Abstract
Introduction:
Hydrogen sulfide (H2S) is found in petroleum, natural gas, and decaying organic matter. Terrorist groups have attempted to use it in enclosed spaces as a chemical weapon. Mass casualty scenarios have occurred from industrial accidents and release from oil field sites. There is no FDA approved antidote for sulfide poisoning. We have previously reported that intravenous cobinamide is effective for sulfide poisoning. A rapid-acting antidote that is easy to administer intramuscularly (IM) would be ideal for use in a prehospital setting. In this study, we assessed survival in sulfide-poisoned swine treated with IM cobinamide.
Methods:
Eleven swine (45–55 kg) were anesthetized, intubated, and instrumented with continuous femoral and pulmonary artery pressure monitoring. After stabilization, anesthesia was adjusted such that animals ventilated spontaneously with a FiO2 of 0.21. Sodium hydrosulfide (NaHS, 8mg/mL) was infused intravenously at 0.9 mg/kg.min until apnea or severe hypotension. Animals were randomly assigned to receive cobinamide (4 mg/kg), or no treatment at the apnea/hypotension trigger. The NaHS infusion rate was sustained for 1.5 min post trigger, decreased to 0.2mg/kg.min for 10 min, and then discontinued.
Results:
The amount of NaHS required to produce apnea or hypotension was not statistically different in both groups (cobinamide: 9.0 mg/kg ±6.1; saline: 5.9 mg/kg ±5.5; mean difference: −3.1, 95% CI: −11.3, 5.0). All of the cobinamide treated animals survived (5/5), none of the control (0/6) animals survived (p <.01). Mean time to return to spontaneous ventilation in the cobinamide treated animals was 3.2 (±1.1) min. Time to return to baseline systolic blood pressure (±5%) in cobinamide-treated animals was 5 min.
Conclusion:
Intramuscular cobinamide was effective in improving survival in this large swine model of severe hydrogen sulfide toxicity.
Keywords: Cobinamide, hydrogen sulfide toxicity, resuscitation, swine
Introduction
Hydrogen sulfide (H2S) is a toxic, flammable, colorless, gas that occurs naturally from the decomposition of organic substances. H2S is easily made by combining chemicals containing hydrochloric acid (i.e., disinfectants) with those that contain sulfur (i.e., pesticides), which led the New York State Homeland Security Office to suggest it could be used as a chemical weapon in a terrorist attack [1]. The notion became fact in 2017 when Australian Federal Police exposed a terrorist plot to release H2S in an enclosed public space [2]. The most recent Department of Homeland Security Chemical of Interest document lists hydrogen sulfide as a weapon of mass effect. Most exposure and deaths caused by H2S, however, are accidental and related to industrial accidents. For example, industrial accidents involving gas pipeline leaks have released H2S causing multiple instances of H2S poisoning [3]. Technological developments in hydraulic fracturing have increased extraction of natural gas from shale [4]. As hydraulic fracturing escalates, the risk of accidental H2S release and subsequent casualties increases [5].
As a byproduct of industrial processes, H2S occurs naturally in crude petroleum and natural gas. It has a rotten egg smell, but olfactory fatigue often occurs; hence, the smell is an unreliable tool for detecting the gas which can result in fatal exposure [6,7]. Exposure to H2S may rapidly produce dyspnea, confusion, nausea and vomiting, hypotension and loss of consciousness, seizures, and death [6,7]. The mechanism by which H2S causes toxicity is via inhibition of cytochrome C oxidase in complex IV of the electron transport chain; similar to cyanide [7-9]. Much like current FDA approved antidotes for cyanide poisoning, treatment recommendations for H2S exposure are aimed at binding H2S and accelerating elimination. To date, there are no FDA approved antidotes to H2S poisoning but research is ongoing.
Previously, we demonstrated that intravenous cobinamide rescued severely sodium hydrosulfide-poisoned swine [8]. Gaining intravenous access can be difficult and time-consuming in potential hydrosulfide exposure situations. Antidotes to H2S poisoning that can be administered by intramuscular injection would be better suited for mass casualty conditions. Cobinamide is the penultimate precursor of hydroxocobalamin (Vitamin B12) and is more water soluble than cobalamin. This allows for a volume small enough to be administered intramuscularly which may be more practical in the prehospital, mass casualty setting [7,8]. It neutralizes sulfide more efficiently, as it has two available binding sites, thus being able to neutralize two moles of sulfide for each mole of cobinamide. The proposed mechanism of action of cobinamide is thought to be two-fold, via direct reversal of complex IV inhibition and neutralization of sulfide-generated reactive oxygen species [9,10]. Cobinamide rapidly reacts with sulfide to form SSH2−. This forms a stable complex with cobinamide which can then be eliminated [7-10]. Intramuscular injections can be given quickly and without much training, and could be self-administered. We evaluated the efficacy of intramuscular cobinamide to treat H2S poisoning in swine.
