Abstract
Objective:
Our goal is to translate lung ultrasound surface wave elastography (LUSWE) for assessing patients with interstitial lung disease (ILD) and various connective tissue diseases including systemic sclerosis (SSc).
Methods:
LUSWE was used to measure the surface wave speed of lung at 100 Hz, 150 Hz and 200 Hz through six intercostal lung spaces for 91 patients with ILD and 30 healthy control subjects. In addition, skin viscoelasticity was measured on both forearms and upper arms for patients and controls.
Results:
The surface wave speeds of patients’ lungs were significantly higher than those of control subjects. Patient skin elasticity and viscosity were significantly higher than those of control subjects. In dividing ILD patients into two groups, ILD with SSc patients and ILD without SSc patients, significant differences between each patient group with the control group were found for both the lung and skin. No significant differences were found between the two patient groups, although there were some differences at a few locations and at 100 Hz. for skin viscoelasticity.
Conclusion:
Significant differences of surface wave speed were found between ILD patients and healthy control subjects for both the lung and skin.
Significance:
LUSWE may be useful for assessing ILD and SSc and screening early stage patients.
Index Terms: interstitial lung disease (ILD), lung ultrasound surface wave elastography (LUSWE), lung, skin, systemic sclerosis
I. INTRODUCTION
Ultrasonography is not widely used for clinically assessing lung disease. Lung tissue is normally filled with air, and the difference in acoustic impedance between air and lung parenchyma is large. Most of the energy of the ultrasound wave is reflected from the lung surface. Ultrasonography evaluation of the thorax is therefore limited to evaluating structures outside of the lung such as pleural fluid, thoracic superficial masses, or adenopathy [1]. We have developed a lung ultrasound surface wave elastography (LUSWE) technique to measure superficial lung tissue stiffness safely and quickly [2],[3]. In LUSWE, a 0.1 second harmonic vibration at a given low frequency is generated by the indenter of a handheld vibrator on the chest wall of a subject. The ultrasound probe is positioned about 5 mm away from the indenter in the same intercostal space to measure the generated surface wave propagation on the lung in that intercostal space. The measurement of surface wave speed on the lung is determined from the change in wave phase with distance and independent of the location of wave excitation.
We have been evaluating LUSWE for assessing patients with interstitial lung disease (ILD) in a prospective clinical research study. Patients with ILD have fibrotic and stiff lungs leading to symptoms, especially dyspnea, and may eventually lead to respiratory failure [4]. Many ILDs typically are distributed in the peripheral, subpleural regions of the lung. The superficial distribution of lung fibrosis is especially suited for LUSWE. Diagnosis of lung fibrosis can be difficult, especially early in the disease course, because the symptoms are nonspecific (most commonly shortness of breath and a dry cough). High-resolution computed tomography (HRCT) is the clinical standard for diagnosing lung fibrosis [5], but it substantially increases radiation exposure for patients. Various HRCT scanning techniques were proposed to reduce the dose [6]. Lung fibrosis results in stiffened lung tissue. However, HRCT does not directly measure lung stiffness.
Both ILD and systemic sclerosis (SSc) are systemic diseases. SSc is a multi-organ connective tissue disease characterized by immune dysregulation and organ fibrosis [7]. Skin involvement in SSc, the major clinical feature, can span from edematous swelling and induration to extensive fibrosis and eventually atrophy. SSc is categorized by variable extent and severity of skin thickening and hardening. The degree of skin involvement is an important measure and predictor of mortality [8]. Severe organ involvement, especially of the skin and lungs, is the cause of morbidity and mortality in SSc [9]. Improvement in skin stiffness is associated with improved survival in many clinical trials [10].
There is a close relationship between lung and skin involvement in both diseases. A large study showed that 35% of patients with SSc had lung fibrosis and 15% had pulmonary hypertension [11]. Survival rates of patients with SSc-ILD, connective tissue disease–associated ILD, and idiopathic pulmonary fibrosis were studied [12]. Patients with SSc-ILD had better survival rates than those with other ILDs, which was at least partly attributable to routine screening and early detection of ILD in patients with SSc.
Lung involvement in patients with SSc may be predicted at early stages by evaluation of skin fibrosis, because skin manifestation is an early and easily detectable marker of SSc disease activity. Recommendations for future clinical trials stress the critical need for noninvasive, clinically applicable biomarkers to improve the evaluation of patients with SSc-ILD [13]. In this paper, we report our results on both lung and skin stiffness measurements using LUSWE on 91 patients with ILD and 30 healthy control subjects.
