Abstract
Fibrous dysplasia is an uncommon mosaic disorder in which bone is replaced by structurally unsound fibro-osseous tissue. It is caused by the sporadic post-zygotic activating mutations in GNAS, resulting in dysregulated GαS-protein signaling in affected tissues. This manifests on a broad clinical spectrum ranging from insignificant solitary lesions to severe disease with deformities, fractures, functional impairment, and pain. Fibrous dysplasia may present in isolation or in association with hyperfunctioning endocrinopathies and café-au-lait macules, known as McCune-Albright Syndrome. This review summarizes current understanding of pathophysiology in fibrous dysplasia, describes key pre-clinical and clinical investigations, and details the current approach to diagnosis and management.
Keywords: bone disorders, bone metabolism, McCune-Albright syndrome, FGF23-mediated hypophosphatemia
Introduction
Fibrous dysplasia (FD) is an uncommon mosaic disorder resulting in replacement of normal bone with fibro-osseous tissue. The resulting skeleton is weakened and prone to fractures and deformity, resulting in pain and functional impairment. FD presents along a broad clinical spectrum due to varying degrees of mosaicism, resulting in disease that can range from asymptomatic to severely disabling. FD may occur in isolation, or in conjunction with extraskeletal disease, including hyperpigmented skin lesions and hyperfunctioning endocrinopathies. The combination of FD and one or more extraskeletal features is termed McCune- Albright syndrome (MAS).
Epidemiology
The epidemiology of FD/MAS is poorly understood. Mild, asymptomatic FD is often noted incidentally on imaging studies, and many patients likely go undiagnosed. In addition, fibro-osseous lesions are poorly characterized, which may result in an overdiagnosis of monostotic FD in patients with isolated bony lesions [1]. Additionally, patients who are diagnosed with “monostotic” FD may not be appropriately evaluated for polyostotic and endocrine disease, making it difficult to determine the relative prevalence of these subtypes. A metanalysis of 18 series including a total of 487 patients diagnosed with craniofacial FD reported that 56% had monostotic disease, 47% had polyostotic disease, and 7% had MAS [2]. It is unclear whether these patients were screened for subclinical endocrine disease, calling into question the generalizability of these findings.
Genetics and molecular mechanisms
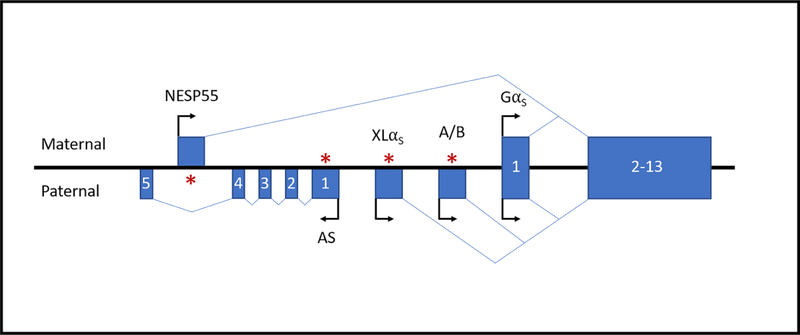
FD/MAS is caused by sporadic, post-zygotic mutations in the GNAS locus on chromosome 20q13.3 [3]. The human GNAS locus is highly complex and encodes four primary transcripts, GαS, XLαS, NESP55, and A/B, as well as an antisense transcript GNAS-AS1 (Fig 1). The sense transcripts are generated through the use of four distinct promoters and alternative splicing of four unique first exons to common exons 2–13. Allelic and tissue-dependent expression of the transcripts is regulated by differential methylation of the alternative promoters. XLsα, A/B, and GNAS-AS1 are selectively expressed from the paternal allele, whereas NESP55 is expressed from the maternal allele. In contrast, the GαS transcript is not imprinted in most tissues and exhibits random asymmetric bi-allelic expression in bone [4].
Figure 1. Schematic representation of the GNAS locus on chromosome 20q13.3.
The GNAS locus encodes four primary sense transcripts, GαS, XLαS,NESP55, and A/B, and an antisense transcript GNAS-AS1. The sense transcripts are generated by alternative splicing of four unique first exons to common exons 2–13. Allelic and tissue-dependent expression of the XLαS, NESP55, A/B, and GNAS-AS1 transcripts is regulated by differential methylation of their promoters. The GαS transcript exhibits random asymmetric bi-allelic expression in bone. Boxes and connecting lines represent the exons and introns, respectively. Arrows indicate the direction of transcription. Asterisks indicate methylation of the imprinted promoters on either the maternal or paternal allele. Figure is not drawn to scale.
All the known pathogenic mutations in FD/MAS result in the production of a constitutively active GαS signaling protein. Heterotrimeric G proteins, composed of Gα, Gα and Gα subunits, function as molecular switches that transduce signals from ligand-bound G protein-coupled receptors (GPCRs) on the cell surface to intracellular effectors. GαS belongs to a class of activating Gα subunits that signals by upregulating cyclic AMP (cAMP) production by adenylyl cyclase and activating protein kinase A. Gα proteins possess intrinsic GTPase activity, which regulates their signal-transducing activity by switching them from the active GTP-bound conformation to the inactive GDP-bound conformation. Agonist binding to GPCRs promotes GDP release from the Gα subunit, allowing GTP to bind and thereby activate the G protein [5]. More than 95% of GαS mutations in FD/MAS occur at the R201 position in exon 8, with the arginine most commonly replaced by either a cysteine or histidine residue [6, 7]. About 5% of mutations occur at the Q227 position in exon 9 [8]. Mutations at either codon inhibit the GTPase activity of GαS and lead to protracted downstream signaling [6, 9–11] (Fig 2). Additionally, recent evidence demonstrates that GDP-bound GαS bearing the R201C mutation is in an active conformation under physiologic conditions and can activate adenylyl cyclase [12]. There is limited published data that directly compares the effects of different pathogenic mutations on the enzymatic activity and downstream effects of GαS [10, 13]. Moreover, the relative clinical pathogenicity of different GαS mutations is unknown.
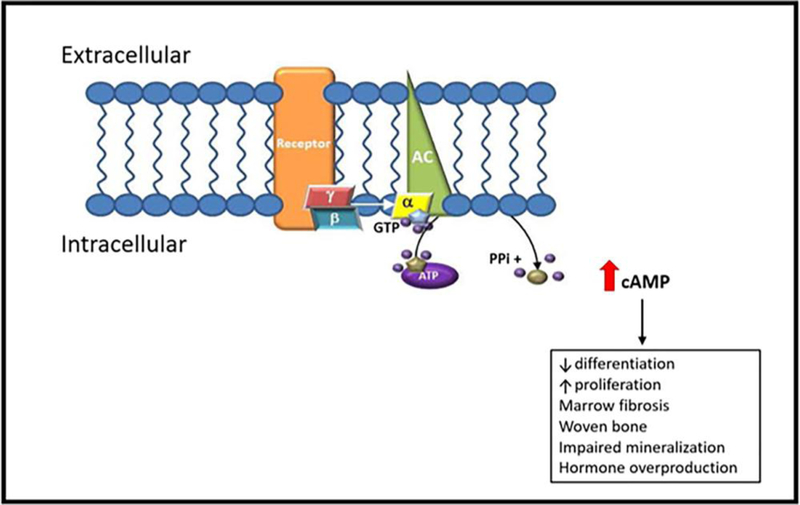
Figure 2. G protein-coupled signaling upregulation in FD/MAS.