Goal of this investigation
The primary hypothesis of our study is that intramuscular cobinamide will rescue animals from H2S toxicity. The swine model we developed produces apnea, significant hypotension, and biomarkers that closely parallel exposure of >500 parts per million (ppm) in humans [8,9]. In humans, exposure to sulfide leads to less oxygen consumption, elevation of inflammatory markers, inhibition of oxidative phosphorylation, and the development of hyperlactatemia, as well as hypotension and tachycardia; similar to the toxicity, demonstrated in the swine model [7-9]. We compared survival of swine treated with intramuscular cobinamide to survival in the control group.
Methods
Study design
A prospective, randomized investigation was conducted. The study was approved by the University of Colorado′s Institutional Animal Care and Use Committee (IACUC) and the Wilford Hall Ambulatory Surgical Center’s Animal Care and Use Committee. The study complied with the regulations and guidelines of the Animal Welfare Act and the American Association for Accreditation of Laboratory Animal Care. Animal housing and experimental procedures took place in the animal care facility at the University of Colorado and the Wilford Hall Ambulatory Surgical Center Clinical Research Division.
Animal subjects and preparation
Eleven female Yorkshire swine (Sus scrofa) weighing 45–55 kg were used. Intramuscular administration of ketamine (10–20 mg/kg) (MWI, Boise, ID) followed by inhaled isoflurane (MWI, Boise, ID) via nose cone was used for induction. To prevent any toxin from escaping into the OR room, all animals had to have urinary catheters with closed systems. Because male pigs have a tortuous urethra, catheterizing them is not ideal, and all animals used were female. Following induction, animals were intubated with an 8.0 mm cuffed endotracheal tube (Teleflex, Morrisville, NC), and peripheral venous access obtained. Sedation was maintained using the Draeger Fabius GS anesthesia machine (Draeger, Houston, TX) with 1–3% isoflurane and 0.4 FiO2. Tidal volume was set at 8–10 mL/kg. A respiratory rate of 10–20 breaths per minute was used to maintain an end-tidal CO2 of 38–42 mmHg. Lead II of the surface electrocardiogram was monitored continuously. Temperature was maintained at 37.5–39 °C.
The external jugular, carotid artery, and femoral artery were visualized using the M9 ultrasound system (Mindray, Mahwah, NJ), and central venous and arterial access was obtained approximately 30 min prior to toxin exposure. A 20 mL/kg bolus of 0.9% saline was administered prior to central line placement. An 8.5 French introducer (Arrow, Reading, PA) was placed in the carotid artery for laboratory sampling and another was placed in the jugular for medication administration. Arterial pressure was measured continuously through the femoral artery. Other continuous measurements included mean arterial pressure (MAP), heart rate (HR), respiratory rate (RR), pulse oximetry (SpO2), electrocardiogram (ECG), and core temperature, which were recorded every 5 min. The Draeger Infinity Delta Monitor (Draeger, Houston, TX) was used for data acquisition at University of Colorado, whereas the Draeger Fabius GS was used for data acquisition at Wilford Hall.
The animals were allowed to stabilize for 10 min after instrumentation. Baseline measurements included oxygen saturation, PaO2, PaCO2, hemoglobin (Hb), pH, bicarbonate, lactate (ABL 800 Flex blood gas analyzer, Radiometer America, Westlake, OH), prothrombin time (PT), partial thromboplastin time (PTT), (STA-R Evolution, Diagnostic Stago Inc., Parsippany, NJ), and platelet count (Advia 120, Siemens, Norwood, MA).
Anesthesia was adjusted such that animals would spontaneously ventilate without mechanical ventilator assistance but were not awake enough to be of danger to laboratory personnel and themselves. Successful weaning from ventilator, defined as spontaneous ventilation with minute ventilation of ≥7.0 L/min, end-tidal CO2 50–53 mmHg, oxygen saturation ≥95%, and respiratory rate 25–35 breaths per minute (BPM), was confirmed prior to the start of the sodium hydrosulfide (NaHS) infusion.
