Abstract
Introduction:
Clinical studies of patients treated with somatostatin-receptor (sstr)-targeted [DOTA0-Tyr3]-octreotide (DOTATOC) labeled with 177Lu and 90Y have shown overall response rates in the range of 9–33%. This study evaluates the potential for combination therapy with gemcitabine in an effort to improve clinical outcomes.
Methods:
Human pancreatic adenocarcinoma Capan-2, rat pancreatic cancer AR42J and human small cell lung cancer NCI-H69 cells were each treated with 1 μg/ml gemcitabine for 4 days followed by replacement of the medium alone for four additional days. Cell cycle and direct receptor-uptake studies were performed with 177Lu-DOTATOC after the total 8-day treatment as described. Cell viability and apoptosis experiments were performed to study the effects of gemcitabine pretreatment and 177Lu-DOTATOC radionuclide therapy. Parallel control studies were performed with receptor-non-targeted 177Lu-DOTA and DOTATOC.
Results:
Cells treated with gemcitabine for 4 days showed a down-regulation of sstr expression as determined by 177Lu-DOTATOC uptake. However, after 4 days of additional growth in absence of gemcitabine, the uptake of 177Lu-DOTATOC was 1.5–3 times greater than that of the untreated control cells. In gemcitabine-pretreated Capan-2 cells, 84% of the cell population was in the G2M phase of the cell cycle. Due to sstr up-regulation and cell cycle modulations, synergistic effects of gemcitabine pretreatment were observed in cell viability and apoptosis assays.177Lu-DOTATOC resulted in two to three times greater apoptosis in gemcitabine-pretreated Capan-2 cells compared to the untreated cells.
Conclusion:
Gemcitabine pretreatment up-regulates sstr expression and acts as a radiosensitizer through cell cycle modulation. The rational combination of gemcitabine and sstr-targeted radiopharmaceuticals represents a promising chemoradiation therapeutic tool with great potential to improve clinical outcomes and, thus, merits further study.
Keywords: Somatostatin receptors, Combination therapy, Gemcitabine, Radionuclide therapy, DOTATOC
1. Introduction
Radiolabeled somatostatin analogues such as 90Y-[DOTA0-Tyr3]-octreotide (DOTATOC) have been used clinically for more than 15 years for the treatment of neuroendocrine tumors. Studies indicate ≥50% tumor regression in 9–33% of patients; in contrast, 177Lu-DOTATATE treatment resulted in tumor regression of ≥50% in 28% of patients and tumor regression of 25–50% in 19% of patients, stable disease in 35% of patients and progressive disease in 18% of patients [1–4]. When renal protective agents such as D-lysine were administrated to the patients, nephrotoxic effects of radiolabeled DOTATOC treatments were decreased. However, the incomplete response in many patients as well as the likelihood of relapse following initial regression suggests that improved therapeutic regimes are required.
The challenges that beta-emitting radionuclide DOTA-TOC therapy faces are renal toxicity in the absence of a renal protective agent and partial or limited response especially in radioresistant tumors.
Somatostatin-targeted alpha-emitting radionuclide therapies have been evaluated in order to overcome the challenges posed by somatostatin-targeted beta-emitting radionuclide therapy. Such studies have demonstrated the advantages of alpha-particle peptide therapy [5–7]. At the same absorbed dose, alpha-emitting 213Bi-DOTATOC was 3.4 times more effective than beta-emitting 177Lu-DOTATOC therapy in human pancreatic adenocarcinoma cells and demonstrated a relative biological effectiveness of 3.4 when compared with external source gamma therapy using 137Cs in combination with DOTATOC [5]. In an animal model, 213Bi-DOTATOC demonstrated dose-dependent reduction of tumor growth with minimal toxicity demonstrating safety and efficacy in vivo [7]. However, alpha-particle therapy poses its own set of problems with respect to commercial availability of the radionuclide, the lack of long-term clinical toxicity data and potential regulatory barriers. In a previous report, gemcitabine pretreatment resulted in overexpression of the somatostatin receptor (sstr) and suggested that it could be used in combination therapy with sstr-targeted radionuclide therapy [8]. Nucleoside analogues, such as gemcitabine, are potent inhibitors of DNA synthesis and, as such, also inhibit various processes involved in the repair of genomic damages induced by radiation [9–12]. Gemcitabine is a pyrimidine analogue of deoxycytidine and has been used clinically as a chemotherapeutic agent both as a radiosensitizer in chemoradiation combination therapy and as first-line drug therapy in patients with pancreatic cancer [13,14].
