Abstract
Objective:
To advance understanding the effectiveness of evidence-based treatments for comorbid posttraumatic stress and substance use disorders (PTSD and SUD), research must provide a more nuanced picture of how substance use affects change in PTSD symptoms over the course of treatments, and whether prolonged exposure techniques can be efficacious during active substance use. A dataset that included patients with PTSD/subthreshold PTSD and SUD treated with an exposure-based intervention provided an opportunity to conduct a secondary analysis to test how patients’ substance use impacted PTSD change over treatment.
Method:
We applied growth models to week-to-week PTSD symptom and substance use changes during treatment and follow-up of a randomized controlled trial of two cognitive behavioral treatments for PTSD and SUD: Concurrent Treatment of PTSD and SUD Using Prolonged Exposure (COPE) and Relapse Prevention Therapy (RPT). Cross-lagged analyses were used to determine whether prior week substance use impacted subsequent PTSD symptom severity.
Results:
Both treatments evidenced significant reductions in PTSD symptom severity. In the context of continued substance use, results suggest that individuals still benefit from exposure-based treatment.
Conclusion:
Results provide evidence that RPT and COPE both led to significant reductions in PTSD, providing further support that exposure-based techniques tailored for SUD can be conducted without jeopardizing PTSD or SUD outcomes. Implications for clinical decision-making around treatment selection are discussed.
Keywords: RCT for posttraumatic stress disorder and substance use disorders, comorbidity, cross-lagged treatment effects, symptom changes during treatment, treatment matching
Two decades of literature clearly document the wide scope of problems associated with comorbid posttraumatic stress disorder (PTSD) in the lives of people seeking treatment for substance use disorders (SUD). These problems include poorer treatment prognosis, longer hospital stays for treatment, lower treatment compliance, higher suicide rates, and less support for achieving and maintaining sobriety than patients with SUD without PTSD (Greenfield et al., 2007; McCauley, Killeen, Gros, Brady, & Back, 2012). Despite the fact that the health care burden of such patients is high, many questions about the optimal treatment practices across the co-occurring PTSD and SUD (PTSD and SUD) population remain unanswered.
Knowledge to date on PTSD and SUD treatment is primarily based on randomized controlled trials (RCT; e.g., Back, Foa, Killeen, Mills, et al., 2014; Hien et al., 2009; Hien, Cohen, Miele, Litt, & Capstick, 2004; Mills et al., 2012), systematic reviews (e.g., Simpson, Lehavot, & Petrakis, 2017; Debora van Dam, Vedel, Ehring, & Emmelkamp, 2012), and several meta-analyses (e.g., Roberts, Roberts, Jones, & Bisson, 2015; van Dam, Vedel, Ehring, & Emmelkamp, 2012). Taken as a whole, findings suggest that trauma processing models such as prolonged exposure may be superior to coping based, present-focused approaches in reducing both PTSD and substance use symptoms (e.g., Roberts, Roberts, Jones, & Bisson, 2015). However, few studies to date have examined how trauma symptoms change over the course of treatment while participants are actively using substances and under what levels of substance use a trauma processing approach like prolonged exposure can be safely used (e.g., Hien et al., 2015; McCauley et al., 2012; Simpson et al., 2017). Researchers have argued that through examining how individuals symptoms change during psychotherapy, we will be better able to elucidate mechanisms of action and inform clinical decision-making (Kahn & Schneider, 2013; Kazdin, 2009; Laurenceau, Hayes, & Feldman, 2007).
To better explicate the relationship between PTSD symptoms and substance use during treatment, one line of research has employed cross-lagged or time-lagged models which can examine the impact of changes in one symptom domain across time and problem area. In an RCT for concurrent PTSD and SUD treatment, Hien et al. (2010) found that SUD improvement reliably followed PTSD improvement, but found no converse association between SUD change and subsequent PTSD improvement. Recently, Kaczkurkin, Asnaani, Alpert, and Foa, (2016) extended growth models to examine the lagged effects of PTSD symptoms on alcohol craving within a trial of integrated treatment combinations for PTSD and SUD (naltrexone vs. placebo, with or without prolonged exposure). When PTSD symptoms (at time t) were compared to subsequent alcohol craving (at time t + 1), improvement in PTSD symptom severity was associated with diminished alcohol craving; however, no interactions between PTSD symptoms and the four treatment combinations were observed. Although growth models have begun to provide important information on course of symptom change, it is largely unknown how active substance use during treatment impacts the delivery of an exposure-based therapy simultaneously targeting PTSD and substance dependence symptoms, such as Concurrent Treatment of PTSD and SUD using Prolonged Exposure (COPE; Back, Foa, Killeen, Teesson, et al., 2014)).
One recently completed RCT (Ruglass et al., 2017) affords the opportunity to explore these questions. Ruglass et al. (2017) compared two cognitive behavioral treatments for PTSD and SUD: COPE, an integrated, trauma- and addiction-focused treatment with modified prolonged exposure, and Relapse Prevention Therapy (RPT; Carroll, 1998), a SUD-focused treatment. The trial provided evidence of the efficacy of both treatments for reducing PTSD and SUD symptom severity relative to an active monitoring control group (AMCG). COPE and RPT significantly decreased PTSD symptoms and days of substance use relative to AMCG at the end of treatment as well as at the 3 month follow-up. Although the difference between COPE and RPT was not significant in the complete sample, the subset of participants with full (versus subthreshold) PTSD demonstrated significantly greater reduction of PTSD severity in COPE relative to RPT. When compared to COPE, RPT showed significantly more improvement in SUD outcome at end-of-treatment. At 3-month follow-up, COPE and RPT maintained their treatment gains and were not significantly different in PTSD severity or days of primary substance use. It remains unclear, however, whether concurrent substance use interacts with PTSD symptom levels to moderate treatment outcomes. In addition to its research implications for understanding the dynamics of PTSD and SUD, this is a clinically salient issue. Therapists conducting exposure therapy with substance abusing patients have voiced concern that continued substance use during PE-based interventions may worsen PTSD symptoms, interfere with the treatment process, and increase risk of relapse (van Minnen, Harned, Zoellner, & Mills, 2012; Van Minnen, Wessel, Dijkstra, & Roelofs, 2002).