Once the animal was breathing spontaneously, sodium hydrosulfide (Sigma Aldrich, St. Louis, MO) was diluted in saline and delivered via intravenous infusion. Animals received an initial infusion of 0.9 mg/kg.min until either reaching apnea defined as >30 s without taking a breath or a systolic blood pressure (SBP) < 75 mmHg. We chose the clinical trigger of apnea or hypotension as sulfide induces toxicity quickly and some animals become apneic before hypotension and vice versa. Either trigger is clinically relevant and represents severe toxicity. Thirty seconds after reaching apnea or SBP <75 mmHg, the infusion rate was decreased to 0.4 mg/kg.min and remained at this rate for 1.5 min. At this point, the infusion was decreased to 0.2 mg/kg.min for 10 min and then discontinued. In inhalational exposure to a poison, the exposed subject experiences rapid systemic absorption and distribution to tissues [8,10]. Because safety issues prevent us from providing inhalational exposure, the adjustment of the intravenous infusion rate is done to approximate the toxicokinetics experienced with inhalation exposure.
Upon reaching apnea or SBP <75 mmHg the animals were injected intramuscularly with either cobinamide (4 mg/kg) or saline and monitored for 60 min post-treatment or until death (if <60min), defined as a mean arterial pressure (MAP) < 30mmHg for 10 min. Animals that survived 60 min were considered survivors and then euthanized.
Outcome parameters and data analysis
The primary outcome parameter for this study was survival. A Kaplan–Meier curve was generated for each group and survival distribution analyzed by log-rank testing. Means and standard deviations were calculated for secondary parameters including cardiovascular parameters (heart rate, mean arterial pressure), respiratory parameters (minute volume, respiratory rate), and biochemical parameters (arterial blood gasses, and lactate). Secondary parameter variables were each analyzed using separate repeated-measure multivariate analysis of variance (RMANOVA) and post hoc analysis with Bonferroni adjustment.
A power analysis (using PASS version 11.0.8; Kaysville, Utah, USA) indicated that a total sample of 11 animals (six in the control group and five in the treatment group) and 18 measurements was sufficient to detect a hazard ratio of 0.0194 (equivalent to 0% survival in the control group and 80% survival in the treatment group) in a log-rank test with 80% power and an alpha of 0.05. Statistical analyses were conducted using IBM SPSS (Armonk, New York, USA).
Cobinamide
Hydroxocobinamide was synthesized from pharmaceutical grade hydroxocobalamin by base hydrolysis. The cobinamide product was >96% pure as determined using high-pressure liquid chromatography. It was converted to histidylcobinamide by adding four molar equivalents of histidine (Sigma-Aldrich) which was the compound administered in these experiments [11].
Results
Characteristics of study subjects
At baseline, the groups had similar vital signs and biochemical variables except for weight and respiratory rate (Table 1). Swine have similar physiology and normal vital signs to humans, all vitals were within normal limits at baseline and drug doses were weight based, so it is unlikely that these differences influenced outcomes [12]. There was no significant difference in isoflurane use between the two study groups during the period of spontaneous ventilation (cobinamide group: 1.2 ± 0.03; control group: 1.2 ± 0.4) as measured using Scio 4 gas analyzer (Draeger, Telford PA). There were no significant differences in the mg/kg dose of NaHS (mean difference: −3.1, 95% CI: −11.3, 5.0) to produce apnea/hypotension among the groups, nor was there a difference in the time (min) required for NaHS to produce apnea/hypotension (mean difference: −3.8, 95% CI: −13.0, 5.3; Table 2).
Table 1.
Swine characteristics at baseline.
| Cobinamide group (n = 5) |
Control group (n = 6) |
Difference between cobinamide and control group (95% CI) |
|
|---|---|---|---|
| Weight (kg) | 44.5 ± 2.5 | 50.0 ± 3.4 | 5.5 [1.4, 9.6] |
| Lactate (mmol/L) | 0.6 ± 0.2 | 0.9 ± 0.39 | 0.3 [−0.1, 0.7] |
| PH | 7.5 ± 0.02 | 7.5 ± 0.07 | 0.02 [−0.5, 0.6] |
| SBP (mmHg) | 109.0 ± 12.8 | 118.8 ± 15.0 | 9.8 [−9.4, 29.1] |
| HR (beats per min) | 78.8 ± 18.5 | 90.8 ± 12.3 | 12.0 [−9.0, 33.1] |
| RR (breaths per min) | 25.4 ± 5.6 | 34.3 ± 5.6 | 8.9 [1.53, 16.3] |
kg: kilograms; mmol/L: millimoles per liter; mmHg: millimeters of mercury; SBP: systolic blood pressure; HR: heart rate; RR, respiratory rate. Data presented as mean ± standard deviation.
Table 2.
Dose of NaHS and time to produce apnea/hypotension.
| Cobinamide group (n = 5) |
Control group (n = 6) |
Difference between cobinamide and control group (95% CI) |
|
|---|---|---|---|
| Total dose NaHS (mg) | 401.6 ± 272.5 | 289.5 ± 263.0 | −112.1 [−478.3, 254.0] |
| mg/kg dose | 9.0 ± 6.2 | 5.9 ± 5.5 | −3.1 [−11.3, 5.0] |
| Time (min:s) | 10:0 ± 6:5 | 6:1 ± 6:2 | −3.8 [−13.0, 5.3] |
NaHS: sodium hydrosulfide; mg, milligram; mg/kg: milligrams per kilogram; min:s: minutes:seconds. Data presented as mean ± standard deviation.
