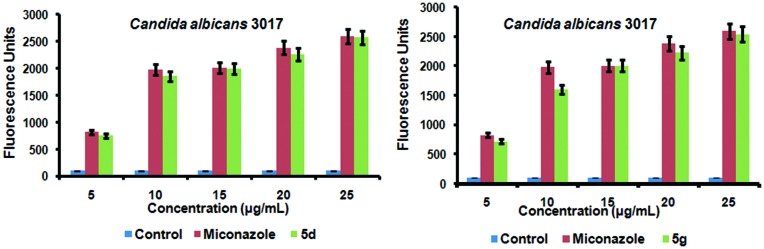
In order to explain whether oxidative stress was also involved in fungal cell death, the intracellular ROS accumulation within the cells of biofilms was evaluated using the fluorescent probe, 2′,7′-dichlorofluorescein-diacetate (DCFH-DA). Based on the DCF-DA fluorometric assay,22 it was observed that Candida albicans MTCC 3017 cells treated with various concentrations of compounds 5d and 5g showed accumulation of ROS in a dose-dependent pattern and the results are shown in Fig. 5. Miconazole was used as the control to monitor the ROS generated.
Fig. 5. Intracellular ROS accumulation of compounds 5d and 5g in Candida albicans MTCC 3017.
Both 5d and 5g accumulated ROS levels equivalent to that of miconazole and showed fluorescence intensities similar to that of miconazole treated Candida albicans MTCC 3017 cells. The ROS accumulation started at 5 μg mL–1 of the test compound as seen in Fig. 6. This concentration is much lower than its MFC (7.8 μg mL–1). At higher concentration (25 μg mL–1), compounds 5d and 5g generated ROS in amounts that were equivalent to that of miconazole. Based on the fluorometric assay, it is evident that compounds 5d and 5g altered the redox state of yeast cells by inducing the ROS accumulation in the C. albicans MTCC 3017 strain and thereby possibly affect the membrane stability and viability of Candida cells thus causing fungal cell death.
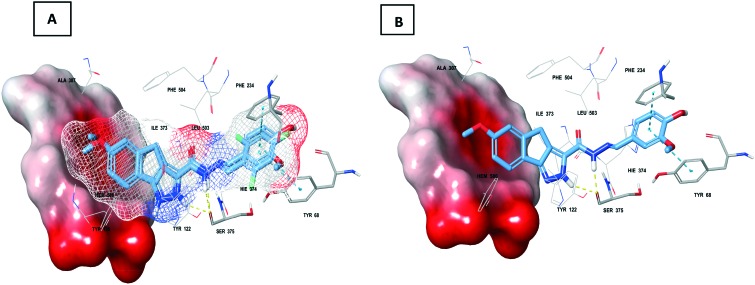
Fig. 6. A. Overlap of 5d and 5g (mesh and indicated by thick sticks) at the active site of sterol 14-α-demethylase (PDB: ; 4UYM); B. Thick sticks 5d at the sterol 14-α-demethylase (PDB: ; 4UYM) prerogative site; note: solid surface indicates heme, thin sticks indicate interacting residues and other macromodel sticks indicate active site residues; yellow dotted line – hydrogen bond and blue dotted line – π–π interaction.
Molecular docking studies
In order to validate the mode of action and predict the binding abilities of compounds 5d and 5g, molecular docking was performed using the Glide module of Schrodinger LLC.23 The heme proteins (CYP51), sterol 14-α-demethylases (PDB: ; 3GW9 and ; 1EA1) of parasites (Trypanosoma brucei), bacteria (Mycobacterium tuberculosis) and fungi (Candida albicans) (PDB: ; 4UYM) along with their co-crystal ligands were retrieved from the protein data bank.24 Molecular docking against three different types of sterol 14-α-demethylases originating from these parasites, bacteria and fungi was compared with validated molecular docking. The docking scores obtained against tested active sites are presented in Table 4. From the initial assessment, the interactions of 5d and 5g with heme (porphyrin) were not observed with respect to the parasite and bacterial sterol 14-α-demethylases. Intriguingly, interactions such as hydrogen bonding and π-cation interactions were observed with fungal sterol 14-α-demethylase which might play a significant role in its high docking efficiency. The combined hydrogen bond of pyrazole and hydrazinyl functionalities with Ser375 and π–π interaction of the substituted phenyl ring with Phe234 and Tyr68 of fungal sterol 14-α-demethylase was observed with the tested ligands (Fig. 6). The docking study was in correlation with the ergosterol inhibition assay thereby validating their antifungal activity.