Purpose of the Present Study
The present study, therefore, examined how PTSD symptom severity changed among individuals with PTSD and SUD over time (i.e., during the treatment and posttreatment phases), and how those changes were influenced by ongoing substance use during treatment. To that end, we conducted a secondary analysis of the RCT of COPE and RPT (Ruglass et al., 2017) by estimating a series of piecewise mixed-effects models. Study aims were two-fold. First, we aimed to examine (a) how PTSD symptom severity changed over the course of the active treatment phase and posttreatment phase by treatment type (ie., COPE, RPT) and (b) whether there were differences in PTSD symptom severity based on ongoing substance use during treatment. Second, by looking at the joint influences of treatment type and ongoing substance use during treatment, we sought to explore whether there was any indication that applying an exposure-based treatment would be more or less beneficial than standard CBT for SUD for addressing PTSD symptom severity for those who continue to use substances. We anticipated that higher weekly substance use would be associated with higher PTSD symptom severity in the subsequent week of treatment regardless of type of treatment.
Method
Participants
Participants were recruited through advertisements and outpatient referrals in New York City between September 2008 and January 2014 and provided written informed consent prior to baseline assessment. To be eligible participants met Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) (American Psychiatric Association (APA), 2000)) criteria for full PTSD or subthreshold PTSD (Grubaugh et al., 2005). We employed the most common definition of subthreshold PTSD which requires an individual to meet Criteria A (exposure to a traumatic stressor), B (re-experiencing symptoms), either C (symptoms of avoidance and/or numbing) or D (increased arousal symptoms), E (symptom duration of at least 1 month), and F (significant distress or impairment of functioning) (Blanchard, Hickling, Taylor, Loos, & Gerardi, 1994). Our decision to include subthreshold PTSD was informed by prior research: using the Blanchard et al. (2004) criteria, various large scale studies have shown that subthreshold PTSD is associated with levels of impairment, distress, and comorbidity comparable to full PTSD and should be considered a clinically relevant diagnostic group. In addition to full or subthreshold PTSD, participants were required to meet DSM-IV-TR criteria for either past or current alcohol or substance dependence and alcohol/substance use in the prior 3 months. Given the chronic, relapsing nature of SUDs in the context of co-occurring mental health disorders (Bradizza, Stasiewicz, & Paas, 2006; Jin, Rourke, Patterson, Taylor, & Grant, 1998), we included individuals with past dependence who were currently using substances. Exclusion criteria were: 1. Psychotic, schizoaffective or bipolar disorder; 2. Current severe depression or suicide risk; 3. Participation in PTSD-specific treatment; 4. Start or regimen change of any psychotropic medications 8 weeks before study participation; 5. Organic mental syndrome. The institutional review board of the City College of New York approved all procedures.
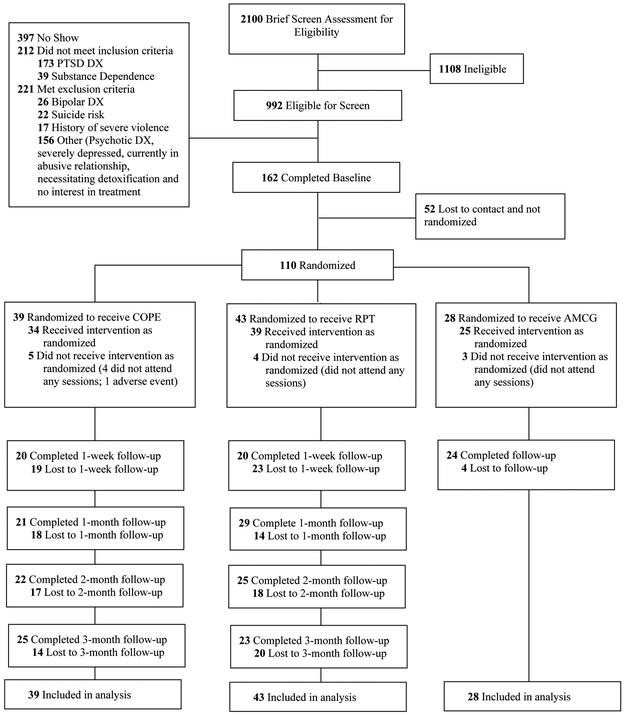
The CONSORT diagram in Figure 1 provides information on study design, participant flow and attrition and Supplementary Table 1 includes information about attendance at each session. The majority of patients received at least 5 sessions (COPE 22/39,56%; RPT 30/43, 70%), while roughly half of those in COPE (17/39, 43.6%) and RPT (22/43, 51.2%) received an adequate dose of treatment, which was defined as attending 8 or more sessions (Najavits, 2015). Moreover, the pattern of findings across treatment groups did not differ statistically by treatment attendance as referenced in the primary outcome paper (Ruglass et al., 2017; see Supplementary Table 1), nor were there differences in who received an adequate dose. All participants met criteria for either current alcohol or substance dependence except for two individuals in the RPT group who met criteria for past alcohol dependence. Further descriptions of all study procedures including randomization, the interventions and fidelity, and data collection are described in Ruglass et al. (2017).
Figure 1.
CONSORT Diagram of participant flow through the protocol. PTSD = posttraumatic stress disorder; DX = diagnosis; COPE = Concurrent Treatment of PTSD and SUD using Prolonged Exposure; RPT = Relapse Prevention Therapy; AMCG = Active Monitoring Control Group
Randomization
Randomization was stratified by sex, baseline severity of substance and alcohol dependence (high or low operationalized from median split of Addiction Severity Index Lite composite scores) and PTSD severity (high or low defined by the cutoff score of 60 on the Clinician-Administered PTSD Scale). Urn randomization procedures were employed to balance these factors across groups. An independent biostatistician conducted the randomization allocation. A research coordinator revealed group allocation to participants after they provided informed consent. All research assessors were blind to group allocation.
Interventions
Two manualized psychotherapy treatments, COPE and RPT, consisted of 12 individual weekly sessions lasting 90 minutes. COPE integrates the empirically supported models of PE for PTSD (Back, Foa, Killeen, Teesson, et al., 2014; Foa, Molnar, & Cashman, 1995; Foa, Chrestman, & Gilboa-Schechtman, 2009) and RPT for SUD (Carroll, 1996; Marlatt & Donovan, 2005). Psychoeducation about the functional relationship between PTSD and SUD is provided during the first three sessions. To address behavioral avoidance and fear associated with traumatic memories, in-vivo and imaginal exposures begin in session four and five, respectively, and continue through session eleven. Imaginal narratives are audio-recorded for daily listening between sessions. Relapse prevention strategies are reviewed during each 90-minute session. Between sessions, participants recorded progress of exposure exercises, substance use cravings, and use of coping skills.