Primary outcome
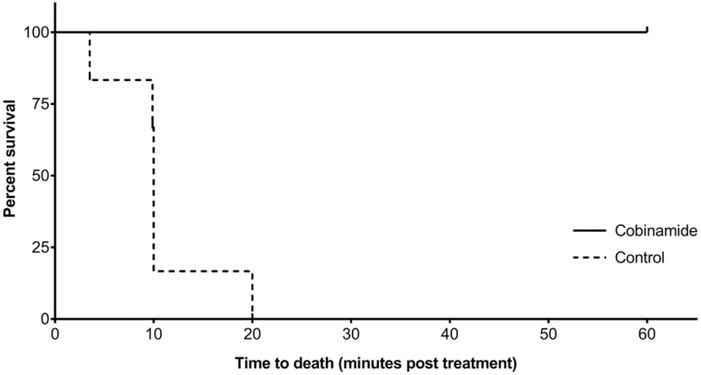
All the animals treated with cobinamide survived through the 60-min observation period, whereas none of the animals in the control group survived past 20 min (mean survival time 10.3± 5.2 min). Kaplan–Meier survival analysis indicated a significant difference in survival between the cobinamide and control groups (log-rank p<.001; Figure 1). In animals where apnea was the trigger for treatment, the mean time to spontaneous ventilations was 3.2 ± 1.1 min in cobinamide-treated animals. In animals where hypotension was the trigger for treatment, the mean time to return of baseline systolic blood pressure was 5 min (±5%) in cobinamide-treated animals.
Figure 1.
Kaplan-Meier curve: survival vs. time (min) in NaHS poisoned swine treated with cobinamide vs. control.
Secondary outcomes
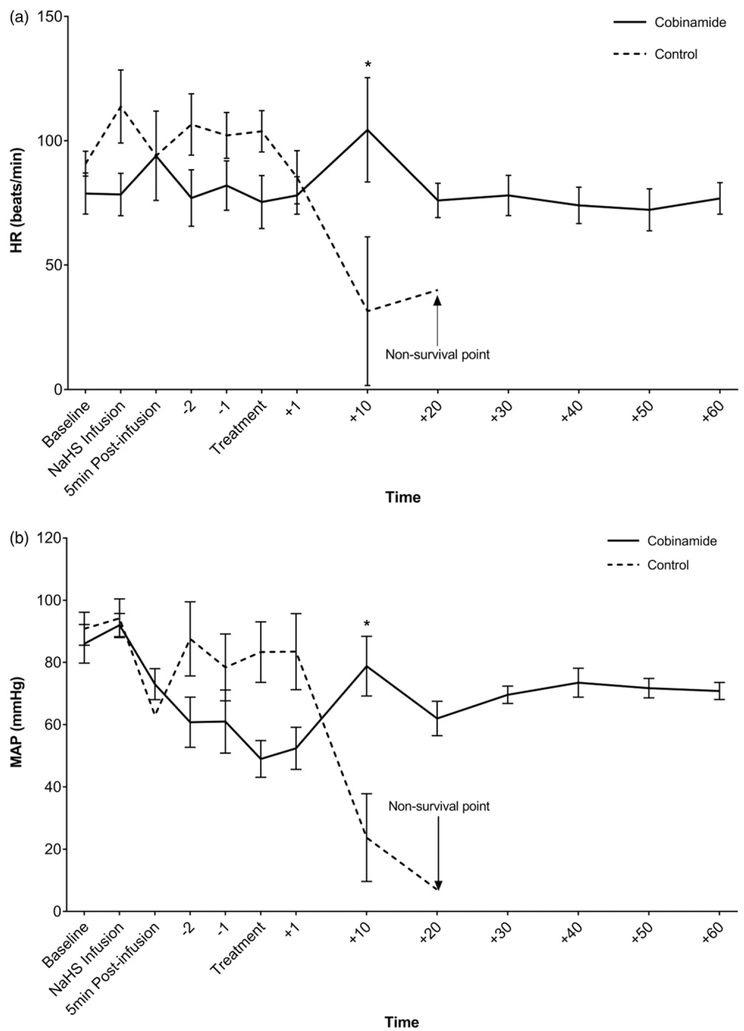
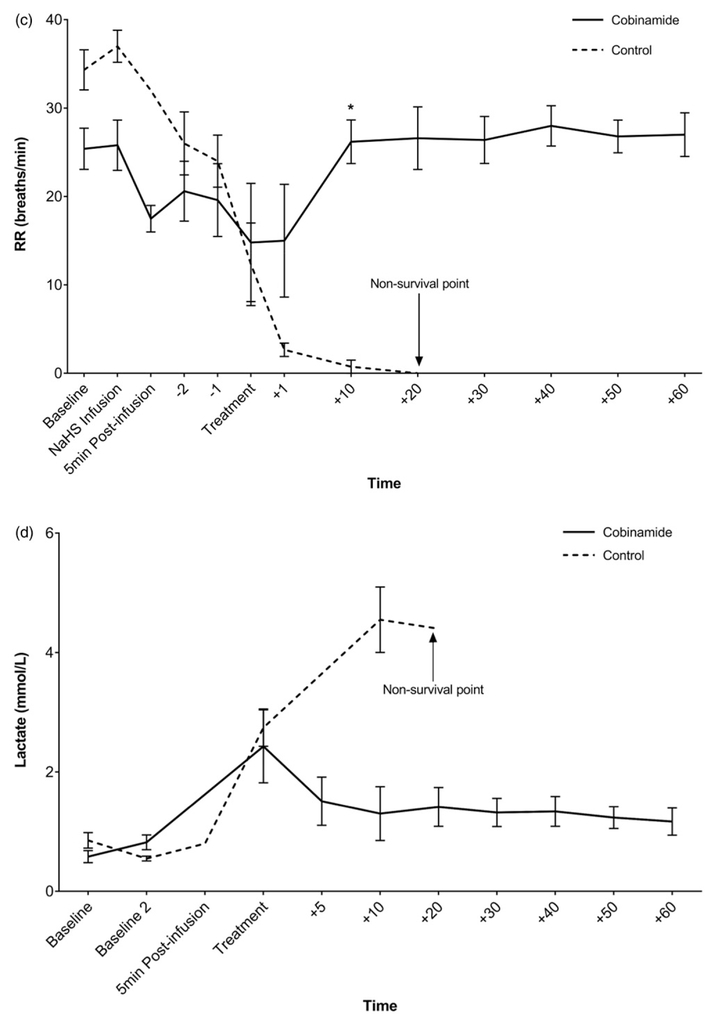
There were no significant differences in vital signs or lactate concentrations among the groups at treatment time. By 10 min post-treatment, the animals treated with cobinamide were recovering, whereas the animals in the control group continued to deteriorate which was reflected in abnormal vital signs (heart rate, blood pressure, and respiratory rate) and increasing lactate concentration (Figures 2(a-d)) pH was trended via ABG (Table 3).
Figure 2.
(a) Heart rate (beats/min) vs. time (min) in NaHS poisoned swine treated with combinamide vs. control. (b) Mean arterial pressure (mmHg) over time (min) in NaHS poisoned swine. (c) Respiratory rate (breaths/min) vs. time (min) in NaHS poisoned swine treated with cobinamide vs. control. (d) Lactate (mmol/L) vs. time (min) in NaHS poisoned swine treated with cobinamide vs. control. Mean values are shown with standard error bars. *Statistically significant.
Table 3.
Results of RMANOVA for ABGs.
| Variable | Time | Cobinamide group (n = 5) | Control group (n = 6) | Difference between cobinamide and control group (95% CI) |
|---|---|---|---|---|