Fibrous dysplasia is caused by post-zygotic mutations in GNAS, which result in the expression of a constitutively signaling GαS protein. The mutant GαS activates adenylyl cyclase in a ligand-independent manner, leading to excess production of cyclic AMP and protracted downstream signaling. In bone, this promotes the proliferation of immature osteoblast progenitor cells, which leads to formation of structurally abnormal matrix, with increased fibrosis, decreased mineralization, cortical thinning, and obliteration of hematopoietic bone marrow. AC, adenylyl cyclase; α/β/γ, alpha/beta/gamma-subunits of Gs; GTP, guanosine triphosphate; ATP, adenosine triphosphate; PPi, pyrophosphate; cAMP, cyclic adenosine monophosphate. Adapted from Burke AB, et al, Oral Dis. 2017 Sep; 23(6): 697–708.
Germline activating mutations in human GαS have not been described in the literature and are therefore presumed to be embryonic lethal. The classic pathogenic hypothesis of Happle posits that somatic mosaicism, which allows for close association of mutation-bearing cells with normal cells, is essential for the survival of less viable mutant cells in FD/MAS [14]. Therefore, the pathogenic mutations in FD/MAS are thought to occur postzygotically at an early stage in embryogenesis.
During development, mutant progeny cells migrate to different parts of the skeleton and extraskeletal tissues, resulting in mosaic expression of constitutively active GαS and leading to a broad spectrum of disease burden in affected individuals. An attractive and widely accepted theory attributes the diversity of disease expression to the developmental timing of the mutation, with earlier mutations resulting in more widespread disease. Most cases of polyostotic FD and MAS involve tissues derived from at least two different germ layers and, therefore, can only result from mutations that occur before gastrulation. For example, the proximal femur and craniofacial bones are the most commonly affected sites in FD, and, since these bones develop from the mesoderm and neural crest ectoderm, respectively, polyostotic FD affecting both sites in one individual can only result from a mutation event that occurs before irreversible lineage commitment [15].
The most common activating amino acid substitutions in GαS (R201C and R201H) result from transition mutations, which arise by deamination of the methylated cytosine base at the conserved CGT codon on either the sense or antisense DNA strand. Genomic imprinting involves a massive demethylation and de novo methylation event, which occurs at CG dinucleotides between the morula and blastocyst stage of embryogenesis. Since the GNAS locus is imprinted, an attractive hypothesis is that FD/MAS-causing mutations occur within this narrow developmental window before gastrulation [15]. However, the precise inciting event for these mutations is unknown. The lack of any obvious increased prevalence in different populations and geographic areas suggests the mutational event likely occurs randomly, however it is unknown if environmental or genetic factors may predispose certain individuals.
Alternative explanations for the broad spectrum of disease burden in FD include differences in the survival or migration capacity of mutant stem cells in different individuals. However, the molecular basis for these differences remains unclear.
Pathophysiology
FD results from the replacement of normal bone with abnormal, structurally unsound fibro-osseous tissue, with concomitant obliteration of the hematopoietic bone marrow. The fibrous tissue is rich in fibroblast-like cells that express markers of early stages of osteogenic maturation [16], suggesting that uncontrolled GαS-mediated signaling impairs the differentiation of osteogenic progenitors into mature osteoblasts and osteocytes. The immature osteoprogenitors proliferate and produce excess amounts of abnormal bone matrix, consisting predominantly of woven bone. Additionally, there is evidence of active osteoclastogenesis and increased bone resorption in the dysplastic bone [16–18]. Both FD lesions and cultured bone marrow stromal cells bearing activating GαS mutations produce high basal levels of IL-6, a cytokine that mediates osteoclastogenesis, likely in response to excess cAMP production by the mutant cells [18]. Therefore, dysplastic bone appears to undergo active remodeling, with accelerated rates of bone formation and resorption.
Nearly 50% of patients with FD/MAS exhibit some degree of renal phosphate wasting [19]. This has been attributed to the elevated levels of circulating FGF23 in patients with FD compared to age-matched controls [20]. FGF23 is an essential regulator of phosphate homeostasis and is normally produced by osteoblasts and osteocytes that are actively involved in bone formation [20]. The hormone inhibits renal phosphate reabsorption by downregulating the expression of renal tubular phosphate transporters, NaPi-2a and NaPi-2c. Additionally, FGF23 decreases the levels of active 1,25-dihydroxy vitamin D and, thereby, decreases intestinal phosphate absorption by suppressing 1α-hydroxylase activity and increasing 24α-hydroxylase activity [21, 22]. In FD lesions, FGF23 is produced by immature osteogenic cells lining the bone trabeculae, as well as by mature osteocytes. FGF23 levels correlate with the skeletal disease burden and with disease activity, suggesting that excess FGF23 production results from the accumulation of active osteogenic cells within FD lesions, rather than from excessive production at the single-cell level [20]. Notably, the incidence of frank hypophosphatemic rickets/osteomalacia is very low, despite the high circulating levels of FGF23. This has been attributed to the increased cleavage and inactivation of intact FGF23 by furin proteases in osteogenic cells expressing the active GαS mutant protein [23].
Histopathology
FD has several unique histologic features which can sometimes be helpful in diagnosis when clinical presentation is ambiguous (Fig 3). Osteogenic cells at the surface of bone trabeculae typically have a retracted cell body, resulting in a stellate appearance. This morphology appears to be dependent on cAMP, as cultured rat osteoblasts have been shown to assume a stellate shape upon exposure to excess cAMP and revert to their normal cuboidal shape after removal of cAMP [16]. The abnormal osteoblasts deposit collagen bundles perpendicularly (instead of parallel) to the forming bone surface, resulting in the formation of comb-like Sharpey’s fibers at the trabecular-stromal interface. The trabeculae consist predominantly of woven bone, with large lacunae accommodating multiple developing osteocytes and excess osteoid, reflecting undermineralization. Interestingly, “normalization” of dysplastic features has been demonstrated on histopathology of FD lesions from older patients, with increased lamellar architecture of bone trabeculae and cuboidal, rather than stellate, morphology of lining osteoblasts [24].
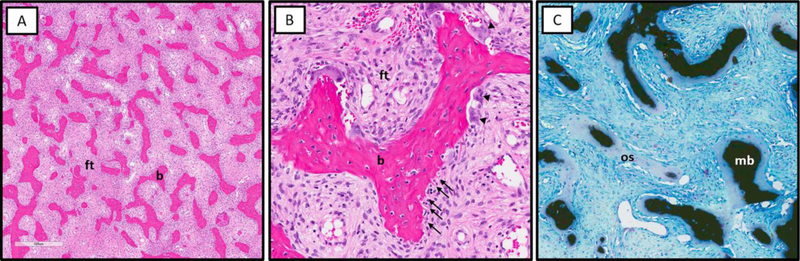
Figure 3. Characteristic histologic features of fibrous dysplasia.
