Abstract
Background:
Opioid abuse is a major problem around the world. Identifying environmental factors that contribute to opioid abuse and addiction is necessary for decreasing this epidemic. In rodents, environmental enrichment protects against the development of low dose stimulant self-administration, but studies examining the effect of enrichment and isolation (compared to standard housing) on the development of intravenous opioid self-administration have not been conducted. The present study investigated the role of environmental enrichment on self-administration of the short-acting μ-opioid remifentanil.
Methods:
Rats were raised in an enriched condition (Enr), standard condition (Std), or isolated condition (Iso) beginning at 21 days of age and were trained to lever press for 1 or 3 μg/kg/infusion remifentanil in young adulthood. Acquisition of self-administration and responding during increasing fixed ratio requirements were assessed and a dose-response curve was generated.
Results:
In all phases, Enr rats lever-pressed significantly less than Std and Iso rats, with Enr rats pressing between 9% and 40% the amount of Iso rats. Enr rats did not acquire remifentanil self-administration when trained with 1 μg/kg/infusion, did not increase responding over increasing FR when trained at either dose, and their dose-response curves were flattened compared to Std and Iso rats. When expressed as economic demand curves, Enr rats displayed a decrease in both essential value (higher α) and reinforcer intensity (Q0) compared to Std and Iso rats at the 1 μg/kg/infusion training dose.
Conclusion:
Environmental enrichment reduced remifentanil intake, suggesting that social and environmental novelty may protect against opioid abuse.
Introduction
While self-reported heroin use has remained steady over the last decade (SAMHSA 2014), heroin overdose is on the rise (Dasgupta et al. 2014). Evidence suggests that most current heroin addicts start abusing heroin after misuse of prescription opioids (Kolodny et al. 2015). The transition to injectable intravenous (i.v.) drug use comes with several health problems (Tavitian-Exley et al. 2015) that cost society an estimated 5 billion dollars in health care costs annually (Mark et al. 2001). As such, understanding the environmental influences contributing to opioid misuse is essential in reducing heroin’s substantial societal impact.
Like other drugs of abuse, a common risk factor for the initiation of problematic opioid use is mild stress during childhood and adolescence (SAMHSA 2014). Thus, adolescents that are experiencing chronic mild stress can be considered at risk for drug abuse. This may be modeled in rodents using social isolation (Iso), a housing condition where animals are kept apart from conspecifics. Conversely, rodents raised in enriched environments (Enr), which typically contain multiple novel objects and conspecifics for social interaction, may model protected individuals. Consistent with this notion, Enr rats self-administer stimulants at lower rates compared to Iso rats or pair-housed rats in standard cages (Std), although this effect is only obtained at low unit doses (Alvers et al. 2012; Green et al. 2010).
A few studies have examined opioid reward in rodents raised in different rearing environments. One study found that Enr rats express greater conditioned place preference (CPP) to low-efficacy μ-opioids, but not high-efficacy μ-opioids, compared to Iso rats (Smith et al. 2005), although other studies suggest that Std rodents demonstrate greater heroin CPP than Enr rodents (El Rawas et al. 2009; Galaj et al. 2016). Limited evidence has been collected regarding the importance of rearing environment on intravenous self-administration of opioids, although several older studies have demonstrated that single-housed animals drink more morphine solution (Alexander et al. 1978; Hill and Powell 1976; Marks-Kaufman and Lewis 1984; Raz and Berger 2010) and self-administer more aerosolized sufentanil (Weinhold et al. 1993) compared to group-housed or Enr animals. In another study, Bozarth (1989) measured i.v. self-administration in group-housed and single-housed rats and found that group-housed rats self-administered less heroin than single-housed rats (Bozarth et al. 1989). Despite this previous work, it is unknown if environmental enrichment applied during the adolescent period alters the development of opioid self-administration via the i.v. route, which is most applicable to human abuse. Additionally, previous studies in this area have not consistently used the same housing protocols, and often they do not include Enr, Std, and Iso conditions together for comparison.