RPT (Carroll, 1996; Marlatt & Donovan, 2005) is a cognitive-behavioral intervention for SUDs that focuses on the acquisition of coping strategies to manage situations that increase risk of substance use relapse. Coping strategies are acquired through psychoeducation, role-plays and active problem-solving exercises combined with at-home assignments all geared towards increasing participants’ self-efficacy in preventing relapse1.
Measures
PTSD.
PTSD diagnosis was assessed at baseline with the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995). PTSD symptom severity was measured at each weekly intervention visit and all pre- and post-treatment assessments, using the modified PTSD Symptom Scale Self-Report (MPSS-SR; Falsetti, Resnick, Resick, & Kilpatrick, 1993) which assessed the past 7 days of self-reported PTSD symptom severity. The MPSS-SR yielded a total score comprised of the sum of frequency and intensity ratings of each of the 17 DSM-IV-TR PTSD symptoms. Scores range from 0 to 119. A recent psychometric study of the MPSS-SR with similar comorbid PTSD and SUD treatment samples demonstrated its high concurrent validity with the CAPS, and suggest it is a reliable and valid tool for monitoring PTSD symptom severity (Lesia M. Ruglass, Papini, Trub, & Hien, 2014). In addition, Ruglass and colleagues (2014) found that the rationally-derived three-category severity classification for the CAPS correctly classified 69% of women with PTSD and SUD at posttreatment. We used this three-category classification to examine whether individuals were in one of three categories at posttreatment: asymptomatic (0 – 17points), mild/subthreshold (17.5-34.99), and threshold (35+).
Substance Use.
Primary SUD diagnosis was assessed using the Structured Clinical Interview for DSM-IV for Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 2002). The primary SUD diagnosis was determined by selecting the SUD diagnosis (if there was more than one) that had the greatest number of dependence criteria from the SCID and/or the highest level of use in the past month. Weekly Days of Substance Use (DSU) was measured using the Substance Use Inventory (SUI; Weiss, Hufford, & Najavits, 1995), a self-report measure of past 7 day substance use. Participants completed the SUI weekly during treatment and at all pre- and post-treatment assessments. Scores represent the number of days of use of the primary substance and range from 0 to 7.
DSM-IV Diagnoses.
The SCID-I was used to assess the presence of current or past anxiety, mood, or psychotic disorders. These diagnoses were assessed for the purposes of determining exclusion criteria in the study.
Demographics.
Age, sex, race/ethnicity, education, employment pattern, and income were collected during the baseline interview. See Ruglass et al. (2017) for more a more detailed description of all study measures.
Data Analysis
Piecewise two-level mixed-effect models were estimated using Stata Statistical Software: Release 14.2 (StataCorp, 2015). All randomized participants were included in analyses and thus models represent the intent-to-treat sample. To examine the first aim, a piecewise linear growth model was specified using the following equation: Yijd =( b0ia + b1ia (week – 15)ija + εija) + ( b0ib + b1ib (week – 15)ijb + εijb) (UCLA Statistical Consulting Group, n.d.) and the mixed command with the nocons option. The constant was suppressed in the model because the model includes two intercepts (i.e., two constants). In this model, Yij represents individual i’s PTSD symptom severity at time j for indicator d. The subscript d, denotes whether the value of Yij is based on one of two indicator variables, either subscript a or b. Time was represented by week 15, so that the intercept in the piecewise model (i.e., when X = 0) would represent individual i’s PTSD symptom severity at either posttreatment (subscript b, information to the right of bolded plus sign) or the time infinitely close to posttreatment, but not yet posttreatment (subscript a, information to the left of the bolded plus sign). Therefore, the intercept with a subscript can be understood as the intercept at the end of the treatment phase, while the intercept with the subscript b can be understood as the intercept at the beginning of the posttreatment phase. A significance test was computed to examine whether the value of the two intercepts was significantly different, indicating the necessity of a piecewise model. The piecewise model also yields two slopes: the slope to the left of the bolded plus sign (subscript a) is the linear rate of change for the treatment period, while the slope to the right of the bolded plus sign (subscript b) is the linear rate of change for the posttreatment period. The main effect and moderating role of treatment (i.e., COPE vs RPT) was then examined by including treatment as a predictor in the model, as well as the interaction between treatment and time during the treatment and posttreatment phases.
To examine the impact of continued substance use, we determined the weekly DSU using the frequency variable of the SUI for each individual’s primary substance. Weekly DSU was lagged by one time point and was then included in the model as a predictor, along with all two - way interactions between lagged DSU and all significant functions of time (e.g., time-treatment phase, time-posttreatment phase).
To examine the second aim, we estimated a final model that included both treatment and lagged DSU, as well as all two- and three-way interactions between treatment, lagged DSU, and significant functions of time (e.g., time-treatment phase, time-posttreatment phase). To present the most parsimonious model, final models only included interactions when p < .10 and/or interactions that were important for interpretation. Model fit was examined using Akaike’s Information criterion (AIC) and Schwarz’s Bayesian information criterion (BIC).
Testing of power in these complex mixed-effects models is exceedingly difficult, and clear conventions are lacking. The unstandardized coefficient estimates convey the difference between treatments, and thus are directly related to the power of hypothesis tests (Raudenbush & Liu, 2000). In line with this view, Dorey (2011) argued that after a study is conducted, confidence intervals are more informative than a post-hoc power analysis: "Once a study has been completed and analyzed, the confidence interval reveals how much, or little, has been learned and the power will not contribute any meaningful additional information (p.620)." Biesanz, Deeb-Sossa, Papadakis, Bollen, and Curran (2004) echo these points and note that confidence intervals can be used to see how power varies across estimates and time in mixed-effects models. As such, we include confidence intervals around all reported estimates along with effect sizes (i.e., Cohen’s (Cohen, 1988) d), where appropriate, using the equation of Feingold (equation 1; 2009), dividing the product of the unstandardized coefficient and assessment length by the pooled within-group standard deviation of PTSD. Effect size confidence intervals were derived from the growth model effect size using the equations of Feingold (2015).