Table 4. Docking scores of 5d and 5g with different sterol 14-α-demethylases (kcal per mole).
In vitro cytotoxicity assay
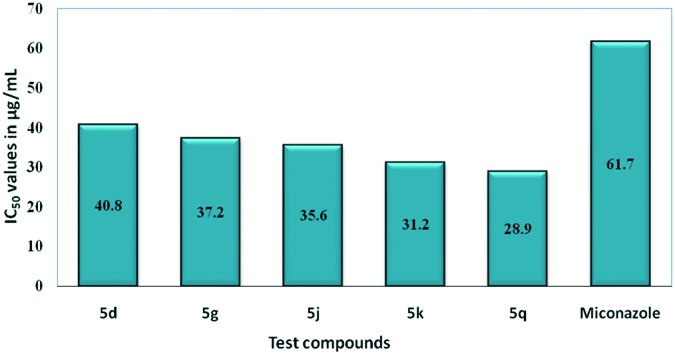
Most active compounds (5d, 5g, 5j, 5k and 5q) were screened for their in vitro cytotoxicity against normal human lung cell line MRC5 (ATCC No. CCL 171) using MTT assay25 and the results are presented in Fig. 7. In this assay, miconazole was used as the positive control along with DMSO as the negative control. IC50 values are presented in μg mL–1 as mean ± S.D. From these results, it is evident that their cytotoxicity was found to be in the range of 28–40 μg mL–1 while their antifungal activity (MIC) was observed in the range of 3.9 to 7.8 μg mL–1. These findings suggest that compounds 5d and 5g exhibited lower cytotoxicity to normal cell lines compared to the antifungal activity and could be considered to have therapeutic potential.
Fig. 7. Cytotoxicity assay of the most active derivatives (5d, 5g, 5j, 5k and 5q) on the MRC5 normal cell line.
In silico drug likeness studies
The in silico drug likeness prediction of 1,4-dihydroindeno[1,2-c] pyrazole tethered carbohydrazides (5a–u) was performed by means of the Molinspiration online property calculation toolkit26 in order to determine the Lipinski parameters27 and the results to this regard are depicted in Table S1.† Drug likeness of the target compounds (5a–u) was predicted using Lipinski's rule of five, which states that an orally administered compound is more likely to be permeable if the molecule satisfies the following rules: (i) molecular weight <500, (ii) calculated log P < 5, (iii) hydrogen bond donors ≤5 (OH and NH groups), and (iv) hydrogen bond acceptors ≤10 (N and O atoms). Compounds obeying the Lipinski rule may have more oral absorption and bioavailability. Fascinatingly, all the conjugates from this series (5a–u) satisfied Lipinski's rule of five. From all these findings, it can be predicted that the compounds can be orally active in humans.
In silico pharmacokinetics (ADME) studies
Pharmacokinetics refers to absorption, distribution, metabolism and excretion of the drug (ADME) in the body. The in silico ADME studies of the most active compounds (5d, 5g, 5j, 5k and 5q) were carried out using the SwissADME web based tool28 and the data generated are presented in Table S2.† High gastrointestinal absorption indicates a drug to be orally active and all the active hybrids (5d, 5g, 5j, 5k and 5q) were found to exhibit high gastrointestinal absorption. Bioavailability is defined as the amount of the drug present in the plasma and is considered as the most vital factor governing absorption. Furthermore, all the hits were found to have high bioavailability scores. Permeability is an index of absorption and distribution of the drug. Permeability of these active compounds was found to be high indicating that these are skin permeable. Moreover, all the active hits were found to be metabolizing via the CYP450 substrate indicating their easier metabolism. Synthetic accessibility scores refer to how effortlessly a compound can be synthesized in a lab on scales of simple to tough ranging between 0 and 10. In addition, the synthetic accessibility scores of all the hits were found to be below 4 demonstrating that they can be effortlessly synthesized on a large scale. In conclusion, the above results indicate that these hybrids demonstrate an appreciable pharmacokinetic profile and drug likeness.