II. METHOD
A. Lung ultrasound surface wave elastography (LUSWE) technique
In LUSWE, a 0.1s harmonic vibration at a frequency is generated on the skin of the chest wall in an intercostal space. The resulting wave propagation at that frequency travels through the intercostal muscle and propagates on the surface of the lung. The wave motions on the selected locations on the lung surface are noninvasively measured using our ultrasound-based method [14]. The phase change with distance of the harmonic wave propagation on the lung surface is analyzed, and from which the surface wave speed is measured [15],
| (1) |
where Δr is the distance of two measuring locations,Δϕ is the wave phase change over distance, and f is the frequency.
The measurement of wave speed can be improved by using multiple phase change measurements over distances [16]. The regression of the phase change Δϕ with distance Δr can be obtained by “best fitting” a linear relationship between them, and the equation is
| (2) |
where denotes the value of Δϕ on the regression for a given distance of Δr, and α is the regression parameter.
The surface wave speed can be estimated by
| (3) |
where csr is the estimation of wave speed from the regression analysis.
The surface wave speed can be related to the elastic modulus of tissue as [17],
| (4) |
where μ is the shear elasticity in Pascals and ρ is the mass density of the tissue in kg/m3.
For soft tissue under low frequency harmonic excitation, Voigt’s model, which consists of a spring of elasticity μ1 and a damper of viscosity μ2 connected in parallel, has been proven to be effective in modeling the linear viscoelastic materials [18–20]. The wave dispersion curve of wave speed cs with respect to the excitation frequency Z can be formulated by,
| (5) |
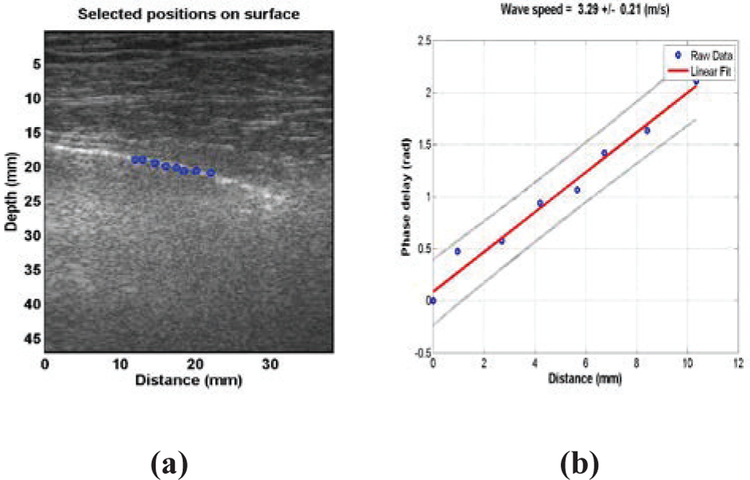
Measurement of lung surface wave speed is noninvasive. Representative LUSWE analysis for a patient is shown in Figure 1(a). The wave motions are measured at eight locations on the lung surface. The normal component of the lung surface motion can be analyzed by cross-correlation analysis of the ultrasound tracking beams [21, 22]. In this study, eight locations over a length of approximately 10 mm on the lung surface were used to measure the normal component of the lung surface motion. The tissue motion is measured at these locations in response to the harmonic wave excitation on the chest wall. A high pulse repetition rate of 2000 pulse/s is used to detect tissue motion in response to wave excitation at 100, 150, or 200 Hz. A Verasonics ultrasound system (Verasonics, Inc; Kirkland, WA) is used and collects up to a few thousand imaging frames per second by using a plane-wave pulse transmission method.
Fig. 1.
(a) Representative LUSWE analysis for a patient subject. The wave motions are measured at eight locations on the lung surface. (b) The wave phase delay of the remaining locations, relative to the first location, is used to measure the surface wave speed.
The surface wave speed on the lung is estimated by determining the change in wave phase with distance along the lung surface. Lung motion at the first location is measured and used as a reference. The wave phase delay of lung motions at the remaining locations, relative to the reference at the first location, is used to measure lung surface wave speed. The surface wave speed is estimated by the phase changes simultaneously at the 8 locations. Figure 1(b) shows a representative wave speed at 100 Hz for a patient in the left second intercostal space. The surface wave speed was 3.29 ± 0.21m/s (mean ± standard error from the regression analysis) at 100 Hz for the patient. The wave speed on the lung surface is determined by analyzing ultrasound data directly from the lung. Therefore, the wave speed measurement is local and independent of the location and amplitude of excitation.