The current study examined opioid self-administration in Enr, Std, and Iso rats. In contrast to studies that use single-housed animals in standard cages to compare to group-housed animals (Bozarth et al. 1989; Raz and Berger 2010), we employed a more extreme Iso condition using a hanging wire mesh cage with solid metal side walls. In addition, in contrast previous work (El Rawas et al. 2009), we did not include a running wheel in the Enr environment, as access to a running wheel alone has robust effects on drug self-administration independent of enrichment (Smith and Pitts 2011). Finally, rather than using heroin or morphine, we tested self-administration of the short-acting synthetic μ-receptor agonist remifentanil. Compared to heroin, remifentanil engenders higher response rates and sharper dose-response functions in rodent self-administration models (Hiranita et al. 2014; Hiranita et al. 2013; Panlilio and Schindler 2000), which makes it ideal for studying potential environment-induced changes in opioid self-administration.
Materials and Methods
Subjects and Housing
Thirty-six male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) arrived in the colony at PND 21 and were randomly separated into 1 of 3 housing environments. Enr rats were placed in a large stainless steel cage (122 × 61 × 45.5 cm) with 5–8 age-matched cohorts and 14 objects rearranged daily with 7 objects replaced daily. Std rats were pair-housed in standard cages (33 × 38 × 20 cm) with bedding but no objects and Iso rats were singly housed in small stainless steel cages (17 × 24 × 20 cm) with grid metal floors and no objects. Rats were housed in their respective environments for the entire study. All rats within the same Enr or Std cage were run simultaneously and all were included in the experiment. Rats were kept on a 12h light-dark cycle (lights on at 7:00AM) and were allowed food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical Procedures
Between PND 55–58, rats underwent jugular catheter implantation surgery. Briefly, rats were anesthetized with a ketamine (Butler Schein, Dublin, OH, USA) /xylazine (Akorn, Inc., Decatur, IL, USA) /acepromazine (Boehringer Ingelheim, St. Joseph, MO, USA) cocktail (75/7.5/0.75 mg/kg; 0.15ml/100g body weight; i.p.). A silastic catheter was inserted into the right jugular vein, threaded under the skin, and exited the body via an incision on the scalp. A cannula was connected to the end of the catheter and secured to the skull with dental acrylic and four jeweler’s screws.
Self-Administration Apparatus
All self-administration sessions were conducted in standard 2-lever operant conditioning chambers (28 × 24 × 21 cm; ENV-008CT; MED Associates, St. Albans, VT, USA) equipped with a cue light located above each lever and syringe pumps for drug delivery (PHM-100; MED Associates). For the self-administration sessions, rats were connected to the syringe pump via tubing strung through a leash (PHM-120; MED Associates) that was attached to a swivel (PHM-115; MED Associates) above the chamber.
Self-Administration Procedure
Acquisition
Seven days after surgery (PND 62–65), rats began training for self-administration of either 1 or 3 μg/kg/infusion remifentanil using an autoshaping procedure. For autoshaping sessions, the active lever was extended on a variable interval 90-sec schedule and remained extended until pressed or after 15 sec, after which the active lever retracted, both cue lights turned on, and a 3.4 sec infusion of remifentanil occurred. Each rat received 10 infusions (regardless of number of lever presses) over the first 15 min of the autoshaping session but remained in the operant chamber for an additional 45 min. Rats were returned to their housing environments after the autoshaping session. One hr later, rats were returned to the operant boxes and were allowed to self-administer their respective remifentanil dose on a FR1 schedule of reinforcement. For these response-contingent sessions, both levers were extended for the entire 60-min session. Each infusion was signaled with the illumination of both cue lights with no scheduled time out. Active lever presses occurring during remifentanil infusion were recorded but had no programmed consequence. Autoshaping occurred for 7 days and the FR1 sessions continued an additional 3 days in the absence of autoshaping. Only responses made during the FR1 portion of acquisition phase were used in the statistical analysis. Position of the active lever was counterbalanced across rats.