Results
All demographic information for the participants randomized to COPE (n = 39) and RPT (n = 43) are presented in Table 1, along with descriptive information on the outcomes variables at baseline and post-treatment. There were no significant differences between the two groups on demographic variables, nor were there differences on PTSD symptom severity or weekly DSU at baseline, post-treatment, or three-month follow-up.
Table 1.
Demographic, baseline clinical characteristics, and baseline and posttreatment outcomes
| Characteristic | COPE n = 39 n or M (% or SD) |
RPT n = 43 n or M (% or SD) |
p-value |
|---|---|---|---|
| Demographic | |||
| Age | 43.08 (10.00) | 44.21 (9.05) | .590 |
| Female | 11 (28.2%) | 16 (37.2%) | .386 |
| Race/Ethnicity | .490 | ||
| Black/African American | 21 (53.8%) | 28 (65.1%) | |
| Hispanic/Latino | 10 (25.6%) | 9 (20.9%) | |
| White | 6 (15.4%) | 6 (14.0%) | |
| Other | 2 (5.1%) | 0 | |
| Education (years) | 13.31 (1.92) | 13.13 (2.46) | .714 |
| Full PTSD | 32 (82.1%) | 35 (81.4%) | .949 |
| Alcohol and substance use | |||
| Alcohol Dependence | 30 (76.9%) | 35 (81.4%) | .618 |
| Substance Dependence | 25 (64.1%) | 30 (69.8%) | .586 |
| Alcohol and Substance Dependence | 16 (41.0%) | 24 (55.8%) | .181 |
| Primary substance | .519 | ||
| Alcohol | 19 (48.7%) | 18 (41.9%) | |
| Cannabis | 3 (7.7%) | 4 (9.3%) | |
| Cocaine | 6 (15.4%) | 6 (14.0%) | |
| Alcohol and stimulants | 8 (20.5%) | 13 (30.2%) | |
| Other polysubstance | 3 (7.7%) | 2 (4.6%) | |
| Major Depressive Disorder | 13 (33.3%) | 16 (37.2%) | .714 |
| PTSD Symptom Severity | |||
| Baseline | 54.26 (24.60) | 57.49 (24.33) | .550 |
| Posttreatment | 36.11 (28.04) | 26.64 (24.30) | .237 |
| 3-month Follow-Up | 26.88 (23.39) | 25.95 (25.60) | .898 |
| Weekly Days of Substance Use (DSU) | |||
| Baseline DSU | 4.21 (2.67) | 4.05 (2.31) | .776 |
| Baseline Abstinence (0 DSU) | 6 (15.4%) | 2 (4.76%) | .146 |
| Posttreatment DSU | 2.11 (2.68) | 1.04 (1.71) | .114 |
| Posttreatment Abstinence (0 DSU) | 8 (44.4%) | 12 (50%) | .764 |
| 3-month Follow-Up DSU | 1.84 (2.30) | 0.90 (1.52) | .116 |
| 3-month Follow-Up Abstinence (0 DSU) | 10 (40.0%) | 13 (65.0%) | .136 |
Note. Descriptive statistics for outcome variables at posttreatment and the three-month follow-up were based on the sample of participants that attended the post-treatment and three-month follow-up assessments. At post-treatment, N = 43 (COPE = 18, RPT = 25). At the three-month follow-up, N = 46 (COPE = 25, RPT = 21).
Aim one: How did PTSD symptom severity change during the active treatment phase and posttreatment?
Parameter estimates and fit statistics for all models are presented in Table 2.
Table 2.
Regression coefficients, effect sizes and confidence intervals for piecewise models examining trajectories of PTSD symptom severity
| Linear Growth Model N = 82 |
Model with Treatment N = 82 |
Model with Lagged DSU N = 75+ |
Final Model with Treatment and Lagged DSU N = 75+ |
|||||
|---|---|---|---|---|---|---|---|---|
| B | d [95%CI] | B | d [95%CI] | B | d [95%CI] | B | d [95%CI] | |
| Initial Status: | ||||||||
| Intercept-Session 12 | 22.24*** | -- | 25.07*** | -- | 20.36*** | 22.95*** | -- | |
| Intercept-Posttreatment | 29.98*** | -- | 32.58*** | -- | 23.42*** | 25.77*** | -- | |
| Treatment | ||||||||
| RPT | -- | -- | Ref | -- | -- | Ref | ||
| COPE | -- | -- | −6.24 | −0.26[−0.67,0.16] | -- | −7.81 | 0.31[0.23,−0.13] | |
| Lagged DSU | -- | -- | -- | 1.50* | 0.84[0.12,1.56] | 1.85** | 1.04 [.30, 1.77] | |
| Rate of Change: | ||||||||
| Time-Treatment Phase | −2.28*** | −1.31[−1.49,−1.12] | −2.04*** | −1.17[−1.39,−0.95] | −2.24*** | −1.26[−1.54,−0.97] | −2.00*** | -- |
| Time-Posttreatment Phase | −0.27 | −0.03 [−0.10,0.03] | −0.23 | −0.03[−0.09,0.04] | −0.04 | 0.00[−0.09,0.08] | 0.05 | -- |
| Interactions | ||||||||
| Treatment x Time-Treatment Phase | -- | -- | −0.52* | −.30 [−.54, −.05] | -- | -- | −0.79** | −.44 [−.75, −.13] |
| Lagged DSU x Treatment | -- | -- | -- | -- | -- | -- | NS | |
| Lagged DSU x Time-Treatment Phase | -- | -- | -- | -- | 0.10 | 0.05[−0.01,0.12] | 0.14* | .08 [.01, .15] |
| Lagged DSU x Time-Treatment Phase x Treatment | -- | -- | -- | -- | -- | -- | NS | -- |
| Fit Statistics | ||||||||
| AIC BIC |
7406.76 7435.35 |
7404.85 7442.97 |
5955.68 5992.05 |
5951.80 5997.27 |
||||
Note. DSU = Days of Substance Use. The intercept represents the expected value of PTSD symptom severity when all other predictors are held constant at 0, while the slope represents the expected difference in PTSD symptom severity given a one-unit change in time (all other variables held constant). All predictors are interpreted similarly to the slope and represent the expected difference due to a one-unit change in the predictor (all other predictors held constant). Treatment was coded dichotomously with RPT equal to 0. Lagged days of use ranged from 0 to 7 and represented the reported days of use for the previous assessment (e.g., at session 1, lagged days of use = days of use at the MI session).
p < .05.
p < .01.
p < .001.