A. Sharpey’s fibers (collagen fibers oriented perpendicular to the bone surface, arrows) and abundant osteoclasts (arrowheads) at the surface of FD bone trabeculae. B. Von Kassa staining showing undermineralization of FD bone with excess osteoid (os) and paucity of mineralized bone matrix (mb). C. Osteogenic cells on the surface of bone trabeculae have a retracted “stellate” appearance (arrows).
Dysplastic lesions exhibit distinct histologic patterns in different types of bone [17]. In the axial and appendicular skeleton, FD lesions commonly have a pattern characterized by thin, irregularly arranged, discontinuous trabeculae, consisting mostly of woven bone, surrounded by abundant fibrous stroma (Fig 4A). These lesions exhibit active osteoclastic resorption, which frequently occurs in the interior of trabeculae (termed dissecting resorption). The sclerotic/pagetoid type of FD is commonly seen in cranial bones and consists of thick, interconnected, non-lamellar trabeculae with less abundant surrounding fibrous tissue (Fig 4B). The trabecular bone matrix contains a rich system of cement/arrest lines, which resembles the Schmorl’s mosaic pattern observed in Paget’s disease.
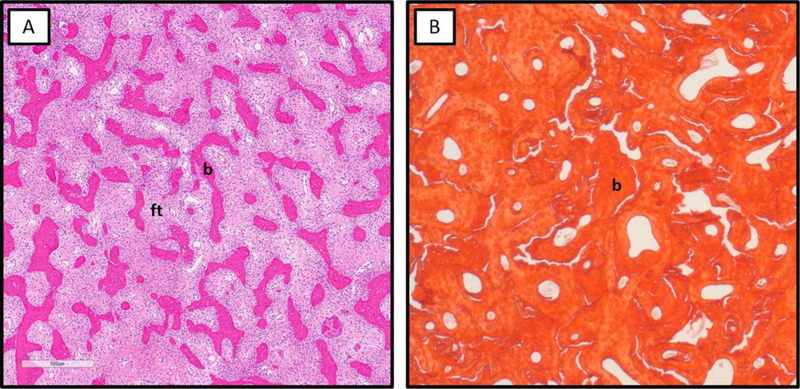
Figure 4. Histologic patterns of fibrous dysplasia.
A. Typical pattern of FD bone with thin discontinuous trabeculae (b) surrounded by abundant fibrous tissue (ft). B. Sclerosing/pagetoid pattern of FD bone with thick, interconnected trabeculae consisting of woven bone and relative paucity of fibrous tissue.
Animal models
Animal models have provided valuable insight into the pathophysiology of FD and have enabled more precise elucidation of the signaling mechanisms involved in the development and persistence of FD lesions. However, development of animal models that accurately replicate the human FD phenotype has been challenging.
The first animal models of FD involved heterotopic xenotransplantation of human bone marrow stromal cells (BMSCs) into the subcutaneous tissue of immunocompromised mice. Transplantation of BMSC from normal human bone generated ossicles comprised of donor-derived bone and marrow, which contained normal adipocytes and supported host-derived hematopoiesis. In contrast, transplantation of a clonal population of mutant BMSCs from FD lesions resulted in loss of the graft without ossicle formation. However, transplantation of a mixed population of mutant and wild-type BMSCs from FD lesions generated dysplastic ossicles, which reproduced several features of human FD, including thin trabeculae consisting largely of woven bone and containing Sharpey’s fibers, perpendicular arrangement of osteogenic cells with respect to the bone surface, abundance of fibrous stroma, and absence of hematopoietic marrow [25]. Together, these findings support Happle’s hypothesis, demonstrating that osteogenic progenitor cells bearing the activating GαS mutation cannot survive on their own and that FD lesions are themselves mosaics of mutant and normal cells. Notably, transplanted BMSCs from “normalized” FD lesions of older individuals led to the formation of normal-appearing ossicles [24]. Moreover, these lesions had a lower mutation burden than classic FD lesions of younger individuals, suggesting that mutation-bearing osteogenic progenitors have a limited replicative lifespan and are gradually lost by apoptosis from the mosaic population within FD lesions.
Hsiao, et al [26] generated a transgenic mouse model expressing an engineered GPCR (“Rs1”) with constitutive Gs signaling. Tetracycline-inducible expression of the mutant receptor in murine osteoblasts led to dramatic expansion of trabecular bone volume, increase in whole body bone mineral density, and gradual obliteration of the cortical shell and bone marrow cavity in the mouse femur. The lesions expressed elevated levels of osteoblast lineage and osteoclast activity markers [26, 27]. Mice expressing the engineered receptor at gestation were phenotypically normal at birth and began showing the first histologic signs of abnormal bone expansion at postnatal day 5, reflecting the age-dependent effects of constitutive Gs signaling. The phenotype was attenuated when expression of the mutant receptor was delayed until after birth and partially reversible upon suppression of mutant GPCR expression [27].
Saggio, et al [28] described the first transgenic mouse model expressing the R201C mutant GαS in all tissues. The transgenic mice postnatally developed skeletal lesions, which evolved through several age-dependent phases before exhibiting the bona fide histologic features of FD in aging mice, including effacement of cortico-trabecular boundaries, discontinuous bony trabeculae, and fibrotic stroma. Overall, the model demonstrated that expression of a constitutively active GαS is sufficient to establish FD in aging mice. However, it failed to replicate the phenotype in juvenile mice. Interestingly, large portions of the skeleton remained unaffected, despite the absence of mosaicism, suggesting that local factors, such as differentially expressed phosphodiesterases, may regulate the downstream signaling of the constitutively active G proteins and phenotypic expression of the disease. Surprisingly, constitutive expression of the mutant GαS was not embryonic lethal, which may potentially be attributed to the intact native GNAS locus in the transgenic animals.
Several groups have recently reproduced the human FD phenotype in the long bones of mice by inducing tissue-specific expression of mutant (R201C or R201H) GαS in the early limb bud osteochondral progenitors [29–31]. Early embryogenic induction of mutant GαS expression led to severe limb deformities at birth with the long bones exhibiting hallmark features of FD and disorganized and expanded growth plate cartilage. Postnatal induction of mutant GαS expression led to the formation of polyostotic FD-like lesions within 2 weeks, and withdrawal of mutant GαS expression led to improvement in the skeletal lesions [31]. The bone lesions showed evidence of increased osteoclastogenesis and bone resorption.
Affected mice exhibited enhanced bone-specific expression of early osteogenic markers and poor expression of late osteogenic markers, consistent with impaired osteoblast maturation. There was also increased expression of β-catenin and its target genes. The importance of Wnt/β-catenin signaling for the development of FD was further confirmed by the rescue of the FD phenotype and partial reversal of the osteogenic marker expression profile in mice missing one copy of the Wnt co-receptor gene LRP6 or by treatment of the mutant mice with a small molecule inhibitor of Wnt signaling [30].
Clinical Features
Skeletal Manifestations
The clinical sequalae in FD are determined primarily by the location and extent of skeletal lesions. There is a wide clinical spectrum, ranging from incidental and insignificant solitary lesions to widespread disease. FD lesions may be monostotic, occurring in one bone, or polyostotic, occurring in multiple bones. By convention, solitary lesions that contain multiple contiguous bones, as found in craniofacial FD, are considered monostotic. Complications of bone lesions may include pain, fractures, deformity, and functional impairment. Clinical bone lesions usually present prior to 5 years old, and the majority of lesions are present by age 15 [32].