Increasing FR and Dose-Response
After the conclusion of the FR1 portion of the experiment, the response requirement was systematically increased across sessions. Rats spent 3 days at a FR2, then 3 days at a FR3, and finally 3 days at a FR5. Rats were then allowed to self-administer different doses of remifentanil (saline, 0.1, 0.3, 1, 3, and 10 μg/kg/infusion) in pseudo-random order (saline was never presented first and rats always ended on their respective training dose: 1 or 3 μg/kg/infusion). Each dose was presented for 3 consecutive days; responding on the last 2 days were averaged together to generate the dose response curve. A demand curve was fit to the dose-response data using the formula: log Q = log(Q0)+k*(e(-αQ0C) −1), where Q equals consumption, Q0 equals consumption at zero cost (i.e., demand intensity; intercept of function), C equals unit price, k equals a scalar constant for consumption range, and 1/α is essential value (Hursh and Silberberg 2008). One day following the last experimental day, rats were administered a bolus infusion of 15 mg/kg morphine to test for catheter patency. If rats failed the patency test, they were excluded from all analyses.
Statistical Analysis
Active and inactive lever presses during acquisition were analyzed using 3 (environment) × 2 (training dose) × 10 (session) mixed ANOVAs. Active and inactive lever presses during increasing FR were analyzed using 3 (environment) × 2 (training dose) × 9 (session) mixed ANOVAs. Active and inactive lever presses during the dose response phase were analyzed using 3 (environment) × 2 (training dose) × 6 (dose) mixed ANOVAs. For the demand curves, the parameters α and Q0 were extracted and analyzed using non-linear mixed effects models (Pinheiro et al. 2007), with subject as a random variable and environment and training dose as fixed, between-subjects variables. Tukey’s HSD post hoc analyses were used in the event of significant interactions; p values less than 0.05 were deemed statistically significant.
Results
Acquisition
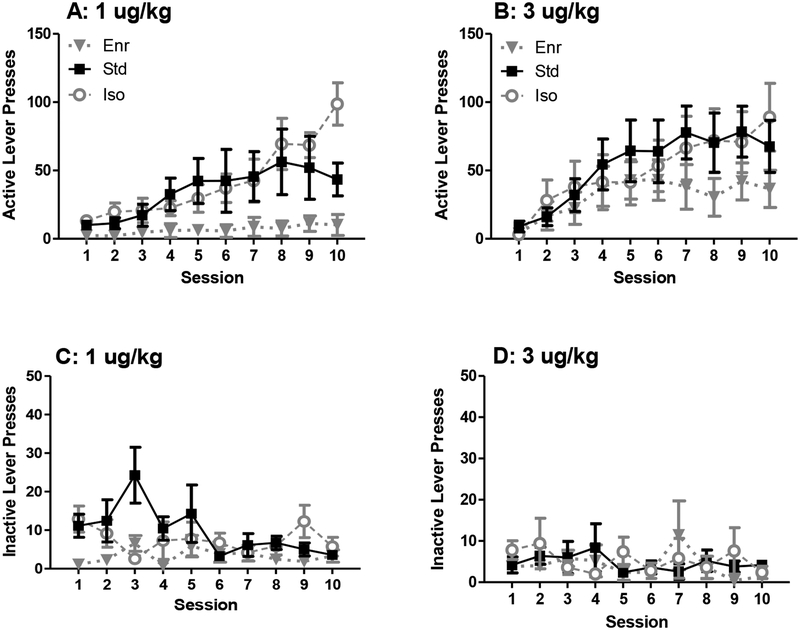
The results from the acquisition phase of the experiment are presented in Figure 1. Analysis of these results revealed a main effect of session (F(9, 270) = 20.61, p < 0.05), a main effect of environment (F(2, 30) = 3.77, p < 0.05), and a session × environment interaction (F(18, 270) = 3.53, p < 0.05) on active lever presses during acquisition. While there were no significant differences among groups on active lever presses during session 1, Enr rats made fewer active lever presses than Iso rats when collapsed across training dose on session 10 (p < 0.05). In addition, Iso and Std rats, but not Enr rats, significantly increased their remifentanil intake over sessions (Iso rats showed significantly more active lever presses on session 10 compared to sessions 1, 2, 3, 4, 5, 6, and 7, p < 0.05; Std rats showed significantly more active lever presses on sessions 5, 6, 7, 8, 9, and 10 compared to both sessions 1 and 2, p < 0.05). For inactive lever presses, there was an interaction between session and environment (F(18, 270) = 1.77, p < 0.05). However, Tukey’s post hoc analysis of this interaction yielded no systematic differences across sessions.
Figure 1: Effect of Environmental Enrichment on Acquisition of Remifentanil Self-Administration.