Concomitant chemoradiotherapy is widely used in the treatment of various cancers. Numerous addictive and synergistic interactions between ionizing radiation and cytotoxic agents are known, which may modify the dose–response relationships in vitro and have demonstrated synergistic effects in clinical studies. The main interactions are spatial cooperation involving localized radiation therapy and systemic chemotherapy, independent cell killing, cell synchronization, inhibition of radiation damage repair, reoxygenation, reduction of the hypoxic fraction of the tumor and increased apoptosis. The combination of chemotherapeutic agent and radiation leads to increases in the number of unrepaired double-strand breaks [9]. Through these mechanisms and DNA damage by both the chemotherapeutic and radiation therapeutic modalities, therapeutic efficiency is enhanced. The main outcomes of chemoradiotherapeutic combination range from inhibition or antagonism, subadditive, additive to synergistic effects [15–17]. The most important outcome for clinical practice is synergistic effects, when the cytotoxic effect of the combination is greater than the sum of the effects of radiotherapy alone and that of chemotherapy alone [9].
Therefore, in an effort to improve clinical outcomes, we sought to investigate the in vitro effects of combination therapy using gemcitabine and 177Lu-DOTATOC on cell viability, apoptosis, cell cycle and receptor expression.
2. Materials and methods
2.1. Cell culture
Human pancreatic adenocarcinoma Capan-2, rat pancreatic cancer AR42J and human small cell lung cancer NCI-H69 cells were cultured in McCoy’s 5a medium, F-12 medium and RPMI-1640, respectively, and supplemented with 1.5 mM L-glutamine, 10% (v/v) fetal bovine serum, 10% of 100 U/ml penicillin and 100 μg/ml streptomycin. All the cell lines, culture media and supplements were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were grown at 37°C, in a humidified atmosphere of 5% CO2 and 95% air.
2.2. Radionuclide and preparation of radiolabeled peptide
177Lu (half-life, 6.64 days; Emax, 0.5 MeV) was obtained from Perkin Elmer (Waltham, MA, USA) in the form of 177Lu-chloride in 0.05 N optima grade HCl with the reported specific activity of 185 GBq/mg at the time of expiration. 177Lu-DOTATOC and 177Lu-DOTA were prepared as previously described [5].
2.3. Gemcitabine treatments
Gemcitabine was obtained as Gemzar (Eli Lilly, Indianapolis, IN, USA).
2.3.1. Cohort 1
Depending upon the cell type, a total of 2–4 million cells were plated overnight in T-75 flasks. On the day of the experiment, cells were washed with phosphate-buffered saline (PBS) and the tissue culture medium was replaced. Gemcitabine was added to the cells at a concentration of 1 μg/ml, and the cells were incubated for 4 days. The cells were washed twice with PBS, removed from the flasks by trypsinization, resuspended as a single-cell suspension and counted for further experimentation.
2.3.2. Cohort 2
Cells in Cohort 2 were treated in the same way as Cohort 1, except that after the 4-day treatment, cells in Cohort 2 were washed twice with PBS and medium (without gemcitabine) was added.
Since one of the objectives of our study was to determine if the increase in binding sites enhances combination therapy with radiolabeled somatostatin analogues, the incubation times and doses of gemcitabine were based on a previously published study [8].
2.4. Radioligand receptor-uptake studies
To evaluate ligand uptake to the sstr expressed on the cells from Cohorts 1 and 2 and untreated control cells, we performed direct uptake experiments with 177Lu-DOTATOC as previously described [5]. Briefly, 100,000 cells/well were transferred to 6-well plates overnight. The cells were washed with 2 ml PBS and incubated in 1 ml HEPES buffer with 6 nM of radiotracer (177Lu-DOTATOC) for 60 min at 37°C. Cells were incubated with excess of unlabeled peptide to determine nonspecific and specific uptake. Cellular uptake was stopped by removing medium from the cells, followed by washing twice with 2 ml PBS. The radioactivity associated with the final pellet was counted using a Wallace Wizard 1480 automatic gamma counter.