B. Skin measurements
This noninvasive technique can be used to measure viscoelastic properties of other tissues. In this research, we also measured the surface wave speed of skin on the arms of patients and controls at the three excitation frequencies. In skin testing, an ultrasound gel pad standoff by Aquaflex® (Parker Laboratories, Inc., Fairfield, NJ 07004, USA) was placed between the ultrasound probe and the skin to improve the imaging quality of skin. The vibration excitation was directly applied on the skin.
C. Patient and healthy control subjects
Human studies were approved by the Mayo Clinic Institutional Review Board (IRB). Each participant completed an informed consent form. Patients were enrolled in this research based on their clinical diagnoses. 91 patients with ILD were enrolled from Mayo Clinic Departments of Rheumatology and Pulmonary and Critical Care Medicine. These patients were confirmed ILD patients with clinical assessments together with pulmonary function tests and high resolution CT scans. These ILD patients also had various diseases including systemic sclerosis (SSc) or scleroderma, rheumatoid arthritis, connective tissue disease, idiopathic pulmonary fibrosis, anti-synthetase, Sjögren’s syndrome, polymyositis, and systemic lupus erythematosus.
We intended to study the relationship between ILD and SSc by measuring both lung and skin. We divided the patients into two groups: patients with SSc (41 patients) and patients without SSc (50 patients). Patients’ mean age was 62.4 ± 13.0 years (range 20–85, 39 male and 52 female). 30 healthy subjects were enrolled as controls if they did not have any lung and skin diseases. Controls’ mean age was 45.4 ± 14.1 years (range 22–73, 14 male and 16 female).
D. Human study protocol
The subject is tested in a sitting position. The subject’s lungs are tested first and then the skin. It takes about 30–40 minutes to finish the testing. Both lungs of the subject are tested through six intercostal spaces. The upper anterior lungs are tested at the second intercostal space in the mid-clavicular line. The lower lateral lungs are tested at one intercostal space above the level of the diaphragm in the mid-axillary line. The lower posterior lungs are tested at one intercostal space above the level of the diaphragm in the mid-scapular line. Ultrasound imaging is used to identify the lungs and select appropriate intercostal spaces to measure the upper and lower lungs. A 0.1-second harmonic vibration is generated by the indenter of the handheld shaker (Model: FG-142, Labworks Inc., Costa Mesa, CA 92626, USA) on the chest wall. The excitation force from the indenter is much less than 1 Newton and the subject only feels a small vibration on his/her skin. The indenter of the handheld shaker is placed on the chest wall in an intercostal space. An L11–4 ultrasound probe with a central frequency of 6.4 MHz is positioned about 5 mm away from the indenter in the same intercostal space to measure the resulting surface wave propagation on the lung. A Verasonics ultrasound system (Verasonics V1, Verasonics, Inc., Kirkland, WA 98034, USA) is used in this research. Images of the lung and skin are acquired by compounding 11 successive angles at a pulse repetition frequency (PRF) of 2 kHz. The lung is tested at total lung capacity when the subject takes a deep breath and holds for a few seconds. The surface wave speeds are measured at three excitation frequencies of 100 Hz, 150 Hz, or 200 Hz. Three measurements are performed at each location and at each frequency. A small tissue motion in tens of μm is enough for sensitive ultrasound detection of the generated tissue motion. The 100 Hz wave motion is stronger than those of higher frequency waves. The higher frequency waves have smaller wave length but decay more rapidly over distance than the lower frequency waves. The frequency ranges chosen in this study consider the wave motion amplitude, spatial resolution, and wave attenuation. The same ultrasound system and probe are used for skin testing. The subject’s arm is placed horizontally on a pillow in a relaxed state. The skin of both left and right forearms and upper arms of subjects are tested. These locations are in the central part of the arms and on the dorsal sides.
E. Statistical analysis
An unpaired, two-tailed t-test between the patients and healthy control subjects was conducted to compare the sample means. Differences in mean values were considered significant when p<0.05. A one-way ANOVA with critical p value = 0.05 and a post hoc Tukey test with α = 0.05 was conducted to compare the sample means among three groups: ILD patients with SSc, ILD patients without SSc, and healthy control subjects.