Active lever presses (A) and inactive lever presses (C) during the self-administration phase of acquisition by rats trained with 1 μg/kg/infusion remifentanil. Active lever presses (B) and inactive lever presses (D) by rats trained with 3 μg/kg remifentanil. Enr: gray triangles (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), Std: black squares (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), Iso: white circles (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg). Note the difference in scales between the upper and lower panels.
Increasing FR
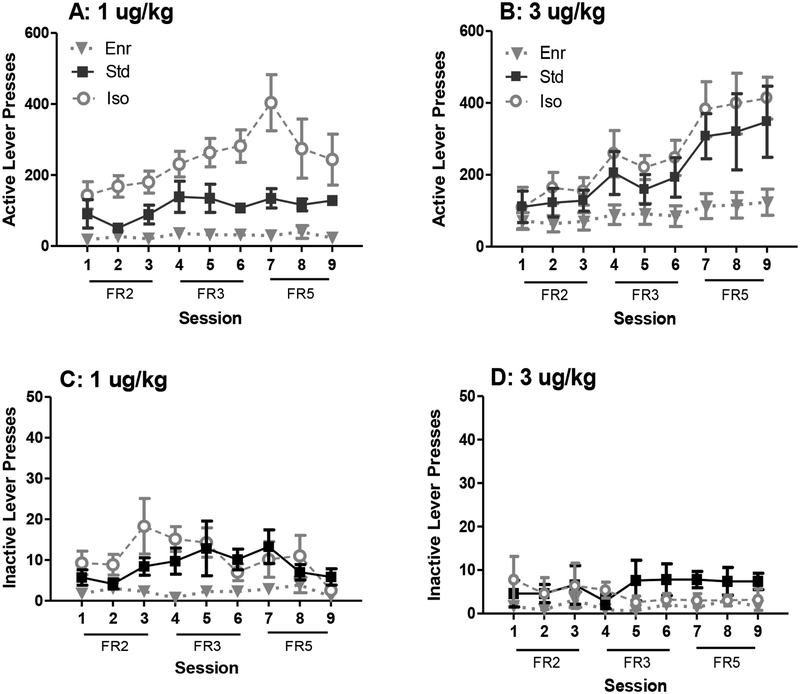
The results from the increasing FR phase of the experiment are presented in Figure 2. There were main effects of environment (F(2, 30) = 14.83, p < 0.05), training dose (F(1, 30) = 4.39, p < 0.05), and session (F(8, 240) = 21.14, p < 0.05) on active lever presses during this phase. There was also a significant environment by session interaction (F(16, 240) = 4.22, p < 0.05) and a training dose by session interaction (F(8, 240) = 21.14, p < 0.05). Across sessions, Tukey’s post hoc analyses revealed significant increases in active lever presses from the FR2 to the FR5 sessions in Iso and Std rats only (all p < 0.05); Enr rats did not significantly increase their responding as the FR requirement increased. Within sessions, Enr rats responded significantly less than Iso rats during the FR3 and FR5 sessions; Std rats also responded less than Iso rats on session 7 (i.e. the first day of FR5).
Figure 2: Effect of Environmental Enrichment on Remifentanil Self-Administration after Increasing Fixed Ratio Requirement.
Active lever presses (A) and inactive lever presses (C) after increasing FR by rats trained with 1 μg/kg/infusion remifentanil. Active lever presses (B) and inactive lever presses (D) by rats trained with 3 μg/kg/infusion remifentanil. Enr: gray triangles (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), Std: black squares (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), Iso: white circles (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg). Note the difference in scales between the upper and lower panels.
For inactive lever presses, there was a main effect of environment (F(2, 30) = 8.85, p < 0.05) and a main effect of training dose (F(1, 30) = 6.11, p < 0.05), indicating overall fewer inactive lever presses for Enr rats compared to Std and Iso rats, as well as overall more inactive lever presses with the 1 μg/kg/infusion training dose compared to the 3 μg/kg/infusion training dose.
Dose-Response
The results from the dose-response phase of the experiment are presented in Figure 3. For active lever presses, there was a main effect of remifentanil dose (F(5, 150) = 34.41, p < 0.05), a main effect of environment (F(2, 30) = 9.34, p < 0.05), and a dose by environment interaction (F(10, 150) = 3.11, p < 0.05). When trained with 1 μg/kg/infusion, Enr rats made significantly fewer active lever presses than Std and Iso rats across the dose-response curve; when trained with 3 μg/kg/infusion, Enr rats made fewer active lever presses than Iso rats across the dose-response curve (all p < 0.05). Regardless of training dose, there were no significant differences between Std and Iso rats at any dose.