2.5. Cell cycle studies
Cells from Cohort 2 and control cells were resuspended with 5 ml PBS, centrifuged, resuspended as single-cell suspension in 0.5 ml PBS, fixed in tubes containing 4.5 ml cold 70% ethanol and stored at −20°C. On the day of cell cycle experiments, the cells were centrifuged and washed once with 5 ml PBS, resuspended in 1 ml propidium iodide solution containing Triton X-100 and RNase and incubated at 37°C for 20 min. Cell cycle was analyzed using a FACSCalibur flow cytometer (10,000 events/sample) and Cell Quest Pro software (BD Biosciences, California, USA).
2.6. Cell viability studies
Cells from Cohort 2 and control cells were incubated with 37 kBq of 177Lu-DOTATOC, 177Lu-DOTA and equimolar DOTATOC. After appropriate incubation times, cell viability was evaluated using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI), a homogeneous method for determining the number of viable cells in culture based on ATP quantification, which reflects the presence of metabolically active cells. For the experiment, 5000 cells (100 μl) were cultured in opaque-walled 96-well plates. After 24 and 48 h of incubation, the plate was equilibrated at room temperature for approximately 30 min. CellTiter-Glo reagent equal to the volume of the cell culture medium was added, and the contents of the plate were mixed on a shaker for 2 min to induce cell lysis. The plate was then incubated for 10 min at room temperature to stabilize the luminescence signal. The luminescence was recorded using Tecan GENios Magellan plate reader (Durham, NC, USA).
2.7. Apoptosis studies
Cells from Cohort 2 and control cells were incubated with 37 kBq of 177Lu-DOTATOC, 177Lu-DOTA and equimolar DOTATOC, and after appropriate incubation times, apoptosis was measured using the Cell Death Detection ELISAPLUS 10X (Roche Applied Sciences, Indianapolis, IN, USA) as previously described [13].
2.8. Statistical analysis
Experiments were performed in triplicates. All numerical data were expressed as the mean of the values±the standard error of mean (S.E.M.). GraphPad Prism version 4 (San Diego, CA, USA) was used for statistical analysis, and a P value <.05 was considered statistically significant.
3. Results
3.1. Preparation of radiolabeled peptide
177Lu-DOTATOC and 177Lu-DOTA were prepared with radiochemical purities ≥95% and incorporation yields ≥99% as determined by HPLC and ITLC, respectively, as previously reported [5].
3.2. Radioligand receptor-uptake studies
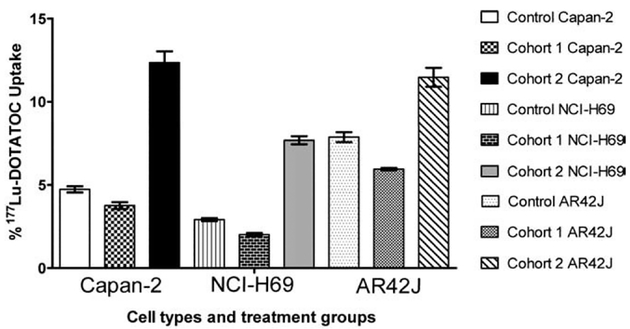
To evaluate the effects of gemcitabine on 177Lu-DOTATOC uptake to sstr, we performed direct cell-uptake studies. As observed in Fig. 1, the percent 177Lu-DOTATOC uptake ranged from 2.9% to 7.9% for gemcitabine-untreated Capan-2, untreated NCI-H69 and untreated AR42J controls.177Lu-DOTATOC uptake and, presumably, the available receptor binding sites decreased in Cohort 1 and increased in Cohort 2. For Cohort 1, 177Lu-DOTATOC uptake for Capan-2 decreased from 4.7±0.2 (n=10) to 3.8±0.2 (n=4) (P<.05), uptake for NCI-H69 decreased from 2.9±0.1 (n=8) to 2.0±0.1 (n=4) (P<.001) and uptake for AR42J from decreased 7.9±0.2 (n=8) to 6.0±0.1 (n=4) (P<.05). For Cohort 2, 177Lu-DOTATOC uptake increased to 12.3±0.7 (n=6) (P<.001) for Capan-2 cells, to 7.7±0.3 (n=4) (P<.001) for NCI-H69 cells and to 11.5±0.6 (n=4) (P<.001) for AR42J cells.