| pH | Baseline | 7.5 ± 0.02 (5) | 7.5 ± 0.07 (2) | 0.02 (−0.05, 0.10) |
| Baseline 2 | 7.4 ± 0.04 (5) | 7.4 ± 0.03 (6) | 0.03 (−0.02, 0.08) | |
| Treatment | 7.5 (1) | 7.3 ±0.04 (4) | – | |
| 5 min | 7.4 ±0.03 (4) | (0) | – | |
| 10 min | 7.5 ± 0.03 (3) | 7.2 ± 0.07 (4) | −0.24 (−0.30, −0.17) | |
| 20 min | 7.5 ± 0.02 (5) | 7.1 (1) | – | |
| 30 min | 7.5 ± 0.04 (5) | (0) | – | |
| 40 min | 7.4 ± 0.02 (5) | (0) | – | |
| 50 min | 7.4 ± 0.02 (5) | (0) | – | |
| 60 min | 7.5 ± 0.01 (5) | (0) | – | |
| pO2 | Baseline | 258.0 ± 71.3 (5) | 215.5 ± 9.2 (2) | −42.5 (−95.2, 10.2) |
| Baseline 2 | 67.6 ± 14.2 (5) | 91.3 ± 20.1 (6) | 23.7 (−14.5, 61.8) | |
| Treatment | 51.6 ± 13.7 (5) | 33.7 ± 8.6 (6) | −17.9 (−56.1, 20.3) | |
| 5 min | 53.3 ± 7.5 (4) | (0) | – | |
| 10 min | 57.0 ± 11.3 (3) | 37.9 ± 25.4 (4) | −19.2 (−67.3, 29.0) | |
| 20 min | 55.8 ± 7.7 (5) | 27.6 (1) | – | |
| 30 min | 58.8 ± 6.1 (5) | (0) | – | |
| 40 min | 64.8 ± 13.0 (5) | (0) | – | |
| 50 min | 65.4 ± 15.0 (5) | (0) | – | |
| 60 min | 70.6 ± 17.1 (5) | (0) | – | |
| pCO2 | Baseline | 33.4 ± 4.3 (5) | 32.0 ± 3.2 (2) | −1.5 (−14.8, 11.9) |
| Baseline 2 | 44.1 ± 2.5 (5) | 44.2 ± 3.1 (6) | 0.2 (−9.5, 9.8) | |
| Treatment | 29.2 (1) | 64.4 ± 7.0 (4) | – | |
| 5 min | 38.9 ± 5.2 (4) | 71.8 ± 9.3 (4) | 32.9 (21.6, 44.2) | |
| 10 min | 37.6 ± 5.7 (3) | 63.1 ± 28.4 (2) | 25.5 (10.9, 40.0) | |
| 20 min | 40.3 ± 6.0 (5) | (0) | – | |
| 30 min | 37.1 ± 4.3 (5) | (0) | – | |
| 40 min | 39.8 ± 3.6 (5) | (0) | – | |
| 50 min | 40.3 ± 4.6 (5) | (0) | – | |
| 60 min | 38.1 ± 3.2 (5) | (0) | – | |
| HCO3 | Baseline | 26.8 ± 2.9 (5) | 28.0 ± 1.5 (6) | 1.2 (−1.8, 4.1) |
| Baseline 2 | 27.5 ± 1.9 (5) | 30.1 ± 0.7 (2) | 2.6 (−1.5, 6.7) | |
| Treatment | 24.5 (1) | 26.2 ± 1.7 (4) | – | |
| 5 min | 26.7 ± 2.2 (4) | (0) | – | |
| 10 min | 26.9 ± 2.7 (3) | 22.7 ± 3.1 (4) | −4.1 (−7.9, −0.4) | |
| 20 min | 26.3 ± 1.9 (5) | 18.3 (1) | – | |
| 30 min | 27.0 ± 2.5 (5) | (0) | – | |
| 40 min | 27.2 ± 2.0 (5) | (0) | – | |
| 50 min | 27.8 ± 2.9 (5) | (0) | – | |
| 60 min | 27.2 ± 2.1 (5) | (0) | – | |