The prototypical FD lesion is homogeneous and radiolucent on radiographs and computed tomography scan, with a “ground glass” appearance (Figs 5 and 6). Other features include cortical thinning, endosteal scalloping and a so-called ‘rind sign’ consisting of a sclerotic bone layer encasing an area of radiolucency (Fig 5C) [33]. The diagnosis can be made based on imaging findings alone, particularly when accompanied by characteristic skin lesions or hyperfunctioning endocrinopathies. However, isolated monostotic FD without accompanying extraskeletal features typically requires a biopsy, preferably with genetic testing on affected tissue, to establish the diagnosis.
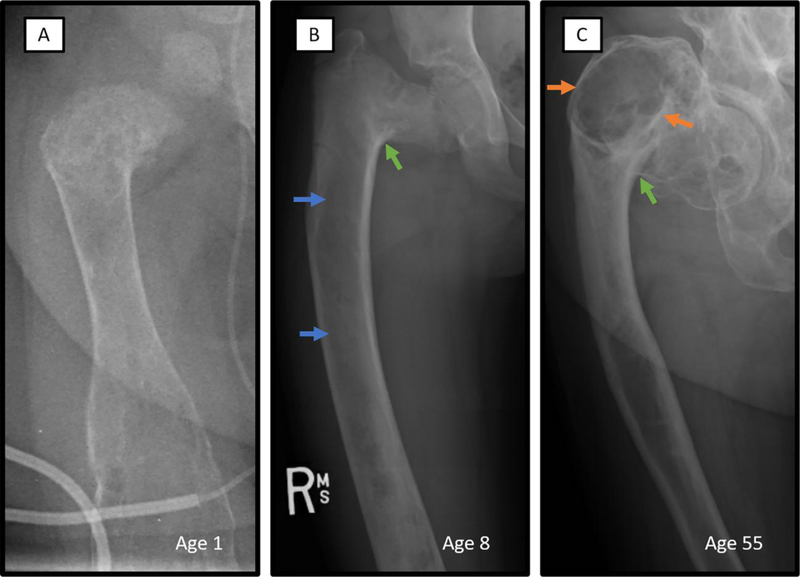
Figure 5. Radiographic features of fibrous dysplasia.
Typical X-ray features of FD, including age-related progression, is demonstrated in three different patients with extensive involvement of the right femur. A. Radiograph of the right femur of a 1-year-old with has the heterogeneous appearance commonly seen in infants with FD. B. Prototypical FD lesion of an 8-year-old demonstrates homogeneous ‘ground glass’ radiolucency (blue arrows) and severe shepherd’s crook deformity (green arrow). C. Sclerotic-appearing FD lesion of older adulthood is shown in the right femur radiograph of a 57-year-old. Typical ‘rind’ sign consisting of a radiolucent lesion surrounded by sclerotic bone (orange arrows) and a severe shepherd’s crook deformity (green arrow) are shown.
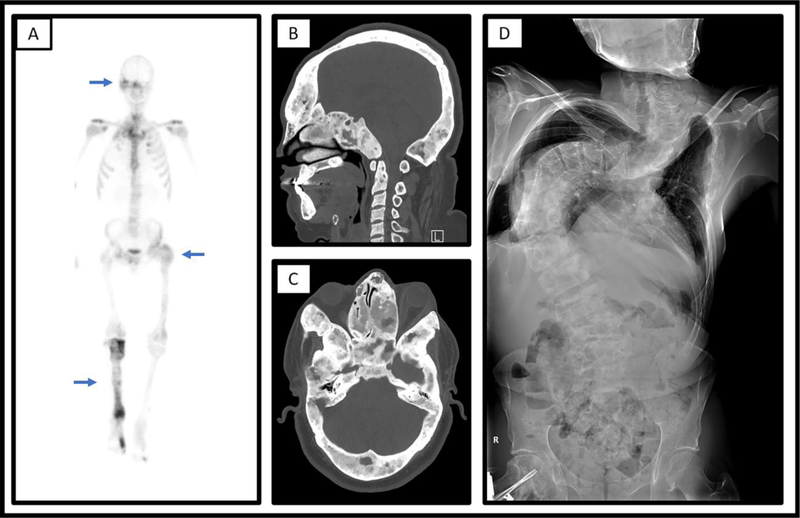
Figure 6. Characteristic imaging findings in fibrous dysplasia.
A. Numerous areas of increased uptake on Technetium-MDP bone scan (blue arrows) consistent with polyostotic fibrous dysplasia in an adult man. B/C. Computed tomography scan shows extensive involvement (blue arrows) of the skull and facial bones in a 28-year-old male with craniofacial FD, with diffuse areas of expansile bone “ground glass” appearing bone. Shown are sagittal (B) and transverse (C) views. D. Radiographs show severe scoliosis in a 36-year-old man with polyostotic fibrous dysplasia/McCune Albright syndrome.
The natural history of FD lesions results in changing imaging appearance over time. In infants and young children, lesions often appear radiographically heterogeneous (Fig 5A). They then take on the typical homogeneous ground glass appearance in late childhood and early adulthood (Fig 5B). In older adults, lesions adopt a more heterogeneous, sclerotic appearance (Fig 5C) [34]. Lesions in the craniofacial region are best visualized with CT or MRI. On CT, FD lesions tend to develop a more heterogeneous appearance with discrete radiolucent regions over time (Fig 7) [33].
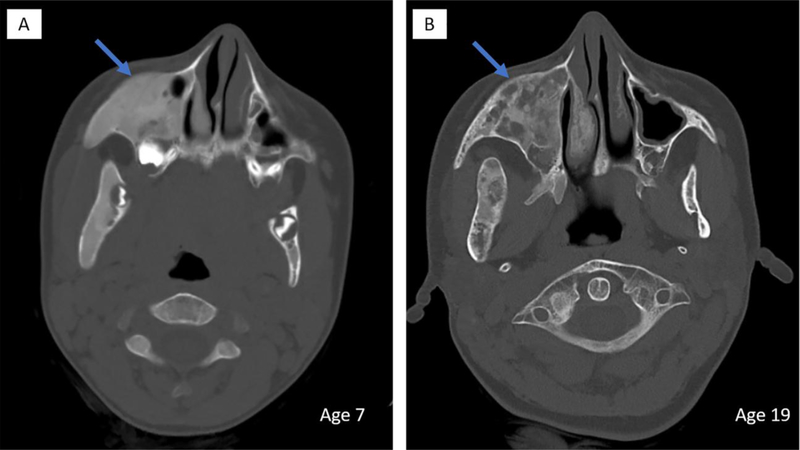
Figure 7. Age-related radiographic changes in craniofacial fibrous dysplasia.
Head CT scans of the same patient at age 7 (A) and age 19 (B) demonstrates typical age-related radiographic changes in the appearance of craniofacial FD. Areas of homogeneous appearing abnormal bone in the right maxilla adopts a more heterogeneous appearance with radiolucent regions with increased age (blue arrows).