Fig. 1.
sstr-targeted radioligand-uptake studies: Effects of gemcitabine treatment from Cohorts 1 and 2 on human pancreatic adenocarcinoma Capan-2, human small cell lung carcinoma NCI-H69 and rat pancreatic carcinoma AR42J cells resulting in either decreased or increased uptake of 177Lu-DOTATOC when compared to untreated control cells.
3.3. Cell cycle studies
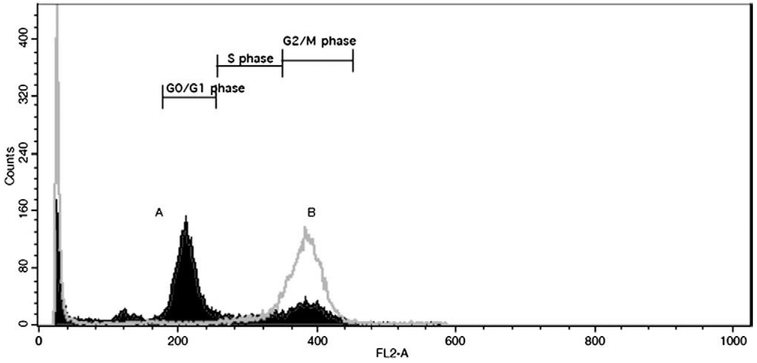
Cell cycle studies were performed to evaluate the role of gemcitabine as a radiosensitizer through modulation of the cell cycle. For control Capan-2 cells (Fig. 2A), approximately 8% of the total cell population (taken as the sum of G1/0, S and G2/M) was in the G2M phase as compared to approximately 84% of the cell population for the gemcitabine-pretreated Capan-2 cells from Cohort 2 (Fig. 2B). Similarly for untreated NCI-H69 cells, approximately 20% of the cell population was in G2M as compared to approximately 54% for gemcitabine-pretreated cells from Cohort 2.
Fig. 2.
Cell cycle analysis: Representative cytogram of gemcitabine-induced effect on human pancreatic adenocarcinoma Capan-2 cells (gray border with no fill, labeled as B) resulting in an increased fraction of cells in G2/M phase as compared to the untreated control cells (black border with fill, labeled as A).
3.4. Cell viability and apoptosis
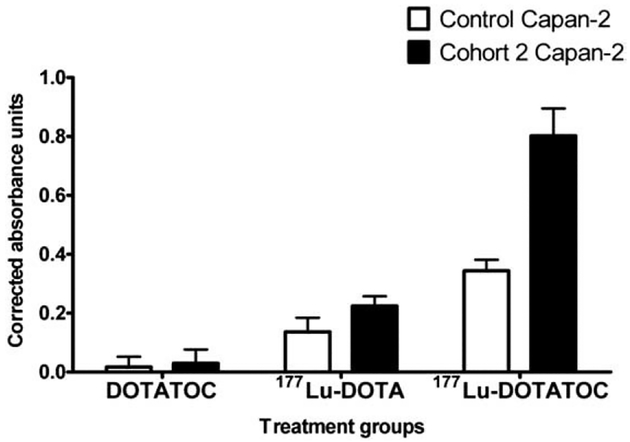
Cell viability and apoptosis experiments were performed on untreated control cells and the cells from Cohort 2 due to increased uptake of 177Lu-DOTATOC and presumably increased absorbed radiation dose from the same amount of added radioactivity. As shown in Fig. 3A, after a 24-h incubation with 177Lu-DOTATOC, there were 1.5 times more viable cells in Capan-2 cells compared to gemcitabine-treated cells from Cohort 2. After 48 h incubation with 177Lu-DOTATOC, there were approximately twice as many viable cells in untreated control Capan-2 cells than gemcitabine-pretreated cells from Cohort 2 (Fig. 3B). For untargeted 177Lu-DOTA, the effects of gemcitabine were less pronounced and equimolar nonradioactive DOTATOC did not have significant effects on cell viability when compared to control cells (Fig. 3).
Fig. 3.