In silico toxicity prediction
Toxicity prediction of a compound is an imperative part of drug discovery and development. Computational toxicity evaluation minimizes the amount of animal experiments than the determination of toxic doses in animals. The best and ideal method to predict the toxicity of drug molecules is by building animal models. The web server Protox29 estimates rodent oral toxicity derived from compounds of known drug candidates and their toxicity by their toxic fragments or chemical structure and compares the similarity of structures of synthesized molecules which will be encumbered in the server with a database of molecules consisting of previously known toxicity and identifies the toxic fragments of loaded molecules and possible toxicity targets.
Toxic doses are calculated as LD50 values in mg per kg body weight and are presented in Table S3.† The LD50 is the median lethal dose, defined as the dose at which 50% of test subjects die upon exposure to the chemical. The predicted LD50 (lethal dose) for compounds 5d and 5k is above 2000 mg kg–1 and less than 5000 mg kg–1 indicating that it comes under class V as compared to the standards used which come under class IV, while the remaining compounds 5g and 5p showed LD50 values above 300 mg kg–1 and below 2000 mg kg–1 depicting that they come under class IV toxicity. This toxicity prediction study summarizes that synthesized derivatives are less toxic.
Conclusion
In conclusion, a series of new 1,4-dihydroindeno[1,2-c] pyrazole-tethered carbohydrazide hybrids (5a–u) were synthesized and evaluated for their in vitro antimicrobial activity against a panel of gram positive and gram negative bacteria along with a fungal strain. Among them, compounds 5d, 5g, 5j, 5k and 5q exhibited promising activity against the entire panel of test pathogens with a MIC ranging from 3.9–7.8 μg mL–1. Further, these potential compounds were evaluated for their antifungal activity against a panel of Candida strains. Compounds 5d and 5g demonstrated promising antifungal activity while the MBC/MFC values were 2-fold the antibacterial/antifungal activity values. Further, compounds 5d and 5g inhibited the ergosterol biosynthesis, elucidating their mode of action which was further validated by molecular docking studies. Furthermore, compounds 5d and 5g caused accumulation of intracellular ROS in a dose-dependent manner equivalent to that of miconazole indicating that they altered the redox state of yeast cells that might possibly affect the membrane stability, viability and efficacy of Candida cells. MTT assay revealed that the most promising hybrids were non-toxic to normal cells and could be considered to have therapeutic potential. In silico drug likeness studies and pharmacokinetics (ADMET) studies revealed that 5d, 5g, 5j, 5k and 5q exhibit drug likeness and an appreciable pharmacokinetic profile. Overall, the findings suggest that compounds 5d and 5g could be further explored as promising leads for further antifungal development.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
Authors would like to thank CSIR and UGC, New Delhi for the funding and CSIR-IICT for the facilities. And also Dr. B. N. and S. S. J. thank SERB-NPDF (Ref: PDF/2017/001556). (Manuscript Communication number: IICT/Pubs./2019/015).