Appendicular skeleton
FD in the appendicular skeleton results in bowing, pain, fractures, gait disturbances, and leg-length discrepancies. Patients frequently present in childhood with a limp, pain, or pathologic fracture. The peak incidence of fractures occurs between 6 and 10 years of age [35]. The proximal femur is commonly affected, and classically develops a coxa vara deformity, sometimes referred to as a “shepherd’s crook” deformity, due to mechanical stress and fractures in the area [36] (Fig 5). Coxa valga and mixed varus/valgus “windswept” deformities are also possible.
Axillary skeleton
Spinal involvement is common in patients with polyostotic FD, with reported prevalence ranging 21–63% [37, 38]. Involvement of the spine or pelvis is associated with development of scoliosis in 33–52% of patients with polyostotic FD (Fig 6D) [37, 38]. In a cohort of 138 patients with FD, progressive scoliosis was positively associated with total skeletal disease burden, FGF23-mediated hypophosphatemia, hyperthyroidism, and leg length discrepancies [39]. Untreated scoliosis can result in significant deformity and, rarely, mortality due to pulmonary compromise [34, 39]. Rib involvement is common, and can be painful.
Craniofacial skeleton
The craniofacial region is the most commonly involved area, affecting as many as 87% of patients with polyostotic FD [40]. Due to the high concentration of vital structures in this area, it can result in serious complications and presents unique challenges to management. FD in this location develops particularly early, with the majority of the lesions established at 3 years of age [32]. It usually presents as a slow-growing, painless mass resulting in facial or cranial asymmetry [41]. Patients are at risk for cosmetic deformity, dental problems, vision and hearing impairment, as well as potentially life-threatening cranial base abnormalities [42]. FD can also involve the sinuses, nasal cavity and jaw bones resulting in congestion, nasal constriction, malocclusion, and dental crowding [41]. One series of 66 patients diagnosed with craniofacial FD found that 36% were asymptomatic [43].
Although encasement of the optic nerve is common, vision loss is a rare occurrence and is often associated with untreated endocrinopathies, particularly growth hormone excess [44]. In a cohort of 38 patients with FD involving the sphenoid bone, 67 optic canals had radiologic evidence of encasement, however only two patients had abnormal neuro-ophthalmologic exams [40]. In patients with optic nerve compression leading to vision loss, optic nerve decompression can result in restoration of vision. However, prophylactic optic nerve decompression is associated with increased risk of vision loss and is contraindicated [45].
Patients with temporal bone FD are at increased risk for both sensorineural and conductive hearing loss. In a cohort of 183 temporal bones affected by FD, 22% were found to have hearing loss, usually mild and non-progressive. Hearing loss was conductive in 66%, sensorineural in 29%, and mixed in 5% of cases [46]. Internal auditory canal elongation and FD involvement of the ossicular chain were the most common causes identified, and external auditory canal stenosis and otic capsule invasion occurred less frequently.
Cranial base abnormalities in FD are of concern due to the potential risk of devastating neurologic sequela. A recent study of 158 patients with craniofacial FD found that 6.3% had Chiari I malformation, while 7.6 % had secondary basilar invagination [47].
FGF23-mediated Hypophosphatemia
Most patients with FD/MAS exhibit some degree of renal phosphate wasting, however overt hypophosphatemia is uncommon and typically affects patients with greater overall disease burden [20]. Affected patients can present in childhood with rickets, or in adulthood with more subtle signs of osteomalacia. Hypophosphatemia has been shown to be associated with an earlier age of first fracture, greater fracture rate, higher risk for skeletal deformities, and may contribute to the development of bone pain [35, 48]. Unlike other MAS-associated endocrinopathies, hypophosphatemia may wax and wane over a patient’s lifespan, depending on skeletal lesion activity and phosphorus requirements. Patients may be particularly vulnerable during periods of rapid linear growth, such as infancy and adolescence.
Bone pain
Pain is a common complication of FD, and in severe cases can be debilitating. Interestingly, bone pain severity does not correlate with the extent of skeletal disease burden [49]. Pain is more common and severe in adults than in children, however children may be less likely than adults to receive treatment for pain [49]. Clinicians should be aware of difficulties in assessing pain in young children, who may complain of feeling ‘tired’ instead of explicitly reporting pain [50].
Extra-skeletal Manifestations
MAS was initially described in 1936 as a classic triad of precocious puberty, FD, and café au lait skin pigmentation [51]. However, over time additional associated features have been identified and are now considered part of this condition. Similar to FD lesions, the extent and presence of extra-skeletal manifestations are also highly variable and dependent on the mosaic distribution of the disease.
Skin Findings
Café-au-lait skin macules are the earliest apparent manifestation of MAS, presenting at or shortly after birth (Fig 8). These hyperpigmented macules typically have characteristic jagged “coast of Maine” borders, in contrast to the smooth “coast of California” borders observed in neurofibromatosis. MAS-associated macules may cross the midline, but characteristically ‘respect’ or have some association to the midline of the body, reflecting patterns of early embryonic cell migration [52]. The distribution of skin lesions does not correlate with the location of bone lesions or other extraskeletal manifestations [52].
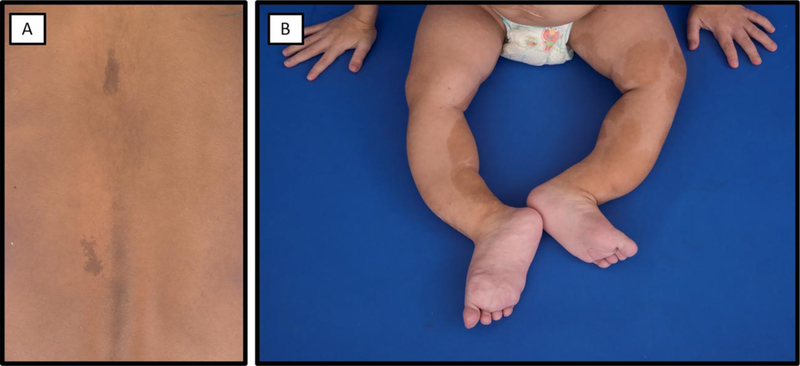
Figure 8. Typical café-au-lait skin macules in McCune-Albright syndrome.
A. An 8-year-old girl has small café-au-lait macules reflecting along the midline of the back. B) A 2-year-old girl has extensive café-au-lait macules on her bilateral lower extremities. Note the bowing deformities resulting from severe fibrous dysplasia.
Endocrinopathies
Gonadotropin-independent precocious puberty is often the presenting feature in girls with MAS, affecting approximately 85% of female patients [52]. Girls develop recurrent ovarian cysts leading to intermittent autonomous estrogen production. Patients may come to attention at the time of cyst resolution, when declining estrogen levels lead to vaginal bleeding [52]. Testicular abnormalities affect approximately 85% of boys and men, and may present with macro-orchidism on exam. Histologic findings include Leydig and Sertoli cell hyperplasia, which on ultrasonography are visible as discrete lesions, microlithiasis, or diffuse heterogeneity [53]. Precocious puberty develops in only a minority of boys (approximately 15%), and presents with continuous testosterone production and signs of progressive androgenization.