Cell viability studies: Decreased cell viability of human pancreatic adenocarcinoma Capan-2 cells from Cohort 2 when treated with sstr-targeted 177Lu-DOTATOC after 24 h (A) and 48 h (B) incubation. The data are expressed as mean±S.E.M. (n≥3).
In the apoptosis study, after a 72-h incubation with 177Lu-DOTATOC, the release of apoptosis-specific mono- and oligonucleosomes was 2.5 times higher in gemcitabine-treated Capan-2 cells from Cohort 2 than in gemcitabine-untreated cells (Fig. 4). In contrast, after a 72-h incubation with 177Lu-DOTA, the release of apoptosis-specific mono- and oligonucleosomes was 1.5 times greater in gemcitabine-treated Capan-2 cells from Cohort 2 than in untreated cells.
Fig. 4.
Apoptosis studies: Increased apoptosis of human pancreatic adenocarcinoma Capan-2 cells from Cohort 2 when treated with sstr-targeted 177Lu-DOTATOC after 72 h incubation. The data are expressed as mean±S.E.M. (n≥3).
4. Discussion
Cancer represents a major challenge to oncologists because of its chemoresistant and metastatic nature and dismal outcomes. In the past decade, tremendous progress has been made in the field of sstr-targeted radionuclide therapy. However, the success of this therapy in routine clinical use in the United States has been limited due to the concerns of toxicity and relatively high relapse rates with radiolabeled DOTATOC [1,18–20]. In addition, the standard treatment option of single-drug chemotherapy has yielded limited success due to low rates of complete remission and chemoresistance [21]. There is a growing consensus that the future of cancer treatment for solid tumors lies in a multimodality approach [14,21–23].
In this project, we have evaluated the combination of gemcitabine and 177Lu-DOTATOC for potential treatment of sstr-expressing carcinomas. It was previously reported that gemcitabine pretreatment increased the high-affinity and low-affinity binding sites for 111In-DOTA-lanreotide binding to human pancreatic carcinoma cells [8]. In our study, we found that gemcitabine treatment alone (Cohort 1) decreased 177Lu-DOTATOC uptake whereas gemcitabine followed by recovery period (Cohort 2) increased 177Lu-DOTATOC uptake by a factor of 1.5–3 in Capan-2, NCI-H69 and AR42J cells (Fig. 1). Cell cycle studies performed on gemcitabine-treated cells from Cohort 2 demonstrated the cell cycle modulation in which most of the viable cells were in G2M phase (Fig. 2). This cell cycle modulation can be critical for radiation therapy as the G2M has been shown to be the most radiosensitive phase of the cell cycle and, therefore, will produce greater radiation-induced cell damage and death [9]. As observed in the cell viability and apoptosis studies, the gemcitabine-treated cells from Cohort 2 exhibited decreased cell viability and increased apoptosis upon treatment with 177Lu-DOTATOC (Figs. 3 and 4). In a previously published report [24], measuring ATP levels, as a cell viability assay, was compared to the clonogenic survival assay. This study revealed no significant difference between the two methods for determining survival fractions upon exposure to radiation using five different cervical carcinoma cell lines. However, considering the observed twofold increase in 177Lu-DOTATOC uptake and the accumulations of cells in G2M, the most radiosensitive cell phase, one might expect a more pronounced effect of 177Lu-DOTATOC than the one observed in this study. In our studies, the cell number was kept constant for untreated control cells and gemcitabine-treated cells. Gemcitabine by itself decreased the cell number by 15–30% depending upon cell type by decreasing proliferation and increasing cell death. Therefore, if the therapeutic effect determined on initial population of cells plated (at Day 0) is considered, then the synergistic effects of the combination are potentially far greater than those presented in this study. Similar observations of sstr up-regulation following irradiation have been reported in vitro and in vivo [25–27]. In CA20948 tumor-bearing rats injected with 185 MBq of 111In-DOTATATE, nearly a twofold increase in the sstr density was observed in escaped tumor cells upon regrowth [26]. Similarly, upon 185 MBq of 177Lu-DOTATATE injection to CA20948 tumor-bearing rats, there were significant decreases in sstr expression after therapy, although tumors escaping from therapy showed two to five times higher expression of sstr as compared to controls [25]. These observations highlight the complex cellular and molecular role of somatostatin in cell survival, response to DNA damage and repair.