III. RESULTS
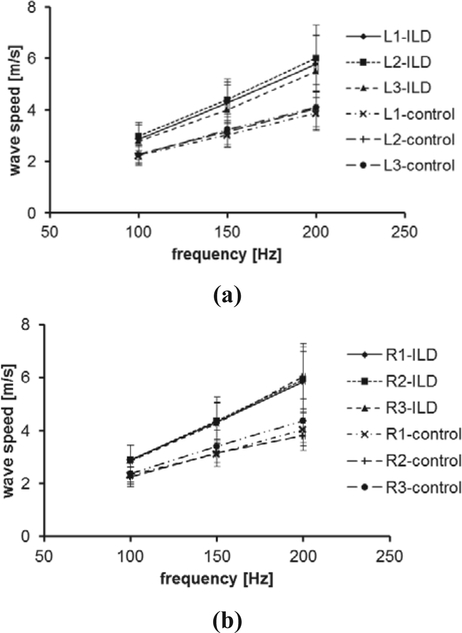
A comparison of lung surface wave speeds between 91 ILD patients and 30 healthy control subjects is shown in Figure 2 for 100 Hz, 150 Hz, and 200 Hz. The three intercostal spaces are designated by a number from 1 to 3. The upper anterior lung is designated by 1. The lower lungs at the lateral and posterior positions are designated by 2 and 3, respectively. The right and left lungs are designated by letters R and L, respectively. Therefore, L1 represents the left anterior lung in the second intercostal space. The p-values for the t -test were less than 0.0001 for all intercostal spaces and for three frequencies between the patients and controls, respectively.
Fig. 2.
Surface wave speed of lung as a function of excitation frequency between patients and healthy control subjects through six intercostal spaces at both (a) left and (b) right sides.
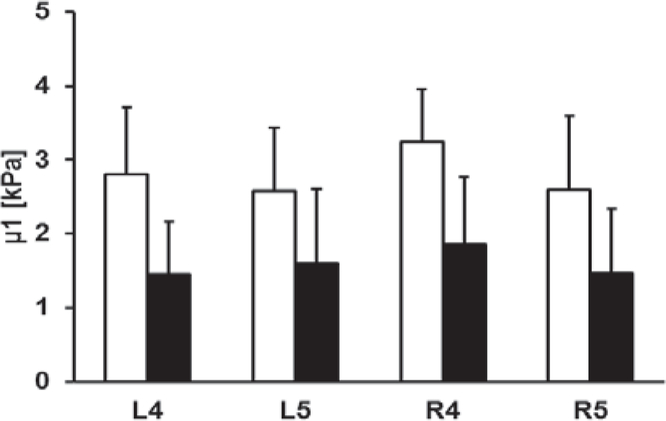
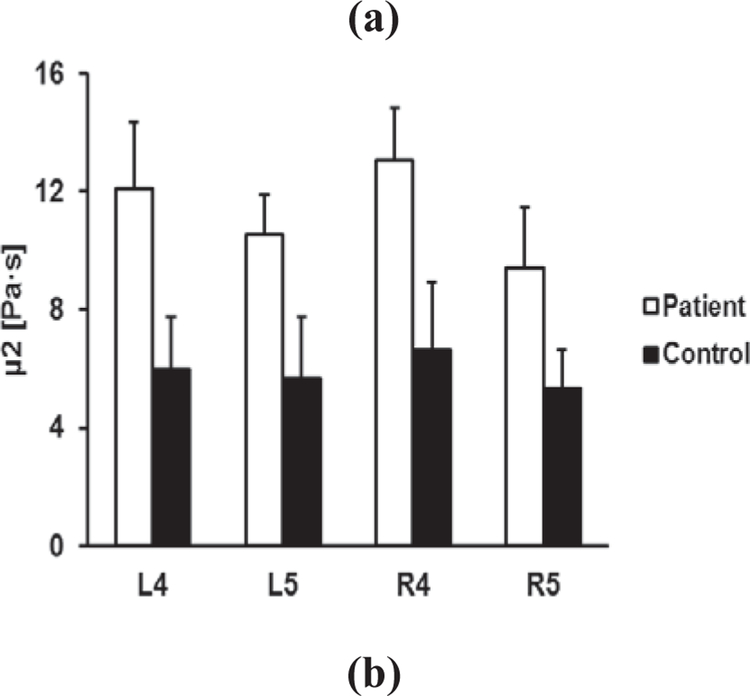
Figure 3 shows the comparison of elasticity and viscosity of skin between 91 ILD patients and 30 healthy control subjects. Viscoelasticity is estimated using equation (5) with wave speed measurements at 100 Hz, 150 Hz, and 200 Hz. Most soft tissues have a mass density close to 1.0 g/cm3. In this study, the mass density of skin is assumed to be 1.0 g/cm3. The forearm and upper arm are designated by numbers 4 and 5, respectively. The right and left arms are designated by letters R and L, respectively. Therefore, R4 represents the skin of the right forearm. The p-values for the t -test were less than 0.0001 for the four locations between patients and controls. Therefore, the magnitudes of both elasticity and viscosity of patients were statistically higher than those of healthy subjects.