Thyroid abnormalities affect approximately 2/3 of patients with MAS, including discrete nodules and diffuse heterogeneity on ultrasonography [13, 54]. Approximately half of patients with ultrasound abnormalities develop frank hyperthyroidism [52], due to a combination of ligand-independent activation of the TSH receptor and increased conversion of T3 to T4 [13, 54].
Growth hormone excess arises due to GNAS mutations in the anterior pituitary and is found in 10–20% of patients with MAS [52, 55]. In most patients, growth hormone excess occurs concurrently with hyperprolactinemia. Growth hormone excess is an important contributor to morbidity in patients with craniofacial FD, and is associated with a higher prevalence of macrocephaly, optic neuropathy, and hearing loss [56–58].
Neonatal hypercortisolism is a very rare but potentially devastating feature of MAS, occurring in up to 7.1% of patients [59]. Hypercortisolism arises due to autonomous hypersecretion from the fetal adrenal gland; for this reason disease typically presents shortly after birth, and almost exclusively in the first 12 months of life [59, 60]. Unlike other MAS-associated endocrinopathies, patients may experience spontaneous remission of their hypercortisolemia if they are able to survive the acute phase of the disease. Patients are subsequently at risk for developmental sequelae and adrenal insufficiency.
Malignant transformation
Skeletal malignancies reported to originate from FD lesions include osteosarcoma, fibrosarcoma, chondrosarcoma, and malignant fibrohistiocytoma [61]. The prevalence is difficult to accurately determine due to rarity of FD, uncertainties in diagnosis, and potential referral bias in tertiary care centers. The largest study addressing this question reported a 2.5% prevalence of sarcomas in a cohort of 1122 cases of FD [61]. This is likely influenced by referral bias, in which case the true prevalence may be considerably lower. Malignancy should be suspected if a patient presents with a rapidly expanding lesion associated with new focal pain or paresthesia. Radiographically, cortical destruction, osteolysis, and rapid growth of a new soft tissue mass are suggestive of malignancy. These symptoms should be distinguished from aneurysmal bone cysts, which are benign, rapidly expanding fluid-filled lesions that can result in significant pain and compressive sequelae. Aneurysmal bone cysts are best visualized with MRI and are treated surgically [33].
Several cancers and pre-cancerous lesions have been associated with MAS. In particular, women with MAS have a 3.4–4.5-fold increased risk of breast cancer with a young age of onset (median age 36–46), suggesting that early breast cancer screening is warranted in this population [62]. Other cancers and premalignant neoplasms have been sporadically reported in association with MAS, often with activating GNAS mutations identified at the focus of the tumor. These include thyroid carcinoma, testicular cancer, intraductal papillary mucinous neoplasms (IPMNs), and rare pancreatic carcinomas and hepatobiliary neoplasms [53, 63–65].
Evaluation and Management
Skeletal disease
Management in FD is supportive, with the goal of optimizing function and minimizing morbidity. There are no known medical therapies capable of altering the course of FD, although anti-resorptive therapy may have a role in reducing pain. Prognosis is largely determined by disease burden, so therapy must be individualized given the wide variability in phenotype. Ideally, treatment should be orchestrated by a multidisciplinary team consisting of endocrinologists, orthopedists, dentists, physiatrists, radiologists, and other subspecialists, including ophthalmologists, otolaryngologists, and neurosurgeons, depending on disease involvement. Treatment often consists of surgery to preserve function and reduce pain, as well as conservative techniques that may include a combination of physical therapy, orthoses, avoidance of prolonged immobilization, and management of underlying endocrinopathies [66].
Once the diagnosis is made, the extent of disease burden should be determined by skeletal scintigraphy, such as a technetium-99 scan. Following baseline studies, plain radiographs are usually sufficient for evaluating the limbs and axial skeleton and should be obtained based on clinical exam. Non-contrast CT of the head is the best imaging modality for assessing craniofacial FD. When clinical suspicion is high, CT or MRI can be used to discover occult fractures, and MRI should be used to evaluate for aneurysmal bone cysts [34]. Disease activity is also reflected in the elevation of bone turnover markers [19]. Alkaline phosphatase is a reasonable cost-conscious option to biochemically track the extent of active disease [34].
Pain in FD may be multifactorial and requires careful clinical evaluation [49]. Any new or focal acute pain should be assessed for underlying fracture or malignant transformation. Patients should be assessed for underlying endocrinopathies, particularly hypophosphatemia, which can contribute to bone pain. Conservative treatments with non-opioid analgesics, heat, massage, and other modalities are often effective. Intravenous bisphosphonates are used to treat moderate to severe bone pain, although the efficacy of this has not been evaluated in a controlled manner, discussed below.
Anti-resorptive agents such as bisphosphonates and denosumab have been advocated as promising potential treatment options for FD due to the overexpression of RANKL and high osteoclastic activity in FD lesions [18, 67]. Studies of bisphosphonate effects on FD pain, progression, and disease activity have shown conflicting results. Initial uncontrolled studies have demonstrated the ability of bisphosphonates, usually intravenous pamidronate, to decrease bone pain and normalize bone turnover markers, and only some studies have shown improvement in the radiographic appearance of FD bone lesions [68–73]. However, the only randomized, double blind placebo-controlled trial of a bisphosphonate in FD showed oral alendronate to have no effect on bone pain or radiographic appearance compared to placebo [74]. There is still a potential role for intravenous bisphosphonates based on previous studies and expert experience, as they may be more effective in treating bone pain; however, this has not yet been demonstrated in a controlled manner [75]. Of note, bisphosphonate-related osteonecrosis of the jaw has been reported in patients with FD, highlighting the need for caution in patients receiving long-term treatment with potent intravenous formulations [76].
The use of denosumab, a humanized monoclonal antibody to RANKL and a strong anti-resorptive agent, has been described in a handful of case reports of FD, which have shown improvements in pain, bone turnover markers, and, in one case, tumor growth [77–79]. However, treatment was complicated by serious side effects, including transient hypocalcemia, hypophosphatemia, and secondary hyperparathyroidism during therapy and severe rebound hypercalcemia on cessation. Future controlled trials are needed to determine the efficacy and safety of denosumab therapy. Given the risk of serious side effects, denosumab should only be used in the setting of a clinical trial.
Optimal surgical management of FD is technically and strategically challenging, so patients should ideally be evaluated by surgeons with significant experience with FD. In general, for treatment of weight-bearing long bones, intramedullary devices are preferred over the previously favored options of curettage, grafting, plates and screws, and external fixation. Curettage of diseased bone followed by either allo- or auto-grafts is of limited value, but can be considered in adult patients with monostotic disease and in selected polyostotic cases where temporary control is required [80, 81]. In young patients with polyostotic disease, however, the majority of grafts fail to incorporate, and the graft eventually resorbs and becomes replaced with FD [82]. The compromised strength of diseased bone limits the effectiveness of other types of internal fixation, such as plates and screws, unless they can be attached to normal bone [80].
Fractures in the weight-bearing lower extremities, or in the upper extremities of patients using assistive devices, may benefit from internal fixation. Non-weight bearing status should be minimized to prevent further bone resorption and fragility from immobilization [80]. Remodeling and correction of residual angulation may not occur spontaneously in bone affected by FD, and angulation should be corrected prior to allowing fractures to heal.