| SO2 | Baseline | 100.0 ± 0.0 (5) | 100.0 ± 0.0 (2) | 0.0 (−4.8, 4.8) |
| Baseline 2 | 92.2 ± 3.7 (5) | 93.5 ± 3.5 (2) | 1.3 (−3.5, 6.1) | |
| Treatment | 88.0 (1) | (0) | – | |
| 5 min | 87.5 ± 3.9 (4) | (0) | – | |
| 10 min | 89.7 ± 4.6 (3) | (0) | – | |
| 20 min | 89.2 ± 3.8 (5) | (0) | – | |
| 30 min | 91.2 ± 2.3 (5) | (0) | – | |
| 40 min | 91.8 ± 4.7 (5) | (0) | – | |
| 50 min | 92.0 ± 4.7 (5) | (0) | – | |
| 60 min | 93.6 ± 4.3 (5) | (0) | – |
Values given are mean ±standard deviation (number of animals with available data). ″(0)″ indicates that no data are available for that variable and time. Comparisons were made at each timepoint if more than 1 animal had data available from each group. Baseline: Measurements upon initial evaluation of animal, Baseline 2: Measurements after animals were instrumented and stabilized before intervention and toxin administration.
Discussion
In our study, intramuscular cobinamide successfully resuscitated all sulfide toxic animals, whereas all animals in the control group died. Respiratory rate returned to normal and blood pressure improved in animals treated with cobinamide. In 2017, we reported on the efficacy of intravenous cobinamide versus hydroxocobalamin or saline in severely NaHS-poisoned swine [8]. We found that all animals treated with cobinamide survived while all animals treated with hydroxocobalamin or control died. This may be secondary to cobinamide′s higher affinity for toxin. The route of administration of cobinamide in that study was intravenous. A major difference between the 2017 study and this study is the route of administration of cobinamide. In this study, we administered cobinamide intramuscularly, which has benefits in the prehospital setting, as it can be administered easily without any advanced procedures such as intravenous access. At baseline, the animals had some differences in baseline weight (Table 1), but it is unlikely these differences influenced outcomes given dosing of toxin and cobinamide were weight based.