Seven participants were excluded from this model because they had insufficient data on the DSU variable.
The shape of change: Piecewise linear growth model.
A piecewise linear growth model was fit to the data with a breakpoint set at posttreatment and a random intercept and results indicated that a piecewise model was a good fit to the data. Specifically, results indicated that the intercept at the end of the treatment phase was (B = 22.24; 95% CI [16.51, 27.97], p < .001), while the intercept at the beginning of the posttreatment phase was (B = 29.98; 95% CI [24.10, 27.97], p < .001). The 7.74 point difference between these two intercepts was found to be significant (p = .002) indicating a discontinuous change between the treatment and posttreatment phase. Results also continued a significant difference in the rate of change between the two phases (p < .001). During the treatment phase, there was a significant linear decline in PTSD symptom severity (B = −2.28; 95% CI [−2.61,−1.95], p < .001, d = −1.31), but there was no significant change during the posttreatment phase (B = −0.27; 95% CI [−0.81,0.26], p = .321, d = −0.03).
Influence of treatment type: Model including treatment type and its interaction with time during the treatment phase.
Treatment type was added to the model as a predictor, as well the interaction between treatment and time during the treatment phase. Given that there was no growth to model during posttreatment phase, we did not include interactions between treatment and time during the posttreatment phase. Results indicated that there was a significant interaction between treatment type and the linear function of time (B = −0.52; 95% CI [−0.95,−0.10], p = .016, d = .−0.30). Inspection of the simple slopes indicated that COPE was associated with a more rapid decline in PTSD symptom severity (B = −2.57; 95% CI [−2.96,−2.17], p < .001, d = −1.47), compared to RPT (B = −2.04; 95% CI [−2.42 ,−1.66], p < .001, d =−1.17). Despite COPE being associated with more rapid decline of PTSD symptom severity, the clinical meaningfulness of this difference is small as it represents less than a point difference of change during each week of treatment. Further, there was no evidence of a main effect of treatment type on PTSD symptom severity at posttreatment (B = −6.24; 95% CI [−16.36, 3.88], p = .227, d = −0.26), suggesting that any difference in the rate of change was not associated with a significant difference in PTSD symptom severity by the end of treatment.
Influence of continued substance use: Model including lagged DSU and its interaction with time during the treatment phase.
Lagged DSU was added to the model as a predictor, as well as the interaction between lagged DSU and time during the treatment phase. Results indicated that lagged DSU did not interact with time during the treatment phase in the prediction of PTSD symptom severity scores (B = 0.10; 95% CI [−0.02,0.22], p = .112, d = .05).There was a main effect of lagged DSU at posttreatment (B = 1.50; 95% CI [0.21,2.80], p = .023, d = .84), indicating that a one-unit change in lagged DSU (e.g., going from 0 to 1 days of use) was associated with 1.50 points higher on PTSD symptom severity.
Aim two: Was one treatment more beneficial for those who continue to use substances?
Joint influences of treatment and continued substance use: Model including lagged DSU, treatment, and all two- and three-way interactions.
The final model was estimated with the inclusion of both treatment type and lagged DSU, as well as all two- and three-way interactions between lagged DSU, treatment type, and time during the treatment phase. To present the most parsimonious model, only interaction terms wherep <.10 were included. The final model indicated that there were no significant three-way interactions between treatment type, lagged DSU, and time during the treatment phase. In addition, the two-way interaction between treatment type and lagged DSU was non-significant. However, there were two significant two-way interactions. Specifically, there was a significant interaction between treatment type and time during the treatment phase (B = −0.80; 95% CI [−1.35,−0.24], p = .005, d = −0.44) and a significant interaction with lagged DSU and time during the treatment phase (B = 0.14; 95% CI [0.02,0.26], p = .026, d = .08).
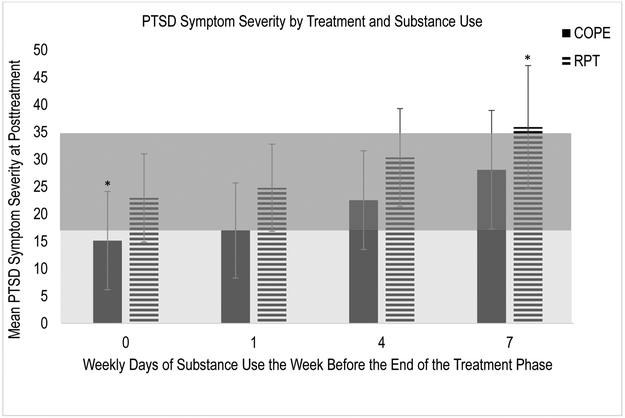
In order to examine the joint impact of the significant two-way interactions between treatment type and time and lagged DSU and time, we examined the simple slopes to determine the mean PTSD symptom severity at posttreatment for those in RPT and COPE at zero, one, four, and seven days of use per week of the primary substance (see Figure 2). Given that this was a piecewise model, we had two possible intercepts to plot. We chose to plot the intercept that represented that time infinitely close to posttreatment, but not posttreatment because (1) the standard errors were smaller at this point allowing for more power to detect an effect and (2) results indicated that there was not significant change in PTSD symptom severity during the posttreatment phase. The bottom shaded region suggests asymptomatic levels of PTSD symptom severity (about 17 points and lower), while the shaded region above it suggests mild/subthreshold PTSD symptom severity on the MPSS-SR (about 17.5-34.99 points) (Ruglass et al., 2014). Examination of the figure suggests that at the end of the treatment phase, the only group that had mean levels of PTSD symptom severity in the asymptomatic range, were those in COPE that were abstinent from their primary substance in the week prior to the end of the treatment phase. The only group that had mean levels of PTSD symptom severity in the threshold range, were individuals in RPT who used their primary substance of abuse daily in the week prior to the end of treatment. For individuals in all other groups, mean levels of PTSD symptom severity were in the mild/subthreshold range. However, error bars are overlapping in each of these groups, except for those in COPE with no days of use and those in RPT with daily use in the week prior to the end of the treatment phase. Therefore, results suggest that those in RPT with daily use fared significantly worse than those in COPE who were abstinent, but all other groups achieved similar levels of PTSD symptom severity by the end of the treatment phase, regardless of treatment or levels of substance use.
Figure 2.