Scoliosis should be monitored regularly, and surgical management should be considered early to prevent progression. Conservative management should focus on balancing forces that have an impact on spinal alignment, such as pelvic girdle weakness and leg length discrepancy. Bracing may be of limited use in patients with concomitant rib and pelvic lesions. Standard surgical fusion has been used successfully and may be lifesaving, with the caveat that fixation should ideally be attached to non-involved vertebra for stability instead of weak FD-involved bone [39, 80]. Bisphosphonate therapy has not been shown to be effective in halting progression of spinal curvature [39].
Physical therapy is an important component of FD management. Aquatic therapy, biking, and other low-impact exercises are appropriate options to maintain muscle strength without further increasing the risk of fractures [66]. Leg length discrepancy should be managed conservatively with lifts and orthotics. Assistive ambulation devices (if needed) should be targeted to each individual’s mechanics and weight-bearing forces to reduce the risk of developing progressive deformities.
Patients with craniofacial FD should undergo regular monitoring for functional deficits, including neuro-ophthalmologic and audiology evaluations at least annually, and periodic CT imaging to evaluate lesion growth. Debulking and recontouring operations can be performed to improve aesthetics and function; however, outcomes are often suboptimal due to frequent postoperative regrowth of lesions, especially in the setting of untreated growth hormone excess [41, 83]. Growth hormone excess and other endocrinopathies should be screened for and treated prior to surgery.
Although previously favored, prophylactic optic nerve decompression is no longer recommended even in the setting of radiographic encasement of the optic canal. A meta-analysis including 241 patients with fibrous dysplasia suggests that surgery in asymptomatic patients actually increases the risk of vision loss [45]. Instead of prophylactic surgery, expectant management consists of routine eye exams with ocular coherence tomography and evoked potentials. Subsequent surgery is only indicated when visual compromise is identified [41]. Untreated growth hormone excess is associated with vision loss, so this should be treated as early as possible [40].
Due to the risk for cranial base abnormalities, a full neurologic exam should be performed regularly in patients with craniofacial FD, and the cranial base should be evaluated radiographically at school age and when symptomatic [47].
Endocrinopathies
All patients with polyostotic FD should be evaluated for MAS-associated endocrinopathies. Management of endocrinopathies is an important aspect of overall care of patients with MAS given that they can often exacerbate bone disease. Precocious puberty in girls is generally treated with the third generation aromatase inhibitor letrozole [84]. The selective estrogen-receptor modulator tamoxifen is also a treatment option, but should be used with caution due to the increased risk of endometrial hyperplasia [85]. In boys, testicular lesions are managed conservatively with serial exam and ultrasound. Palpable, locally invasive, or rapidly growing lesions are suspicious for malignancy and may require surgical removal [53]. Precocious puberty in boys can be adequately treated with aromatase inhibitors and androgen receptor blockers [53]. Children of both sexes may develop secondary central precocious puberty and benefit from the addition of leuprolide [53, 84]. Hyperthyroidism is treated with anti-thyroid medications or total thyroidectomy [13, 54]. Radioablation may also be effective, however there is a theoretical risk of subtherapeutic radiation exposure to unaffected areas of the gland. Growth hormone excess typically responds to treatment with somatostatin analogues or growth hormone receptor blockers [86]. However patients with severe disease may require surgical treatment, which typically necessitates total hypophysectomy due to diffuse involvement with GNAS mutation-bearing somatolactotroph cells [86, 87]. Pituitary radiation should be used only as final recourse in patients with severe disease, due to the risk of subsequent malignant transformation in skull-base FD [88, 89]. Hypophosphatemia is managed similarly to other diseases of FGF23 excess with oral phosphorous and activated vitamin D analogues [90].
Future Directions
The development of medical therapies to treat FD is a critical area of need. Prospective controlled studies are needed to determine the efficacy and safety of anti-resorptive medications, including bisphosphonates and denosumab. Inhibition of IL-6 is a potential treatment strategy that has been advocated based on increased IL-6 production in FD tissue [18, 91, 92], and studies using the monoclonal inhibitor tocilizumab in FD are ongoing. Altering the activity of the mutant Gαs provides the opportunity to selectively target involved tissues, and an ongoing project at the NIH involves screening a small molecule library of >350,000 compounds to identify those able to modulate Gαs activity [93]. Compounds have been identified and are undergoing additional screening and preclinical testing.
Conclusions
FD/MAS is a rare disorder of striking complexity. Variable tissue effects of mutant Gαs combined with mosaic involvement result in a uniquely broad clinical spectrum. Clinicians should take a systematic approach to evaluate the extent of disease involvement and initiate treatment. Effective therapies are available for many extraskeletal features of MAS, however there is a critical need to develop treatments capable of altering the disease course in FD.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.El-Mofty SK, Fibro-osseous lesions of the craniofacial skeleton: an update. Head Neck Pathol, 2014. 8(4): p. 432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L, et al. , Prevalence of Different Forms and Involved Bones of Craniofacial Fibrous Dysplasia. J Craniofac Surg, 2017. 28(1): p. 21–25. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein LS, et al. , Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med, 1991. 325(24): p. 1688–95. [DOI] [PubMed] [Google Scholar]
- 4.Michienzi S, et al. , GNAS transcripts in skeletal progenitors: evidence for random asymmetric allelic expression of Gs alpha. Hum Mol Genet, 2007. 16(16): p. 1921–30. [DOI] [PubMed] [Google Scholar]
- 5.Hilger D, Masureel M, and Kobilka BK, Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol, 2018. 25(1): p. 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumbroso S, et al. , Activating Gsalpha mutations: analysis of 113 patients with signs of McCune-Albright syndrome--a European Collaborative Study. J Clin Endocrinol Metab, 2004. 89(5): p. 2107–13. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P, et al. , Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res, 2000. 15(1): p. 120–8. [DOI] [PubMed] [Google Scholar]
- 8.Idowu BD, et al. , A sensitive mutation-specific screening technique for GNAS1 mutations in cases of fibrous dysplasia: the first report of a codon 227 mutation in bone. Histopathology, 2007. 50(6): p. 691–704. [DOI] [PubMed] [Google Scholar]
- 9.Graziano MP and Gilman AG, Synthesis in Escherichia coli of GTPase- deficient mutants of Gs alpha. J Biol Chem, 1989. 264(26): p. 15475–82. [PubMed] [Google Scholar]
- 10.Landis CA, et al. , GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature, 1989. 340(6236): p. 692–6. [DOI] [PubMed] [Google Scholar]
- 11.Masters SB, et al. , Mutations in the GTP-binding site of GS alpha alter stimulation of adenylyl cyclase. J Biol Chem, 1989. 264(26): p. 15467–74. [PubMed] [Google Scholar]
- 12.Hu Q and Shokat KM, Disease-Causing Mutations in the G Protein Galphas Subvert the Roles of GDP and GTP. Cell, 2018. 173(5): p. 1254–1264.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celi FS, et al. , The role of type 1 and type 2 5’-deiodinase in the pathophysiology of the 3,5,3’-triiodothyronine toxicosis of McCune-Albright syndrome. J Clin Endocrinol Metab, 2008. 93(6): p. 2383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Happle R, The McCune-Albright syndrome: a lethal gene surviving by mosaicism. Clin Genet, 1986. 29(4): p. 321–4. [DOI] [PubMed] [Google Scholar]