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9md00155g
References
- (a) Outterson K., Powers J. H., Daniel G. W., McClellan M. B. Health Aff. 2015;34:277–285. doi: 10.1377/hlthaff.2014.1003. [DOI] [PubMed] [Google Scholar]; (b) Levy S. B., Marshall B. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- (a) Garibotto F. M., Garro A. D., Masman M. F., Rodriguez A. M., Luiten P. G., Raimondi M. M., Zacchino S. A., Somlai C., Penke B., Enriz R. D. Bioorg. Med. Chem. 2010;18:158–167. doi: 10.1016/j.bmc.2009.11.009. [DOI] [PubMed] [Google Scholar]; (b) Latge J. P. Clin. Microbiol. Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Steenbergen J. N., Casadevall A. J. J. Clin. Microbiol. 2000;38:1974–1976. doi: 10.1128/jcm.38.5.1974-1976.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Brown G. D., Denning D. W., Levitz S. M. Science. 2012;336:647–648. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]; (b) Crunkhorn S. Nat. Rev. Drug Discovery. 2016;15:604. doi: 10.1038/nrd.2016.169. [DOI] [PubMed] [Google Scholar]; (c) Vandeputte P., Ferrari S., Coste A. T. Int. J. Microbiol. 2012;2012:1–26. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Saint S., Chenoweth C. E. Infect. Dis. Clin. North Am. 2003;17:411–432. doi: 10.1016/s0891-5520(03)00011-4. [DOI] [PubMed] [Google Scholar]; (b) Burmolle M., Thomsen T. R., Fazli M., Dige I., Christensen L., Homoe P., Tvede M., Nyvad B., Tolker-Nielsen T., Givskov M., Moser C., Kirketerp-Møller K., Johansen H. K., Hoiby N., Jensen P. O., Sorensen S. J., Bjarnsholt T. FEMS Immunol. Med. Microbiol. 2010;59:324–336. doi: 10.1111/j.1574-695X.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- Sheehan D. J., Hitchcock C. A., Sibley C. M. Clin. Microbiol. Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Kathiravan M. K., Salake A. B., Chothe A. S., Dudhe P. B., Watode R. P., Mukta M. S., Gadhwe S. Bioorg. Med. Chem. 2013;21:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]; (b) Jiang Z., Gu J., Wang C., Wang S., Liu N., Jiang Y., Dong G., Wang Y., Liu Y., Yao J., Miao Z., Zhang W., Shen C. Eur. J. Med. Chem. 2014;82:490–497. doi: 10.1016/j.ejmech.2014.05.079. [DOI] [PubMed] [Google Scholar]
- (a) Baillie G. S., Douglas L. J. Antimicrob. Agents Chemother. 1998;42:2146–2149. doi: 10.1128/aac.42.8.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chandra J., Kuhn D. M., Mukherjee P. K., Hoyer L. L., McCormick T., Ghannoum M. A. J. Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Pitman S. K., Drew R. H., Perfect J. R. Expert Opin. Emerging Drugs. 2011;16:559–586. doi: 10.1517/14728214.2011.607811. [DOI] [PubMed] [Google Scholar]; (b) Obach R. S., Walsky R. L., Venkatakrishnan K., Gaman E. A., Brian Houston J. B., Tremaine L. M. J. Pharmacol. Exp. Ther. 2006;316:336–348. doi: 10.1124/jpet.105.093229. [DOI] [PubMed] [Google Scholar]; (c) Kale P., Johnson L. B. Drugs Today. 2005;41:91–105. doi: 10.1358/dot.2005.41.2.882661. [DOI] [PubMed] [Google Scholar]
- Boyd G. V. Tetrahedron Lett. 1965;6:1421–1426. [Google Scholar]
- (a) Lemke T. L., Cramer M. B., Shanmugam K., Ahsan M. J., Samy J. G., Dutt K. R., Agrawal U. K., Yadav B. S., Vyas S., Kaur R., Yadav G. J. Pharm. Sci. Bioorg. Med. Chem. Lett. 1978;2011;6721:1377–1381. 4451–4453. doi: 10.1002/jps.2600671012. [DOI] [PubMed] [Google Scholar]; (b) Khan I., Garikapati K. R., Shaik A. B., Makani V. K. K., Rahim A., Shareef M. A., Reddy V. G., Pal-Bhadra M., Kamal A., Kumar C. G. Eur. J. Med. Chem. 2018;144:104–115. doi: 10.1016/j.ejmech.2017.12.010. [DOI] [PubMed] [Google Scholar]; (c) Bonacorso H. G., Cavinatto S., Campos P. T., Porte L. M. F., Navarini J., Paim G. R., Martins M. A. P., Zanatta N., Stuker C. Z. J. Fluorine Chem. 2012;135:303–314. [Google Scholar]; (d) Angelone T., Caruso A., Rochais C., Caputo A. M., Cerra M. C., Dallemagne P., Filice E., Genest D., Pasqua T., Puoci F., Saturnino C., Sinicropi M. S., El-Kashef H. Eur. J. Med. Chem. 2015;92:672–681. doi: 10.1016/j.ejmech.2015.01.037. [DOI] [PubMed] [Google Scholar]
- Narang R., Narasimhan B., Sharma S. Curr. Med. Chem. 2012;19:569–612. doi: 10.2174/092986712798918789. [DOI] [PubMed] [Google Scholar]
- (a) Leal L. F., Bueno A. C., Gomes D. C., Abduch R., de Castro M., Antonini S. R. Oncotarget. 2015;6:43016–43032. doi: 10.18632/oncotarget.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bhandari R., Eguchi T., Sekine A., Ohashi Y., Kakinuma K., Ito M., Mizoue K. J. Antibiot. 1999;2:231–234. doi: 10.7164/antibiotics.52.231. [DOI] [PubMed] [Google Scholar]
- (a) Prajapti S., Shrivastava S., Bihade U., Gupta A. K., Naidu V. G. M., Banerjee U. C., Babu B. N. MedChemComm. 2015;6:839–845. [Google Scholar]; (b) Kamal A., Hussaini S. M. A., Sucharita M. L., Poornachandra Y., Sultana F., Kumar C. G. Org. Biomol. Chem. 2015;13:9388–9397. doi: 10.1039/c5ob01353d. [DOI] [PubMed] [Google Scholar]
- Amsterdam D., Susceptibility testing of antimicrobials in liquid media, in Antibiotics in Laboratory Medicine, ed. V. Loman, Williams and Wilkins, Baltimore, MD, 4th edn, 1996, pp. 52–111. [Google Scholar]
- Genital/vulvovaginal candidiasis (VVC) www.cdc.gov. February 13, 2014. Retrieved 28 December 2017.