In this study, we used a chemotherapeutic agent that essentially mimics DNA damage and may induce the same repair signaling pathways as that resulting from radiation-induced DNA damage. Hypothetically, the repair mechanism may play a role in the up-regulation of sstr in response to DNA damage induced by radiation and gemcitabine. The increased accumulation of 177Lu-DOTATOC may result from increased endocytosis of agonist-bound sstrs, an ATP-dependent process. The precise mechanisms of sstr regulation are an interesting question and worthy of future investigation at the cellular and molecular level as well as in animal models. Although we have investigated a specific treatment regime, many permutations and combinations of dosing and scheduling would be required to optimize increased in vivo accumulation of radiolabeled octreotide in tumors. The ultimate goal of any therapy is to have maximum therapeutic efficacy with minimal or acceptable toxicity. The dose-limiting factor for 177Lu-DOTATOC or 90Y-DOTATOC used in clinical treatment is renal and hematological toxicity, and as a result, lower doses are given, resulting in incomplete tumor response. The rational combination of gemcitabine, a first-line treatment option for pancreatic cancer, and sstr-targeted radionuclide therapy may not only improve clinical outcomes but also decrease renal toxicity as less radioactivity may be required due to higher receptor-mediated accumulation of the radionuclide in the tumor, resulting in greater absorbed radiation dose.
In conclusion, gemcitabine pretreatment up-regulates sstr expression and acts as a radiosensitizer. The combination of gemcitabine and sstr-targeted radiotherapy has a great potential to improve clinical outcomes and merits further study in animal models.
Acknowledgments
This study was performed under the auspices of the U.S. Dept. of Energy, Office of Science, Office of Biological and Environmental Research under contract 7405-ENG-36 (R.W.A.) and Grant DE-FG01–001NE23554 from the U.S Department of Energy (J.P.N.). The cell cycle data were generated in the Flow Cytometry Shared Resource Center supported by the University of New Mexico Health Sciences Center and the University of New Mexico Cancer Center. Technical assistance by Kamalika Nag (University of New Mexico, Albuquerque, NM) in the operation of flow cytometer is appreciated.
References
- [1].Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide Receptor Radionuclide Therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol 2007;46(6):723–34. [DOI] [PubMed] [Google Scholar]
- [2].Krenning EP, Kwekkeboom DJ, Valkema R, Pauwels S, Kvols LK, De Jong M. Peptide receptor radionuclide therapy. Ann N Y Acad Sci 2004;1014:234–45. [DOI] [PubMed] [Google Scholar]
- [3].Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncology 2005;23(12):2754–62. [DOI] [PubMed] [Google Scholar]
- [4].Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999;26(11):1439–47. [PubMed] [Google Scholar]
- [5].Nayak TK, Norenberg JP, Anderson TL, Prossnitz ER, Stabin MG, Atcher RW. Somatostatin-receptor-targeted alpha-emitting (213)Bi is therapeutically more effective than beta(−)-emitting (177)Lu in human pancreatic adenocarcinoma cells. Nucl Med Biol 2007;34(2):185–93. [DOI] [PubMed] [Google Scholar]
- [6].Nayak T, Norenberg J, Anderson T, Atcher R. A comparison of high-versus low-linear energy transfer somatostatin receptor targeted radionuclide therapy in vitro. Cancer Biother Radiopharm 2005;20(1):52–7. [DOI] [PubMed] [Google Scholar]
- [7].Norenberg JP, Krenning BJ, Konings IR, Kusewitt DF, Nayak TK, Anderson TL, et al. 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res 2006;12(3 Pt 1):897–903. [DOI] [PubMed] [Google Scholar]
- [8].Fueger BJ, Hamilton G, Raderer M, Pangerl T, Traub T, Angelberger P, et al. Effects of chemotherapeutic agents on expression of somatostatin receptors in pancreatic tumor cells. J Nucl Med 2001;42(12):1856–62. [PubMed] [Google Scholar]