Estimated mean levels of PTSD symptom severity for COPE and RPT at the point that is infinitely close to posttreatment, at different levels of lagged DSU (i.e., DSU during the last week of treatment). The bottom shaded region suggests asymptomatic levels of PTSD symptom severity (about 17 points and lower) on the MPSS-SR, while the top shaded region suggests mild/subthreshold levels of PTSD symptom severity (about 17.5 to 34.99 points) (Ruglass et al., 2014). The asterisks indicate that those estimates are significantly different from one another.
Discussion
The present study examined pathways of change in PTSD symptom severity as well as the impact of ongoing substance use levels on PTSD severity by comparing an exposure-based trauma-processing, integrated PTSD and SUD treatment approach (COPE) to a cognitive behavioral intervention for SUD alone (RPT). Given the need for a nuanced analysis of psychotherapy process in this relatively understudied clinical population, we investigated the following: 1) how patients’ PTSD symptom severity changed over time and whether there were differences based on (a) treatment type or (b) ongoing use of the primary substance of abuse during treatment; and 2) whether there was any evidence that either COPE or RPT were more favorable for PTSD outcomes for individuals who continue to use substances.
Impact of Treatment on PTSD Symptom Severity
Over the course of both treatments, PTSD symptoms significantly decreased throughout the trial with each treatment showing a large effect of time. In contrast, the present study took into consideration in-treatment PTSD changes, as well as the rate of such change, and found that patients who received COPE had a significantly faster rate of PTSD change over treatment than did those who received RPT; however, this difference was not judged to be clinically meaningful (i.e., only about a 2 point difference each week, interaction between treatment and time was a small effect) and was not associated with a main effect of treatment type at posttreatment. The findings do provide additional support for the use of exposure-based approaches with individuals with PTSD who also have SUD. Our results demonstrate that in contrast to commonly held beliefs that addressing trauma directly using prolonged exposure techniques might cause a patient to relapse or become more symptomatic (e.g., van Minnen, Harned, Zoellner & Mills, 2012), patients can tolerate and quickly benefit from these skills and techniques. Patients receiving RPT, with its unitary focus on building refusal skills and self-efficacy, also derived benefits in their PTSD symptoms, albeit at a slightly slower pace. By posttreatment, patients in both groups had significantly reduced PTSD severity, regardless of the differences in rate of change observed during treatment.
Impact of Continued Substance Use on PTSD Symptom Severity
Previous studies with PTSD and SUD samples (e.g., Hien et al., 2010; Kaczkurkin et al., 2016; Ouimette, Read, Wade, & Tirone, 2010) have provided support for within treatment changes in PTSD symptoms influencing substance use in the proximal session. Similarly, changes in PTSD during treatment overall have been shown to impact longer-term substance use outcomes over time (Hien et al., 2010). In contrast, the present study addressed a gap in the existing research on the topic of symptom domain influences through testing the impact of previous week’s substance use upon the current week’s PTSD symptoms (e.g., association between substance use in session 1 on PTSD symptom severity on session 2, etc.). Our analysis focused on answering an important clinical question for therapists considering the delivery of prolonged exposure-based interventions to active substance users. How does the level of substance use during treatment impact PTSD reductions? Coffey, Schumacher, Brady, and Cotton (2007) identified that those who were able to be abstinent at baseline demonstrated significant reductions in PTSD during the first two weeks of treatment. Evidence from the present study revealed that levels of substance use at the end of treatment (i.e., session 12) were associated with weekly PTSD symptom severity at posttreatment. These results suggest that patients who can reduce their use, or initiate and maintain abstinence during treatment, may experience lower levels of PTSD symptom severity at the end of the treatment phase and into the posttreatment phase. However, it is worth noting that this difference suggested that each additional day of use of the primary substance of abuse was only associated with 1.50 higher points on the MPSS-SR (without taking treatment type into account), a 1.3% difference on the scale of PTSD symptom severity.
Did one treatment provide greater benefit for PTSD symptom severity when individuals continued to use substances?
Our final model attempted to examine whether either COPE or RPT would be more beneficial for patients with PTSD and SUD who continue to use their primary substance of abuse during treatment. We did not find evidence of a three-way interaction between treatment, continued use of substances, and the rate of change, suggesting that there were no differences in how levels of continued substance use influenced the rate of change between the two treatments. In addition, there was not a two-way interaction between treatment and continued use of substances, indicating that the way continued substance use impacts treatment outcomes, is not different between the two treatments. However, there were two significant two-way interactions that indicated the following: (1) individuals in COPE experienced a more rapid change in PTSD symptom severity, albeit a small effect; and (2) those with greater continued substance use had a slower change in their PTSD symptom severity, although it should be noted that the effect size was negligible therefore limiting generalizability to clinical populations. For these exploratory findings, we examined mean levels of PTSD symptom severity for those in COPE and RPT at various levels of substance use to examine whether these two two-way interactions could approximate clinically-meaningful differences in endpoints using the simple slopes for and examined significance using the confidence intervals around each estimate. Overall, results highlight that under certain conditions, treatment in general can reduce PTSD symptom severity to subthreshold levels even with continued substance use. Importantly, there were two exceptions to this: (1) individuals in COPE who were abstinent from their primary substance of abuse or used once in the week prior to the end of the treatment phase achieved levels in the asymptomatic range; and (2) individuals in RPT who used daily in the week prior to the end of treatment remained in the threshold range. Only the estimates for individuals in COPE who were abstinent and those in RPT who used daily were found to be significantly different from one another. Overall, our findings provide, initial, key support for three main points: 1) both RPT and COPE were associated with significant reductions in PTSD symptom severity for those with PTSD and SUD; 2) exposure-based processing can be accomplished in patients with SUD regardless of frequency of use during treatment, and 3) for those who use daily during treatment, substance-use focused only treatment may not provide enough support to reduce PTSD symptom severity. However, we emphasize that neither inferences nor conclusions should be made based on these two small effects, particularly given the sample size of the study. Instead, these should be taken as exploratory analyses that may guide hypotheses in future studies with larger samples.