- Chandra J., Mukherjee P. K. Microbiol. Spectrum. 2015;3:1–14. doi: 10.1128/microbiolspec.MB-0020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI, Reference method for broth dilution antifungal susceptibility testing of yeasts, CLSI standard M27, Clinical and laboratory standard Institute, P.A. Wayne, 4th edn, 2017. [Google Scholar]
- CLSI, Methods for dilution Antimicrobial susceptibility tests for bacteria that grow aerobically, CLSI document M-07-A10, Clinical and laboratory standard Institute, P.A. Wayne, 10th edn, 2015. [Google Scholar]
- (a) Fuoli L. A., Mellado E. Front. Microbiol. 2013;3:1–6. [Google Scholar]; (b) Onyewu C., Blankenship J. R., Del P. M., Heitman J. Antimicrob. Agents Chemother. 2003;47:956–964. doi: 10.1128/AAC.47.3.956-964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik O. N., Owades J. L. J. Agric. Food Chem. 1957;5:360–363. [Google Scholar]
- (a) Francois I. E., Cammue B. P. A., Borgers M., Ausma J., Dispersyn G. D., Thevissen K. Curr. Med. Chem. 2006;5:3–13. [Google Scholar]; (b) Delattin N., Cammue B. P., Thevissen K. Future Med. Chem. 2014;6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]
- VandenBosch D., Braeckmans K., Nelis H. J., Coeyne Y. J. Antimicrob. Chemother. 2010;65:694–700. doi: 10.1093/jac/dkq019. [DOI] [PubMed] [Google Scholar]
- Friesner R. A., Murphy R. B., Repasky M. P., Frye L. L., Greenwood J. R., Halgren T. A., Sanschagrin P. C., Mainz D. T. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- (a) Hargrove T. Y., Friggeri L., Wawrzak Z., Qi A., Hoekstra W. J., Schotzinger R. J., York J. D., Guengerich F. P., Lepesheva G. I. J. Biol. Chem. 2017;292:6728–6743. doi: 10.1074/jbc.M117.778308. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Podust L. M., Poulos T. L., Waterman M. R. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3068–30673. doi: 10.1073/pnas.061562898. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hargrove T. Y., Wawrzak Z., Lamb D. C., Guengerich F. P., Lepesheva G. I. J. Biol. Chem. 2015;290:23916–23934. doi: 10.1074/jbc.M115.677310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta M., Armaroli S., Castagnolo D., Fontana G., Perad P., Bombardelli E. Bioorg. Med. Chem. Lett. 2007;17:1579–1583. doi: 10.1016/j.bmcl.2006.12.101. [DOI] [PubMed] [Google Scholar]
- Ertl P., Calculation of Molecular Properties and Bioactivity Score, Available online http://www.molinspiration.com.
- Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. Adv. Drug Delivery Rev. 1997;23:1–3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Daina A., Michielin O., Zoete V. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Basu S. K., Sen K. K. Indian J. Exp. Biol. 2006;44:123–127. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.