Fig. 3.
Comparison of elasticity μ1 (a) and viscosity μ2 (b) between patients and healthy control subjects at four locations.
One purpose of the research was to study if the relationship between the lung stiffness and the skin stiffness could be identified for patients with ILD or SSc. Both ILD and SSc are systemic diseases. We divided the patients into two groups. Group 1 is ILD patients with SSc, and group 2 is ILD patients without SSc. We analyzed the data for these two groups of patients and the control subjects.
Figure 4 shows the one-way ANOVA analyses of surface wave speeds of lung for 100 Hz, 150 Hz, and 200 Hz among the three groups: ILD patients with SSc, ILD patients without SSc, and healthy controls. The p-values for the one-way ANOVA test were less than 0.05 for all intercostal spaces and for three frequencies among the three groups. The post hoc Tukey test was performed after ANOVA to analyze the subgroups. Significant differences of wave speed between either group 1 patients or group 2 patients with controls were found. No significant differences were found between group 1 patients and group 2 patients for most locations and the three frequencies. However, there are statistically significant differences at 100 Hz for locations L1 (p = 0.003), R1 (p = 0.002) and R3 (p = 0.001) between group 1 patients and group 2 patients. There is a significant difference at 150 Hz for location R1 (p = 0.026) between group 1 patients and group 2 patients. These findings are interesting because ILD affects the lower lungs more and the upper lungs less at L1 or R1. Although, we can generally conclude that there are no significant differences of wave speed between ILD patients with SSc and ILD patients without SSc. The upper lungs and lower frequencies may provide more information to separate the two groups of patients.
Fig. 4.
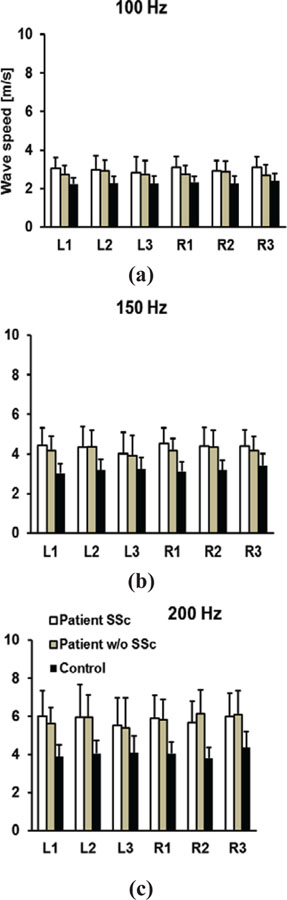
Comparison of wave speeds between patients with SSc, patients without SSc, and healthy control subjects through six intercostal spaces. Surface wave speeds were measured at (a) 100 Hz, (b) 150 Hz, and (c) 200 Hz.
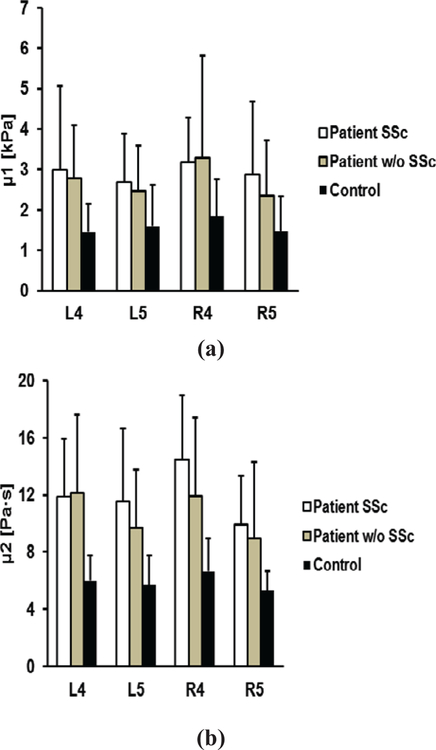
Figure 5 shows the one-way ANOVA analyses of elasticity and viscosity of skin among the three groups. The p-values for the one-way ANOVA test were less than 0.05 for all locations among the three groups. The post hoc Tukey tests indicated that significant differences of elasticity or viscosity between either group 1 patients or group 2 patients with controls were found. In addition, no significant differences were found between group 1 patients and group 2 patients for most locations. There is one significant difference in viscosity at R4 (p = 0.019) between group 1 patients and group 2 patients. We may generally conclude that there are no significant differences of skin elasticity and viscosity between ILD patients with SSc and ILD patients without SSc. Some locations such as R4 may be useful to separate the two groups of patients.