Limitations
Several limitations should be noted. Like all controlled trials with PTSD and SUD (as noted most recently by Roberts, Roberts, Jones, and Bisson, [2015]), attrition over the entire study period may have reduced power. However, data analytic techniques (such as the ability to include all 82 cases in the PTSD analysis and lose only 7 in the DSU analysis) may outweigh this relative limitation. In addition, the degree of missingness at each week may impact the power to detect the influence of either predictor at the intercept (which is dependent on how time is centered in the model), depending on how much missingness occurred at each week. For example, there was more power to detect the impact of treatment assignment at session 1, than at the end of the treatment phase, where fewer participants attended. This difference in power is accounted for in the width of the confidence interval around the estimates. Nonetheless, due to power considerations, we underscore that the findings we report should be considered preliminary and in need of replication. We utilized retrospective self-report of PTSD and SUD symptoms, which are subject to biases in recall and social desirability. We employed only DSU for the primary substance of abuse, which may have limited our sensitivity in capturing relevant associations of other substances of abuse with PTSD symptoms: for example, the potential for an increase in cannabis use to manage sleep or anxiety with a decrease in observed primary substance of abuse (alcohol or cocaine). These changes in other drug use are potentially important to know in relation to treatment type. Analysis of the quantity or severity of substance use may shed further light on the relationship between PTSD symptoms and substance use. Because of our exclusion criteria, findings may not be generalizable to individuals who are within 8 weeks of starting psychotropic or substance abuse medication treatments. Finally, the lack of follow-up assessments beyond three months limited our ability to examine the durability of changes observed.
Conclusion and Future Directions
Taken as a whole, our growth curve analyses revealed important details bearing on the process of psychotherapy change in two potent treatments for PTSD and SUD. The findings provide more support for the use of RPT as a beneficial treatment for this comorbid group (i.e., Hien, Cohen, Miele & Capstick, 2004; Ruglass et al., 2017). Analyses also provide solid evidence that trauma processing exposure-based techniques can lead to significant reductions in PTSD symptoms, even when individuals continue to use substances. Moreover, our findings suggest that when individuals continue to use their primary substance of abuse daily, a substance-use focused only treatment may not provide enough support to reduce PTSD symptom severity. Future replication studies must examine these differences with prospective tracking over longer periods of time, as well as examining cross-lagged impact of PTSD changes on substance use outcomes over time. Contrary to concerns which have traditionally led those conducting controlled trials of PE for PTSD to exclude active substance users from their research, these findings provide further support that exposure-based techniques tailored for SUD can be conducted without jeopardizing PTSD or SUD recovery.
Supplementary Material
Public Health Significance Statement: Study findings provide important support for three main points: 1) both RPT and COPE were associated with significant reductions in PTSD symptom severity for those with PTSD and SUD; 2) exposure-based techniques can be accomplished in patients with SUD regardless of frequency of use during treatment, and 3) for those who use daily during treatment, substance-use focused only treatment may not provide enough support to reduce PTSD symptom severity.
Footnotes
Participants randomized to AMCG received weekly assessments during the in-treatment period but not followed during the post-treatment period. They were not included in the present study as the aims were to compare the two cognitive behavioral therapies over the in-treatment and post-treatment phases.
References
- American Psychiatric Association (APA). (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Association; 10.1176/appi.books.9780890423349 [DOI] [Google Scholar]
- Back SE, Foa EB, Killeen TK, Mills KL, Teesson M, Cotton BD, … Brady KT (2014). Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE): Therapist Guide. New York: Oxford University Press. [Google Scholar]
- Back SE, Foa EB, Killeen TK, Teesson M, Mills KL, Cotton BD, & Carroll KM (2014). Concurrent treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE): Therapist Guide. New York, NY: Oxford University Press. [Google Scholar]
- Biesanz JC, Deeb-Sossa N, Papadakis AA, Bollen KA, & Curran PJ (2004). The Role of Coding Time in Estimating and Interpreting Growth Curve Models. Psychological Methods, 9(1), 30–52. 10.1037/1082-989X.9.1.30 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, & Gerardi RJ (1994). Psychological morbidity associated with motor vehicle accidents. Behav Res Ther, 32(3), 283–290. https://doi.org/0005-7967(94)90123-6 [pii] [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, & Paas ND (2006). Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: A review. Clinical Psychology Review. 10.1016/j.cpr.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Carroll KM (1996). Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology, 4(1), 46–54. 10.1037/1064-1297.4.1.46 [DOI] [Google Scholar]
- Carroll KM (1998). A Cognitive Behavioral Approach: Treating Cocaine Addiction (Vol. 1). Rockville, MD: National Institute on Drug Abuse. [Google Scholar]
- Coffey SF, Schumacher JA, Brady KT, & Cotton BD (2007). Changes in PTSD symptomatology during acute and protracted alcohol and cocaine abstinence. Drug and Alcohol Dependence, 87(2–3), 241–248. 10.1016/j.drugalcdep.2006.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Dorey F (2011). Statistics in brief: Interpretation and use of p values: All p values are not equal. Clinical Orthopaedics and Related Research. 10.1007/s11999-011-2053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, & Kilpatrick DG (1993). The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behaviour Therapist, 16, 161–162. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen. New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Foa EB, Chrestman KR, & Gilboa-Schechtman E (2009). Prolonged exposure therapy for adolescents with PTSD: Emotional processing of traumatic experiences: Therapist guide. New York, NY: Oxford University Press; 10.1093/med:psych/9780195308501.001.0001 [DOI] [Google Scholar]
- Foa EB, Molnar C, & Cashman L (1995). Change in rape narratives during exposure therapy for posttraumatic stress disorder. Journal of Traumatic Stress, 8(4), 675–690. 10.1002/jts.2490080409 [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, … Miele GM (2007). Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug and Alcohol Dependence. 10.1016/j.drugalcdep.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, & Frueh BC (2005). Subthreshold PTSD in primary care: Prevalence, psychiatric disorders, healthcare use, and functional status. The Journal of Nervous and Mental Disease, 193(10), 658–664. [DOI] [PubMed] [Google Scholar]