Figure 3: Effect of Environmental Enrichment on the Remifentanil Dose Response Curve.
Active lever presses (A) and inactive lever presses (C) at saline, 0.1, 0.3, 1, 3, or 10 μg/kg/infusion remifentanil by rats trained with 1 μg/kg remifentanil. Active lever presses (B) and inactive lever presses (D) by rats trained with 3 μg/kg remifentanil. Enr: gray triangles (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), Std: black squares (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), Iso: white circles (n = 7 for 1 μg/kg; n = 5 for 3 μg/kg). Note the difference in scales between the upper and lower panels.
For inactive lever presses, there was a main effect of dose (F(5, 150) = 22.07, p < 0.05), a main effect of environment (F(2, 30) = 22.06, p < 0.05), a main effect of training dose (F(1, 30) = 7.61, p < 0.05), an environment by training dose interaction (F(2, 30) = 3.83, p < 0.05), a dose by training dose interaction (F(5, 150) = 5.07, p < 0.05), and a dose by environment by training dose interaction (F(10, 150) = 2.06, p < 0.05). When trained with 1 μg/kg/infusion, Enr rats pressed the inactive lever significantly less than Iso rats; when trained with 3 μg/kg/infusion, Enr rats pressed the inactive lever significantly less than Std and Iso rats. Enr rats also pressed the inactive lever significantly less than Iso and Std rats when self-administering saline (all p < 0.05).
Demand Curve
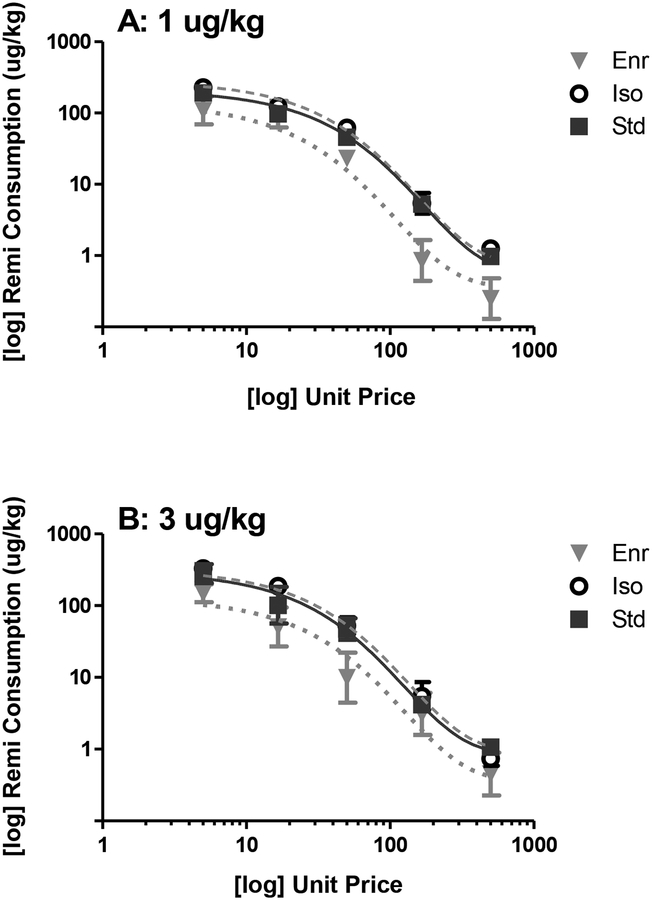
Conversion of the dose-response results to demand curves is depicted in Figure 4. Using a k value of 2.59, analyses revealed a significant main effect of environment on both Q0 (F(2, 121) = 11.38, p < 0.05) and α values (F(2, 121) = 3.10, p < 0.05), but there were no significant effects of training dose and no interactions. Enr rats had the lowest Q0 values when trained at 1 μg/kg (Enr: 2.07, Std: 2.35, Iso: 2.48) but not when trained at 3 μg/kg (Enr: 2.10, Std: 2.20, Iso: 2.08). Additionally, Enr rats had the highest α values (lowest essential value) when trained at 1 μg/kg (Enr: 0.0036, Std: 0.0026, Iso: 0.0027), but not when trained at 3 μg/kg (Enr: 0.0036, Std: 0.0044, Iso: 0.0036).