- [9].Wardman P Chemical radiosensitizers for use in radiotherapy. Clin Oncol 2007;19(6):397–417. [DOI] [PubMed] [Google Scholar]
- [10].Latz D, Fleckenstein K, Eble M, Blatter J, Wannenmacher M, Weber KJ. Radiosensitizing potential of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) within the cell cycle in vitro. Int J Radiat Oncol Biol Phys 1998;41(4):875–82. [DOI] [PubMed] [Google Scholar]
- [11].Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys 1996;34(4):867–72. [DOI] [PubMed] [Google Scholar]
- [12].Feng L, Achanta G, Pelicano H, Zhang W, Plunkett W, Huang P. Role of p53 in cellular response to anticancer nucleoside analog-induced DNA damage. Int J Mol Med 2000;5(6):597–604. [DOI] [PubMed] [Google Scholar]
- [13].Burris HA III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15(6):2403–13. [DOI] [PubMed] [Google Scholar]
- [14].Orditura M, Martinelli E, Galizia G, Vitiello F, Fasano M, et al. Chemoradiotherapy as adjuvant treatment of gastric cancer. Ann Oncol 2007;18(Suppl 6):vi133–5. [DOI] [PubMed] [Google Scholar]
- [15].Fields MT, Eisbruch A, Normolle D, Orfali A, et al. Radiosensitization produced in vivo by oncevs. twice-weekly 2′2′-difluoro-2′-deoxycytidine (gemcitabine). Int J Radiat Oncol Biol Phys 2000;47(3):785–91. [DOI] [PubMed] [Google Scholar]
- [16].Shewach DS, Hahn TM, Chang E, Hertel LW, Lawrence TS. Metabolism of 2′,2′-difluoro-2ô-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res 1994;54(12): 3218–23. [PubMed] [Google Scholar]
- [17].Shewach DS, Lawrence TS. Gemcitabine and radiosensitization in human tumor cells. Invest New Drugs 1996;14(3):257–63. [DOI] [PubMed] [Google Scholar]
- [18].Forrer F, Uusijarvi H, Storch D, Maecke HR, Mueller-Brand J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med 2005;46(8):1310–6. [PubMed] [Google Scholar]
- [19].Lewington VJ. Targeted radionuclide therapy for neuroendocrine tumours. Endocr Relat Cancer 2003;10(4):497–501. [DOI] [PubMed] [Google Scholar]
- [20].Lambert B, Cybulla M, Weiner SM, Van De Wiele C, Ham H, Dierckx RA, et al. Renal toxicity after radionuclide therapy. Radic Res 2004; 161(5):607–11. [DOI] [PubMed] [Google Scholar]
- [21].Blackstock AW, Cox AD, Tepper JE. Treatment of pancreatic cancer: current limitations, future possibilities. Oncology (Williston Park, NY) 1996;10(3):301–7 [discussion: 308–23]. [PubMed] [Google Scholar]
- [22].Crane CH, Varadhachary G, Pisters PW, Evans DB, Wolff RA. Future chemoradiation strategies in pancreatic cancer. Semin Oncol 2007;34: 335–46. [DOI] [PubMed] [Google Scholar]
- [23].Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small-cell lung cancer. J Clin Oncol 2007;25(26):4146–52. [DOI] [PubMed] [Google Scholar]
- [24].Tam KF, Ng TY, Liu SS, Tsang PC, Kwong PW, Ngan HY. Potential application of the ATP cell viability assay in the measurement of intrinsic radiosensitivity in cervical cancer. Gynecol Oncol 2005;96: 765–70. [DOI] [PubMed] [Google Scholar]
- [25].Melis M, Forrer F, Capello A, Bijster M, Bernard BF, Reubi JC, et al. Up-regulation of somatostatin receptor density on rat CA20948 tumors escaped from low dose [(177)Lu-DOTA(0),Tyr(3)]octreotate therapy. Q J Nucl Med Mol Imaging 2007;51(4):324–33. [PubMed] [Google Scholar]
- [26].Capello A, Krenning E, Bernard B, Reubi JC, Breeman W, de Jong M. 111In-labelled somatostatin analogues in a rat tumour model: somatostatin receptor status and effects of peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2005;32(11): 1288–95. [DOI] [PubMed] [Google Scholar]
- [27].Oddstig J, Bernhardt P, Nilsson O, Ahlman H, Forssell-Aronsson E. Radiation-induced up-regulation of somatostatin receptor expression in small cell lung cancer in vitro. Nucl Med Biol 2006;33(7):841–6. [DOI] [PubMed] [Google Scholar]