- Hayes AM, Feldman G, Laurenceau J-P, Feldman G, Strauss JL, & Cardaciotto L (2007). Discontinuous Patterns of Change in Psychotherapy. Clin Psychol Rev., 27(6), 715–723. 10.1016/j.cpr.2007.01.008.Change [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Campbell ANC, Ruglass LM, Saavedra L, Mathews AG, Kiriakos G, & Morgan-Lopez A (2015). Maximizing Effectiveness Trials in PTSD and SUD Through Secondary Analysis: Benefits and Limitations Using the National Institute on Drug Abuse Clinical Trials Network “Women and Trauma” Study as a Case Example. Journal of Substance Abuse Treatment, 56, 23–33. 10.1016/j.jsat.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Cohen LR, Miele GM, Litt LC, & Capstick C (2004). Promising treatments for women with comorbid PTSD and substance use disorders. American Journal of Psychiatry, 161(8), 1426–1432. 10.1176/appi.ajp.161.8.1426 [DOI] [PubMed] [Google Scholar]
- Hien DA, Wells EA, Jiang H, Suarez-Morales L, Campbell ANC, Cohen LR, … Nunes EV (2009). Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. Journal of Consulting and Clinical Psychology, 77(4), 607–619. 10.1037/a0016227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien D. a, Jiang H, Campbell ANC, Hu M, Miele GM, Cohen LR, … Ed M (2010). Do Treatment Improvements in PTSD Severity Affect Randomized Clinical Trial in NIDA’s Clinical Trials Network. Am J Psychiatry, 167(1), 95–101. 10.1176/appi.ajp.2009.09091261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawickreme N, Cahill SP, Riggs DS, Rauch SAM, Resick PA, Rothbaum BO, & Foa EB (2014). Primum non nocere (first do no harm): Symptom worsening and improvement in female assault victims after prolonged exposure for ptsd. Depression and Anxiety, 31(5), 412–419. 10.1002/da.22225 [DOI] [PubMed] [Google Scholar]
- Jin H, Rourke SB, Patterson TL, Taylor MJ, & Grant I (1998). Predictors of relapse in long-term abstinent alcoholics. Journal of Studies on Alcohol, 59(6), 640–6. 10.15288/jsa.1998.59.640 [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Asnaani A, Alpert E, & Foa EB (2016). The impact of treatment condition and the lagged effects of PTSD symptom severity and alcohol use on changes in alcohol craving. Behaviour Research and Therapy, 79, 7–14. 10.1016/j.brat.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JH, & Schneider WJ (2013). It’s the Destination and It’s the Journey: Using Multilevel Modeling to Assess Patterns of Change in Psychotherapy. Journal of Clinical Psychology, 69(6), 543–570. 10.1002/jclp.21964 [DOI] [PubMed] [Google Scholar]
- Kazdin AE (2009). Understanding how and why psychotherapy leads to change. Psychotherapy Research : Journal of the Society for Psychotherapy Research, 19(4–5), 418–28. 10.1080/10503300802448899 [DOI] [PubMed] [Google Scholar]
- Laurenceau J-P, Hayes AM, & Feldman GC (2007). Some methodological and statistical issues in the study of change processes in psychotherapy. Clinical Psychology Review, 27(6), 682–95. 10.1016/j.cpr.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, & Donovan DM (Eds.). (2005). Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors (Second). New York: The Guilford Press. [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, & Back SE (2012). Posttraumatic Stress Disorder and Co-Occurring Substance Use Disorders: Advances in Assessment and Treatment. Clinical Psychology : A Publication of the Division of Clinical Psychology of the American Psychological Association, 19(3). 10.1111/cpsp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Brady KT, Baker AL, Hopwood S, Clin M, Sannibale C, … Hons B (2012). Integrated Exposure-Based Therapy for Co-occurring Posttraumatic Stress Disorder. JAMA, 308(7), 690–699. [DOI] [PubMed] [Google Scholar]
- Najavits LM (2015). The problem of dropout from “gold standard” PTSD therapies. F1000prime Reports, 7, 43 10.12703/P7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette P, Read JP, Wade M, & Tirone V (2010). Modeling associations between posttraumatic stress symptoms and substance use. Addictive Behaviors, 35(1), 64–67. 10.1016/j.addbeh.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, & Liu X (2000). Statistical power and optimal design for multisite randomized trials. Psychological Methods, 5(2), 199–213. 10.1037/1082-989X.5.2.199 [DOI] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, & Bisson JI (2015). Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: A systematic review and meta-analysis. Clinical Psychology Review, 38, 25–38. 10.1016/j.cpr.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Ruglass LM, Lopez-Castro T, Papini S, Killeen TK, Back SE, & Hien DA (2017). Concurrent treatment with prolonged exposure for co-occurring full or subthreshold posttraumatic stress disorder and substance use disorders: A randomized clinical trial. Psychotherapy and Psychosomatics, 86, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruglass LM, Papini S, Trub L, & Hien DA (2014). Psychometric Properties of the Modified Posttraumatic Stress Disorder Symptom Scale among Women with Posttraumatic Stress Disorder and Substance Use Disorders Receiving Outpatient Group Treatments. Journal of Traumatic Stress Disorders and Treatment, 4(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Lehavot K, & Petrakis IL (2017). No Wrong Door: Findings from a Critical Review of Behavioral Randomized Clinical Trials for Individuals with co-occurring Alcohol/Drug Problems and PTSD. Alcoholism: Clinical and Experimental Research. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2015). Stata Statistical Software: Release 14. 2015. 10.2307/2234838 [DOI]
- UCLA Statistical Consulting Group. (n.d.). How Can I Run a Piecewise Regression in Stata. Retrieved April 9, 2018, from https://stats.idre.ucla.edu/stata/faq/how-can-i-run-a-piecewise-regression-in-stata/
- van Dam D, Vedel E, Ehring T, & Emmelkamp PMG (2012). Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: A systematic review. Clinical Psychology Review, 32(3), 202–214. 10.1016/j.cpr.2012.01.004 [DOI] [PubMed] [Google Scholar]
- van Dam D, Vedel E, Ehring T, & Emmelkamp PMG (2012). Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: A systematic review. Clinical Psychology Review, 32, 202–214. 10.1016/j.cpr.2012.01.004 [DOI] [PubMed] [Google Scholar]
- van Minnen A, Harned MS, Zoellner L, & Mills K (2012). Examining potential contraindications for prolonged exposure therapy for PTSD. European Journal of Psychotraumatology, 3(1), 18805 10.3402/ejpt.v3i0.18805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Minnen A, Wessel I, Dijkstra T, & Roelofs K (2002). Changes in PTSD patients’ narratives during prolonged exposure therapy: A replication and extension. Journal of Traumatic Stress, 15(3), 255–258. 10.1023/A:1015263513654 [DOI] [PubMed] [Google Scholar]
- Weiss RD, Hufford C, & Najavits LM (1995). Weekly Substance Use Inventory (Unpublishe). Boston, MA: Harvard Medical School. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.