Figure 4: Effect of Environmental Enrichment on Demand Curves for Remifentanil.
Remifentanil demand curves for Enr (gray triangles; n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), Std (black squares; n = 7 for 1 μg/kg; n = 5 for 3 μg/kg), and Iso (white circles; n = 7 for 1 μg/kg; n = 5 for 3 μg/kg) rats that were trained with 1 μg/kg remifentanil (A) or 3 μg/kg remifentanil (B).
Discussion
The present results demonstrate that, similar to stimulant self-administration (Alvers et al. 2012; Bardo et al. 2001; Green et al. 2010), environmental enrichment starting in adolescence protects against opioid self-administration. Enr rats showed decreased acquisition and demonstrated a downward shift in the dose-response curves compared to both Std and Iso rats, regardless of initial training dose. Because Enr rats often have low rates of responding and sometimes do not acquire self-administration, no acquisition criterion was set for inclusion in the rest of the study. As such, the interpretation that Enr rats self-administer less remifentanil during the increasing FR phase and the dose-response assessment is complicated by the fact that their responding during acquisition was negligible at 1 μg/kg. Using common acquisition criteria, it is likely that most Enr rats would not have been included in this study. However, Enr rats trained at 1 μg/kg changed their responding across doses during the dose-response assessment, suggesting that while their response rates were low, they did learn the remifentanil-reinforced response contingency.
To fully elucidate the effect of enrichment on remifentanil intake over changing price, dose-response data were converted to demand curves. Analysis of these curves showed significant environment-induced changes in α, indicating that rearing environment altered the essential value of remifentanil; this is typically interpreted as a change in the demand for a reinforcer as its price increases. Additionally, Q0 significantly differed between Enr, Std, and Iso rats, indicating that rearing environment altered consumption of remifentanil as price approached zero (Bickel et al. 2010; Hursh and Silberberg 2008). Although α and Q0 can vary independently (Bickel et al. 2010), the fact that Enr rats had the lowest Q0 (when remifentanil would be free), and the greatest α (demand elasticity) at 1 μg/kg, demonstrates the benefit of enrichment in reducing opioid abuse liability. This is in contrast to stimulants, where Enr rats show only greater demand elasticity compared to Iso rats (Yates et al. under review). This can also be observed when comparing the dose response curves of remifentanil (Figure 3) to that of stimulants (Figure 5). Enr rats had lower response rates than Iso rats at low doses, regardless of drug. However, responding at high doses is lower for Enr rats taking remifentanil, unlike what has been observed for methylphenidate (MPD, Figure 5 top, used with permission from Alvers et al. 2012) and cocaine (Figure 5 bottom, used with permission from Green et al. 2010), where no significant differences are found between Enr and Iso rats at high doses.
Figure 5: Effect of Environmental Enrichment on Stimulant Dose Response Curves.
(Top) Methylphenidate dose response curve for Enr (EC, black circles) and Iso (IC, white squares) rats that were trained with 0.3 mg/kg/infusion. MPD: methylphenidate. Used with permission from (Alvers et al. 2012). (Bottom) Cocaine dose response curve for Enr (EC, black circles) and Iso (IC, white circles) rats that were trained with 0.5 mg/kg/infusion. Used with permission from (Green et al. 2010).