Fig. 5.
Comparison of elasticity μ1 (a) and viscosity μ2 (b) between patients with SSc, patients without SSc, and healthy control subjects at four locations.
IV. DISCUSSION
LUSWE is a safe technique. Shear wave elastography (SWE) techniques use ultrasound radiation force (URF) to generate tissue motion. However, URF may not be applied to some tissues such as the lung because the relatively high-intensity ultrasound energy may cause alveolar hemorrhage or lung injury [23]. In addition, long periods of ultrasound pulses with relatively high-intensity ultrasound energy may cause damage to the ultrasound system itself, e.g., high voltage drop and probe element damage. In LUSWE, the wave generation is safely produced using a gentle mechanical vibration on the skin. URF is not used for generating shear or surface waves. Diagnostic ultrasound is only used for detection of wave propagation. Therefore, LUSWE can be used for the lung [2] and eye [24].
Diagnosis of ILD can be difficult at early stages because a patient’s symptoms such as shortness of breath and dry cough are nonspecific. The findings of physical examinations are usually nonspecific and chest radiography typically shows nonspecific or nondiagnostic findings. HRCT is the clinical standard for diagnosing lung fibrosis [5, 25], but it is expensive and involves radiation.
Because ILD is a systemic disease, patients enrolled in this research presented with other various diseases. We divided the ILD patients into two groups: ILD patients with SSc (41 patients) and ILD patients without SSc (50 patients). Our results demonstrate that there are significant differences between either patient group with the control group for both lung and skin stiffness. There are no statistically significant differences between ILD patients with SSc and ILD patients without SSc. However, there are differences between the two patient groups at the upper lungs at 100 Hz. We plan to study some early stage ILD patients or SSc patients without ILD symptoms. LUSWE may be useful to screen patients at early stages. Testing both lung and skin may provide more information to identify ILD at early stages. LUSWE may be easily integrated into a clinical test and takes about 30–40 minutes to finish both lung and skin tests. We may significantly reduce the testing time if we could identify sensitive intercostal spaces and frequencies for assessing specific ILD. However, the current testing time is still good for a clinical study. LUSWE can be used to quantitatively monitor disease progression for ILD patients. We are continuing to follow the 91 ILD patients; however, a few ILD patients have already passed away.
In this research, we provide surface wave speeds at 100 Hz, 150 Hz, and 200 Hz. The elasticity and viscosity of the lung can be estimated from equation (5) if the lung mass density is known. However, we cannot find data of lung mass density for fibrotic lungs. Also, lung density is dependent on the pulmonary pressure. In this research, a subject is tested at total lung capacity (TLC) when taking a deep breath and holding. We expect that the lung density of ILD would be higher than that of healthy lungs. However, there are no data of lung density for ILD patients. Lung mass density is directly associated with lung pathology. Computed tomography (CT) is the major clinical imaging modality for assessing various lung diseases. The mechanism of CT is based on the changes of tissue mass density, but the CT system uses the Hounsfield unit (HU) and not lung density to image the lung. We are developing a deep neural network (DNN) model to predict lung mass density on lung phantoms based on the LUSWE measurements [26]. We will study how to develop and apply the DNN models for the patient’s data.
We have found statistically significant differences for both lung and skin stiffness between the patients and controls. One of the reasons may be due to the fact that the patient group is relatively older than the control group. We plan to study members of the control group around age 60. Since both ILD and SSc are systemic diseases, often multiple organs are involved for most patients with either ILD or SSc or both. One of the purposes of this research is to determine if it is possible to follow skin stiffness changes for identifying early lung involvement. We found no statistically significant differences between ILD patients with and without SSc. However, we found some statistically significant differences at a few locations at 100 Hz. We will further study this by enrolling early stage patients with ILD or SSc to see if it is possible to identify lung involvement at early stages by tracking the changes of skin viscoelasticity. In this study, we measured lungs at six locations and measured skin at four locations. We will further study how to relate both skin and lung measurements and how to use the correlation for clinical use.
The surface wave measurement is local and only dependent on the local tissue material properties. The intercostal muscle should not affect the lung measurement. However, thick muscle can attenuate wave propagation through the muscle. The advantage of LUSWE is that the wave propagation is safely generated by a mechanical vibration on the chest wall. We can easily increase the amplitude of vibration. A patient just feels a short gentle vibration on his/her chest.