Previously, cobinamide administered intramuscularly has been shown to be efficacious in treating cyanide toxicity in mice and rabbits [11]. This study differs in that we examined intramuscular cobinamide in a large animal model and studied intramuscular cobinamide in animals with sulfide, not cyanide toxicity. For scenarios where human studies are not feasible, such as with sulfide poisoning, the FDA can approve new therapeutics studied in well characterized animal models. The FDA Animal Rule (2015) is expected to necessitate two animal models (at least one large animal) for testing the efficacy of sulfide countermeasures. A large animal model like swine is an excellent choice to study potential treatments for human toxicity as they have similar physiology compared to humans and are also similar in size [13]. The dose for the cobinamide was derived from preliminary experiments using the lowest effective dose. We have used adolescent pigs, weighing approximately 50 kg. This approximates human size, thus the dose in this study is the same or very similar to the dose needed in humans. From an allometric perspective, the dose of drugs used for swine is similar to humans (1.1–1) and thus the effective dose in our swine will likely be the effective dose used in humans [14].
In 2018, Anantharam et al. reported preliminary data on the possible efficacy of midazolam for reducing sulfide induced toxicity [15]. The authors reported the group of mice treated with midazolam before exposure had 10% mortality versus 100% mortality in the control group. Anantharam et al. also evaluated midazolam during exposure; however, to treat the mice, investigators removed the study animals from the exposure to receive the medication, and then subsequently returned to them to sulfide exposure. Our study differs in that the animals were treated after exposure and toxicity developed, thus demonstrating rescue from acute sulfide poisoning. Additionally, no interruption of exposure to toxin took place in order to administer treatment.
Limitations
Although a large animal model like swine has similarities to humans, an animal model does not completely reproduce human toxicity. Additionally, we used anesthesia in the study, which may have effects on the toxicity of H2S. However, this was required by our IACUC to proceed with the study. Moreover, because both animal groups received anesthesia, any protective effects of anesthesia should have been seen in the control arm animals as well. In this study, H2S toxicity was induced by an intravenous infusion of NaHS solution which may have different effects than toxicity induced via an inhalational exposure, particularly in terms of pulmonary toxicity. However, NaHS salt is dissolved in vivo, produces sulfide and causes toxicity similar to the toxicity of inhaled exposures. The intravenous infusion enables tight control of exposure dose compared to inhaled exposures in spontaneously breathing animals and is safer for laboratory personnel compared to inhalational exposures [8]. The study period was short, 60 min. There are several mechanisms of toxicity of hydrogen sulfide and potential long-term sequelae associated with H2S poisoning that we did not examine given this short study period [10]. For our primary outcome, which was survival, the study time was adequate. We administered intramuscular antidote when the systolic blood pressure was <75 mmHg. It is possible that intramuscular absorption of antidote may be reduced in exposure victims if blood pressure is substantially lower than the 75 mmHg threshold used, in this study, as the trigger for antidote injection. To address this, a potential future direction of study would be to understand the effects of cobinamide administered intraosseously (IO) in a model of hydrogen sulfide toxicity. IO is a potential way to address absorption and distribution of antidote in situations where severe shock compromises pharmacokinetics. Additionally, cobinamide was administered shortly after the exposure and development of toxicity. In a real-world scenario, administration of an antidote may not be as readily available. Although lactate concentrations rose in our study, the values did not rise as significantly as reported in other studies. This may be secondary to early administration of antidote in the treatment group, and a short time to death in the control group, not allowing further accumulation of lactate. In future studies, we plan to generate data to understand what the optimal timing of cobinamide administration is and further understand the natural history of the exposure. Given that there is potential for it to be administered intramuscularly without specialized training, we envision that, if approved as an antidote for H2S, it would be readily available in situations where the risk of H2S exposure is high and available to first responders, thus minimizing time to administration. Additionally, cobinamide is still in the exploratory/ developmental phases of use. For it to be used clinically, there are several steps toward regulatory body approval and commercialization that have to take place. Furthermore, PK-PD data as well as toxicology, efficacy, GLP, and phase 1 safety data will need further characterization. Further studies are ongoing by our group and collaborators to further assess these important aspects of this potential antidote. We found cobinamide to be effective, however, in some patients chelation alone may not be enough and prehospital supportive care with vasoactive medications such as epinephrine may be contributory in improving H2S survival despite not binding sulfide directly.