While Enr rats differed from both Std and Iso rats across all phases of the experiment, there was relatively little difference between Std and Iso rats in remifentanil self-administration, except during the increasing FR training phase using the lower training dose (1 μg/kg/infusion). In previous work with stimulant self-administration, some studies have shown group-housed or Std rats to have lower rates of self-administration compared to single-housed or Iso rats (Bardo et al. 2001; Boyle et al. 1991; Schenk et al. 1987), whereas other reports have shown no effect (Bozarth et al. 1989; Schenk et al. 1988). In a more relevant study, heroin self-administration was reduced in group-housed rats compared to single-housed rats (Bozarth et al. 1989). Similar to that latter study using heroin, the current study found remifentanil self-administration to be reduced in Std rats compared to Iso rats when the FR requirement was increased from FR1 to FR5. Since social interaction is known to activate endogenous opioid systems (Bertrand et al. 1997; D’Amato and Pavone 2012; Trezza et al. 2011), one potential explanation for the reduced self-administration in Std rats (and Enr rats) is that repeated social interaction may have reduced sensitivity to the reinforcing effect of remifentanil (Hofford et al. 2016). Regardless of the precise mechanism, however, reliable differences between Std and Iso rats did not occur in the dose-response evaluation. Thus, the current results indicate that, in addition to social interaction, repeated exposure to novel objects in the home cage is a critical determinant for altering remifentanil self-administration.
One limitation of the current study is that it only included males. As such, results cannot be extrapolated to females. Based on the available literature, it is possible that sex differences would be present in remifentanil self-administration using the current environmental enrichment paradigm. For example, female rodents are less sensitive than males to the antinociceptive and locomotor-sensitizing effects of prototypical opioids such as morphine (Baker and Ratka 2002; Hofford et al. 2010). In addition, females engage in different social behavior compared to males during adolescence (Pellis et al. 1997), which is the developmental period when rats were first placed in their respective environments in the current study. Interestingly, exposure to social play, which is more prevalent in males, is thought to release endogenous opioids in nucleus accumbens (Trezza et al. 2011) and this exposure to endogenous opioids is hypothesized to reduce sensitivity to other opioids (Hofford et al. 2016). However, potential sex differences in play-induced endogenous opioid release have not been examined. Future studies are needed to determine if environmental enrichment also protects against remifentanil self-administration in females.
Conclusion
This preclinical study enhances our understanding of the role of environmental enrichment on opioid self-administration, knowledge that may be useful for the development and implementation of effective prevention interventions in at-risk populations. While the extension of rodent data to humans should be done with caution, the current studies suggest that behavioral prevention programs that incorporate enriching and social activities may be especially beneficial, at least in males. Consistent with this idea, prevention programs have been developed that use enriching activities, peer influence, and stress reduction training to target children, adolescents and emerging adults (Barnett et al. 2014; D’Silva et al. 2001; Pentz 2014). However, longitudinal data are needed to determine if these environmental interventions reduce opioid abuse vulnerability later in life.
Funding and Disclosure:
This work was funded by NIH: DA012964, DA016176, DA035200, DA036291, and DA033373. The authors declare no financial conflicts of interest.
References
- Alexander BK, Coambs RB, Hadaway PF (1978) The effect of housing and gender on morphine self-administration in rats. Psychopharmacology (Berl) 58: 175–179. [DOI] [PubMed] [Google Scholar]
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT (2012) Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol 23: 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L, Ratka A (2002) Sex-specific differences in levels of morphine, morphine-3-glucuronide, and morphine antinociception in rats. Pain 95: 65–74. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C (2001) Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology 155: 278. [DOI] [PubMed] [Google Scholar]
- Barnett NP, Ott MQ, Rogers ML, Loxley M, Linkletter C, Clark MA (2014) Peer associations for substance use and exercise in a college student social network. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 33: 1134–42. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Smadja C, Mauborgne A, Roques BP, Dauge V (1997) Social interaction increases the extracellular levels of [Met]enkephalin in the nucleus accumbens of control but not of chronic mild stressed rats. Neuroscience 80: 17–20. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Mueller ET, Jones BA, Christensen DR (2010) The Behavioral Economics of Drug Dependence: Towards the Consilience of Economics and Behavioral Neuroscience In: Self WD, Staley Gottschalk KJ (eds) Behavioral Neuroscience of Drug Addiction. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 319–341 [DOI] [PubMed] [Google Scholar]