LUSWE measures the phase velocity at each frequency. For example, a 0.1 second 100 Hz signal of 10 cycles is used to measure the wave speed at 100 Hz. Therefore, LUSWE provides much higher signal to noise ratios (SNRs) for wave speed measurements compared with other elastography techniques using a short pulse excitation.
In this study, viscoelasticity of skin was calculated using Voigt’s model with surface wave speeds at three frequencies. We developed a technique to measure the wave attenuation [27]. We are still working on various models to study wave attenuation and hopeful to apply them to this research.
V. CONCLUSION
Lung ultrasound surface wave elastography is a novel noninvasive technique for measuring superficial lung tissue stiffness. The results show that the surface wave speeds of patients’ lungs were significantly higher than those of control subjects. Patient skin elasticity and viscosity were significantly higher than those of control subjects. In dividing ILD patients into two groups, ILD patients with SSc and ILD patients without SSc, statistically significant differences between each patient group with the control group were found for both the lung and skin. No significant differences were found between the two patient groups, although there were some differences of skin viscoelasticity at a few locations and at 100 Hz. LUSWE may be useful for assessing ILD and SSc and screening early stage patients.
Acknowledgments
This study is supported by NIH R01HL125234 from the National Heart, Lung, and Blood Institute.
Contributor Information
Xiaoming Zhang, Department of Radiology, Mayo Clinic, Rochester, MN, USA.
Boran Zhou, Department of Radiology, Mayo Clinic..
Thomas Osborn, Department of Rheumatology, Mayo Clinic..
Brian Bartholmai, Department of Radiology, Mayo Clinic..
Sanjay Kalra, Department of Pulmonary and Critical Care Medicine, Mayo Clinic..
REFERENCES
- [1].Volpicelli G, “Lung sonography,” J Ultrasound Med, vol. 32, pp. 165–71, January 2013. [DOI] [PubMed] [Google Scholar]
- [2].Zhang X, Osborn T, Zhou B, Meixner D, Kinnick RR, Bartholmai B, et al. , “Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study,” IEEE Trans Ultrason Ferroelectr Freq Control, vol. 64, pp. 1298–1304, September 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang X, Zhou B, Kalra S, Bartholmai B, Greenleaf J, and Osborn T, “An Ultrasound Surface Wave Technique for Assessing Skin and Lung Diseases,” Ultrasound Med Biol, vol. 44, pp. 321–331, February 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Coultas DB, Zumwalt RE, Black WC, and Sobonya RE, “The epidemiology of interstitial lung diseases,” Am J Respir Crit Care Med, vol. 150, pp. 967–72, October 1994. [DOI] [PubMed] [Google Scholar]
- [5].Mathieson JR, Mayo JR, Staples CA, and Muller NL, “Chronic diffuse infiltrative lung disease: comparison of diagnostic accuracy of CT and chest radiography,” Radiology, vol. 171, pp. 111–6, April 1989. [DOI] [PubMed] [Google Scholar]
- [6].Mayo JR, “CT evaluation of diffuse infiltrative lung disease: dose considerations and optimal technique,” J Thorac Imaging, vol. 24, pp. 252–9, November 2009. [DOI] [PubMed] [Google Scholar]
- [7].Steen VD and Medsger TA Jr., “Severe organ involvement in systemic sclerosis with diffuse scleroderma,” Arthritis Rheum, vol. 43, pp. 2437–44, November 2000. [DOI] [PubMed] [Google Scholar]
- [8].Clements PJ, Lachenbruch PA, Ng SC, Simmons M, Sterz M, and Furst DE, “Skin score. A semiquantitative measure of cutaneous involvement that improves prediction of prognosis in systemic sclerosis,” Arthritis Rheum, vol. 33, pp. 1256–63, August 1990. [DOI] [PubMed] [Google Scholar]
- [9].Steen VD and Medsger TA, “Changes in causes of death in systemic sclerosis, 1972–2002,” Ann Rheum Dis, vol. 66, pp. 940–4, July 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Steen VD and Medsger TA Jr., “Improvement in skin thickening in systemic sclerosis associated with improved survival,” Arthritis Rheum, vol. 44, pp. 2828–35, December 2001. [DOI] [PubMed] [Google Scholar]
- [11].Hudson M, Fritzler MJ, and Baron M, “Systemic sclerosis: establishing diagnostic criteria,” Medicine (Baltimore), vol. 89, pp. 159–65, May 2010. [DOI] [PubMed] [Google Scholar]