Conclusion
Intramuscular cobinamide was effective in improving survival in this large swine model of severe hydrogen sulfide toxicity. Sulfide is a potentially deadly toxin. Currently, there are no established antidotes. Given the potential exposures in industry, in mass casualty settings, and by terrorists as described by national organizations like the Department of Homeland Security, an ideal antidote would be one that can be administered easily in the prehospital setting. An antidote that can be administered intramuscularly, in small volumes, is ideal. Cobinamide is a promising agent that can potentially fill this need [16] . Future studies will be directed at further understanding cobinamide in various doses and its effects on sulfide toxicity. Additionally, our group is developing an inhalational model for sulfide toxicity. We plan to study the efficacy of cobinamide in this model.
Acknowledgments
We would like to acknowledge Ms. Susan Boudreau and Ms. Maria Castaneda of the CREST Research Program in San Antonio, TX for their contributions to this research.
Funding
This work was supported by NINDS-CounterACT grant # 5 U01 NS087964-04.
Footnotes
Disclosure statement
There is no prior publication, conflict of interest, or copyright constraint. These data have not been published as a peer-reviewed article previously, and are not under consideration for publication by another journal. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the U.S. Air Force, Department of Defense, or the U.S. government.
Presented at North American Congress of Clinical Toxicology (NACCT) annual toxicology conference, Vancouver, BC; awarded the 2017 Taylor and Francis Best Research Award.
References
- [1].Department of Homeland Security NY. (U//FOUO) Hydrogen sulfide: a potential first responder hazard 2008. [cited 2018 February 09]. Available from: https://publicintelligence.net/ufouo-hydrogen-sul-fide-a-potential-first-responder-hazard/ 2018
- [2].Williams J, Australia details ′sophisticated′ plot by ISIS to take down plane. New York Times [News] 2017. [cited 2018 March 14]. Available from: https://www.nytimes.com/2017/08/04/world/aus-tralia/sydney-airport-terror-plot-isis.html. [Google Scholar]
- [3].Wald M US Labor Department′s OSHA cites Enbridge G&P in Douglasville, Texas, following worker fatality from release of hydrogen sulfide. In: Labor Udo, ed. Vol Region 6 News Release: 10-943-DAL (412). Washington, DC: Occupational Safety and Health Administration; 2010:1. [Google Scholar]
- [4].Carpenter DO. Hydraulic fracturing for natural gas: impact on health and environment. Rev Environ Health. 2016;31:47–51. [DOI] [PubMed] [Google Scholar]
- [5].Jianwen Z, Da L, Wenxing F. Analysis of chemical disasters caused by release of hydrogen sulfide-bearing natural gas. Procedia Eng. 2011;26:1878–1890. [Google Scholar]
- [6].Centers for Disease Control and Prevention: The National Institute for Occupational Safety and Health (NIOSH). Hydrogen Sulfide (H2S). 2012. [cited 2018 May 31]. Available from: https://www.cdc.gov/niosh/topics/hydrogensulfide/default.html. [Google Scholar]
- [7].Anantharam P, Whitley EM, Mahama B, et al. Cobinamide is effective for treatment of hydrogen sulfide-induced neurological sequelae in a mouse model. Ann NY Acad Sci. 2017;1408:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bebarta VS, Garrett N, Brenner M, et al. Efficacy of intravenous cobinamide versus hydroxocobalamin or saline for treatment of severe hydrogen sulfide toxicity in a swine (Sus scrofa) model. Acad Emerg Med. 2017;24:1088–1098. [DOI] [PubMed] [Google Scholar]
- [9].Doujaiji B, Al-Tawfiq JA. Hydrogen sulfide exposure in an adult male. Ann Saudi Med. 2010;30:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. [DOI] [PubMed] [Google Scholar]
- [11].Chan A, Jiang J, Fridman A, et al. Nitrocobinamide, a new cyanide antidote that can be administered by intramuscular injection. J Med Chem. 2015;58:1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Swindle MM, Makin A, Herron AJ, et al. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49:344–356. [DOI] [PubMed] [Google Scholar]
- [13].Helke KL, Swindle MM. Animal models of toxicology testing: the role of pigs. Expert Opin Drug Metab Toxicol. 2013; 9:127–129. [DOI] [PubMed] [Google Scholar]
- [14].U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Starting dose in initial clinical trials for therapeutics in adult healthy volunteers. July 2005. [cited 2018 June 21]. Available from: https://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf%23search=%27guidekines+for+industry+sfe+starting%27.
- [15].Anantharam P, Kim D, Whitley EM, et al. Midazolam efficacy agaisnt acute hydrogen sulfide-induced mortality and neurotoxicity. J Med Toxicol. 2018;14:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jiang J, Chan A, Ali S, et al. Hydrogen sulfide-mechanisms of toxicity and development of an antidote. Sci Rep. 2016;6:20831. [DOI] [PMC free article] [PubMed] [Google Scholar]