- Boyle AE, Gill K, Smith BR, Amit Z (1991) Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav 39: 269–74. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Murray A, Wise RA (1989) Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav 33: 903–907. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Pavone F (2012) Modulation of nociception by social factors in rodents: contribution of the opioid system. Psychopharmacology (Berl) 224: 189–200. [DOI] [PubMed] [Google Scholar]
- D’Silva MU, Harrington NG, Palmgreen P, Donohew L, Lorch EP (2001) Drug use prevention for the high sensation seeker: the role of alternative activities. Substance use & misuse 36: 373–85. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Creppage K, Austin A, Ringwalt C, Sanford C, Proescholdbell SK (2014) Observed transition from opioid analgesic deaths toward heroin. Drug and Alcohol Dependence 145: 238–241. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M (2009) Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology 203: 561–570. [DOI] [PubMed] [Google Scholar]
- Galaj E, Manuszak M, Ranaldi R (2016) Environmental enrichment as a potential intervention for heroin seeking. Drug and Alcohol Dependence 163: 195–201. [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DEH, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ (2010) Environmental Enrichment Produces a Behavioral Phenotype Mediated by Low Cyclic Adenosine Monophosphate Response Element Binding (CREB) Activity in the Nucleus Accumbens. Biol Psychiatry 67: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Powell BJ (1976) Cocaine and morphine self-administration: effects of differential rearing. Pharmacol Biochem Behav 5: 701–704. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL (2014) Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. The Journal of pharmacology and experimental therapeutics 348: 174–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL (2013) Stimulants as specific inducers of dopamine-independent sigma agonist self-administration in rats. The Journal of pharmacology and experimental therapeutics 347: 20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Beckmann JS, Bardo MT (2016) Rearing environment differentially modulates cocaine self-administration after opioid pretreatment: A behavioral economic analysis. Drug and Alcohol Dependence 167: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Roberts KW, Wellman PJ, Eitan S (2010) Social influences on morphine sensitization in adolescent females. Drug and Alcohol Dependence 110: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychological Review 115: 186–198. [DOI] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC (2015) The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction. Annual Review of Public Health 36: null. [DOI] [PubMed] [Google Scholar]
- Mark TL, Woody GE, Juday T, Kleber HD (2001) The economic costs of heroin addiction in the United States. Drug and Alcohol Dependence 61: 195–206. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Lewis MJ (1984) Early housing experience modifies morphine self-administration and physical dependence in adult rats. Addictive Behaviors 9: 235–243. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW (2000) Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology 150: 61. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, Pellis VC (1997) Multiple Differences in the Play Fighting of Male and Female Rats. Implications for the Causes and Functions of Play. Neuroscience & Biobehavioral Reviews 21: 105–120. [DOI] [PubMed] [Google Scholar]
- Pentz MA (2014) Integrating mindfulness into school-based substance use and other prevention programs. Substance use & misuse 49: 617–9. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D (2007) Linear and nonlinear mixed effects models R package version. 3: 57. R Foundation for Statistical Computing, Vienna, Austria: 1–89. [Google Scholar]
- Raz S, Berger BD (2010) Social isolation increases morphine intake: behavioral and psychopharmacological aspects. Behav Pharmacol 21: 39–46. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2014) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, Rockville, MD. [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z (1987) Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neuroscience letters 81: 227–31. [DOI] [PubMed] [Google Scholar]
- Schenk S, Robinson B, Amit Z (1988) Housing conditions fail to affect the intravenous self-administration of amphetamine. Pharmacol Biochem Behav 31: 59–62. [DOI] [PubMed] [Google Scholar]
- Smith M, Chisholm K, Bryant P, Greene J, McClean J, Stoops W, Yancey D (2005) Social and environmental influences on opioid sensitivity in rats: importance of an opioid’s relative efficacy at the mu-receptor. Psychopharmacology 181: 27–37. [DOI] [PubMed] [Google Scholar]
- Smith MA, Pitts EG (2011) Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol Biochem Behav 100: 237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavitian-Exley I, Vickerman P, Bastos FI, Boily M-C (2015) Influence of different drugs on HIV risk in people who inject: systematic review and meta-analysis. Addiction: n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ (2011) Nucleus Accumbens μ-Opioid Receptors Mediate Social Reward. The Journal of Neuroscience 31: 6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold LL, Sharpe LG, Jaffe JH (1993) Housing conditions influence acquisition of sufentanil aerosol self-administration in rats. Pharmacology Biochemistry and Behavior 44: 141–144. [DOI] [PubMed] [Google Scholar]
- Yates JR, Bardo MT, Beckmann JS (under review) Self-administration of drug and natural reinforcers in differentially reared rats: a behavioral economic analysis. Addictive Behaviors. [Google Scholar]