- [12].Su R, Bennett M, Jacobs S, Hunter T, Bailey C, Krishnan E, et al. , “An analysis of connective tissue disease-associated interstitial lung disease at a US Tertiary Care Center: better survival in patients with systemic sclerosis,” J Rheumatol, vol. 38, pp. 693–701, April 2011. [DOI] [PubMed] [Google Scholar]
- [13].Khanna D, Brown KK, Clements PJ, Elashoff R, Furst DE, Goldin J, et al. , “Systemic sclerosis-associated interstitial lung disease-proposed recommendations for future randomized clinical trials,” Clin Exp Rheumatol, vol. 28, pp. S55–62, Mar-Apr 2010. [PubMed] [Google Scholar]
- [14].Zhang X, Zhou B, Miranda AF, and Trost LW, “A Novel Noninvasive Ultrasound Vibro-elastography Technique for Assessing Patients With Erectile Dysfunction and Peyronie Disease,” Urology, vol. 116, pp. 99–105, June 2018. [DOI] [PubMed] [Google Scholar]
- [15].Kubo K, Zhou B, Cheng YS, Yang TH, Qiang B, An KN, et al. , “Ultrasound elastography for carpal tunnel pressure measurement: A cadaveric validation study,” J Orthop Res, vol. 36, pp. 477–483, January 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kubo K, Cheng YS, Zhou B, An KN, Moran SL, Amadio PC, et al. , “The quantitative evaluation of the relationship between the forces applied to the palm and carpal tunnel pressure,” J Biomech, vol. 66, pp. 170–174, January 3 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou B and Zhang X, “Comparison of five viscoelastic models for estimating viscoelastic parameters using ultrasound shear wave elastography,” Journal of the Mechanical Behavior of Biomedical Materials, vol. 85, pp. 109–116, 2018/September/01/ 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Catheline S, Gennisson JL, Delon G, Fink M, Sinkus R, Abouelkaram S, et al. , “Measuring of viscoelastic properties of homogeneous soft solid using transient elastography: an inverse problem approach,” J Acoust Soc Am, vol. 116, pp. 3734–41, December 2004. [DOI] [PubMed] [Google Scholar]
- [19].Prim DA, Zhou B, Hartstone-Rose A, Uline MJ, Shazly T, and Eberth JF, “A mechanical argument for the differential performance of coronary artery grafts,” Journal of the mechanical behavior of biomedical materials, vol. 54, pp. 93–105, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng Y-S, Zhou B, Kubo K, An K-N, Moran SL, Amadio PC, et al. , “Comparison of two ways of altering carpal tunnel pressure with ultrasound surface wave elastography,” Journal of Biomechanics, vol. 74, pp. 197–201, 2018/June/06/ 2018. [DOI] [PubMed] [Google Scholar]
- [21].Hasegawa H and Kanai H, “Improving accuracy in estimation of artery-wall displacement by referring to center frequency of RF echo,” IEEE Trans Ultrason Ferroelectr Freq Control, vol. 53, pp. 52–63, January 2006. [DOI] [PubMed] [Google Scholar]
- [22].Clay R, Bartholmai BJ, Zhou B, Karwoski R, Peikert T, Osborn T, et al. , “Assessment of Interstitial Lung Disease Using Lung Ultrasound Surface Wave Elastography: A Novel Technique With Clinicoradiologic Correlates,” J Thorac Imaging, June 5 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zachary JF, Blue JP Jr., Miller RJ, Ricconi BJ, Eden JG, and O’Brien WD Jr., “Lesions of ultrasound-induced lung hemorrhage are not consistent with thermal injury,” Ultrasound Med Biol, vol. 32, pp. 1763–70, November 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou B, Sit AJ, and Zhang X, “Noninvasive measurement of wave speed of porcine cornea in ex vivo porcine eyes for various intraocular pressures,” Ultrasonics, vol. 81, pp. 86–92, November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Verschakelen JA, “The role of high-resolution computed tomography in the work-up of interstitial lung disease,” Curr Opin Pulm Med, vol. 16, pp. 503–10, September 2010. [DOI] [PubMed] [Google Scholar]
- [26].Zhou B and Zhang X, “Lung mass density analysis using deep neural network and lung ultrasound surface wave elastography,” Ultrasonics, vol. 89, pp. 173–177, May 23 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang X, “A surface wave elastography technique for measuring tissue viscoelastic properties,” Med Eng Phys, vol. 42, pp. 111–115, April 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]