- 15.Riminucci M, et al. , Fibrous dysplasia as a stem cell disease. J Bone Miner Res, 2006. 21 Suppl 2: p. P125–31. [DOI] [PubMed] [Google Scholar]
- 16.Riminucci M, et al. , Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol, 1997. 151(6): p. 1587–600. [PMC free article] [PubMed] [Google Scholar]
- 17.Riminucci M, et al. , The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol, 1999. 187(2): p. 249–58. [DOI] [PubMed] [Google Scholar]
- 18.Riminucci M, et al. , Osteoclastogenesis in fibrous dysplasia of bone: in situ and in vitro analysis of IL-6 expression. Bone, 2003. 33(3): p. 434–42. [DOI] [PubMed] [Google Scholar]
- 19.Collins MT, et al. , Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res, 2001. 16(5): p. 806–13. [DOI] [PubMed] [Google Scholar]
- 20.Riminucci M, et al. , FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest, 2003. 112(5): p. 683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada T, et al. , FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res, 2004. 19(3): p. 429–35. [DOI] [PubMed] [Google Scholar]
- 22.Shimada T, et al. , Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A, 2001. 98(11): p. 6500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharyya N, et al. , Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res, 2012. 27(5): p. 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuznetsov SA, et al. , Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res, 2008. 23(11): p. 1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianco P, et al. , Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha- mutated skeletal progenitor cells. J Clin Invest, 1998. 101(8): p. 1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsiao EC, et al. , Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci U S A, 2008. 105(4): p. 1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao EC, et al. , Gs G protein-coupled receptor signaling in osteoblasts elicits age-dependent effects on bone formation. J Bone Miner Res, 2010. 25(3): p. 584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saggio I, et al. , Constitutive expression of Gsalpha(R201C) in mice produces a heritable, direct replica of human fibrous dysplasia bone pathology and demonstrates its natural history. J Bone Miner Res, 2014. 29(11): p. 2357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karaca A, et al. , Constitutive stimulatory G protein activity in limb mesenchyme impairs bone growth. Bone, 2018. 110: p. 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SK, et al. , Induced Gnas(R201H) expression from the endogenous Gnas locus causes fibrous dysplasia by up-regulating Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A, 2018. 115(3): p. E418–e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, et al. , Expression of an active Galphas mutant in skeletal stem cells is sufficient and necessary for fibrous dysplasia initiation and maintenance. Proc Natl Acad Sci U S A, 2018. 115(3): p. E428–e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart ES, et al. , Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res, 2007. 22(9): p. 1468–74. [DOI] [PubMed] [Google Scholar]
- 33.Kushchayeva YS, et al. , Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights Imaging, 2018. 9(6): p. 1035–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leet AI and Collins MT, Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop, 2007. 1(1): p. 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leet AI, et al. , Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res, 2004. 19(4): p. 571–7. [DOI] [PubMed] [Google Scholar]
- 36.Ippolito E, et al. , Radiographic classification of coronal plane femoral deformities in polyostotic fibrous dysplasia. Clin Orthop Relat Res, 2014. 472(5): p. 1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leet AI, et al. , Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. 2004. 86(3): p. 531–537. [PubMed] [Google Scholar]
- 38.Mancini F, et al. , Scoliosis and spine involvement in fibrous dysplasia of bone. Eur Spine J, 2009. 18(2): p. 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berglund JA, et al. , Scoliosis in Fibrous Dysplasia/McCune-Albright Syndrome: Factors Associated With Curve Progression and Effects of Bisphosphonates. J Bone Miner Res, 2018. 33(9): p. 1641–1648. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, et al. , Normal vision despite narrowing of the optic canal in fibrous dysplasia. N Engl J Med, 2002. 347(21): p. 1670–6. [DOI] [PubMed] [Google Scholar]
- 41.Burke AB, Collins MT, and Boyce AM, Fibrous dysplasia of bone: craniofacial and dental implications. Oral Dis, 2017. 23(6): p. 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapurlat RD, Orcel P.J.B.p., and rheumatology r.C., Fibrous dysplasia of bone and McCune–Albright syndrome. 2008. 22(1): p. 55–69. [DOI] [PubMed] [Google Scholar]
- 43.Becelli R, et al. , Surgical treatment of fibrous dysplasia of the cranio- maxillo-facial area. Review of the literature and personal experience form 1984 to 1999. Minerva Stomatol, 2002. 51(7–8): p. 293–300. [PubMed] [Google Scholar]
- 44.Boyce AM, et al. , Optic neuropathy in McCune-Albright syndrome: effects of early diagnosis and treatment of growth hormone excess. J Clin Endocrinol Metab, 2013. 98(1): p. E126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amit M, et al. , Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia--a meta-analysis. PLoS One, 2011. 6(9): p. e25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyce AM, et al. , Association of Hearing Loss and Otologic Outcomes With Fibrous Dysplasia. JAMA Otolaryngol Head Neck Surg, 2018. 144(2): p. 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan KS, et al. , Chiari I malformation and basilar invagination in fibrous dysplasia: prevalence, mechanisms, and clinical implications. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berglund JA, et al. , Scoliosis in Fibrous Dysplasia/McCune-Albright Syndrome: Factors Associated with Curve Progression and Effects of Bisphosphonates. J Bone Miner Res, 2018. [DOI] [PubMed] [Google Scholar]
- 49.Kelly MH, Brillante B, and Collins MT, Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int, 2008. 19(1): p. 57–63. [DOI] [PubMed] [Google Scholar]
- 50.Dumitrescu CE and Collins MT, McCune-Albright syndrome. Orphanet J Rare Dis, 2008. 3: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCune D, Osteitis fibrosa cystica; the case of a nine year old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child, 1936. 52 p. 743–4. [Google Scholar]
- 52.Collins MT, Singer FR, and Eugster E, McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis, 2012. 7 Suppl 1: p. S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyce AM, et al. , Characterization and management of testicular pathology in McCune-Albright syndrome. J Clin Endocrinol Metab, 2012. 97(9): p. E1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tessaris D, et al. , Thyroid abnormalities in children and adolescents with McCune-Albright syndrome. Horm Res Paediatr, 2012. 78(3): p. 151–7. [DOI] [PubMed] [Google Scholar]
- 55.Salenave S, et al. , Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab, 2014. 99(6): p. 1955–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tessaris D, et al. , Growth hormone-Insulin-like growth factor 1 axis hyperactivity on bone fibrous dysplasia in McCune-Albright Syndrome. Clin Endocrinol (Oxf), 2018. 89(1): p. 56–64. [DOI] [PubMed] [Google Scholar]
- 57.Boyce AM, et al. , Optic Neuropathy in McCune-Albright Syndrome: Effects of Early Diagnosis and Treatment of Growth Hormone Excess. J Clin Endocrinol Metab, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyce AM, et al. , Association of Hearing Loss and Otologic Outcomes With Fibrous Dysplasia. JAMA Otolaryngol Head Neck Surg, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown RJ, Kelly MH, and Collins MT, Cushing syndrome in the McCune-Albright syndrome. J Clin Endocrinol Metab, 2010. 95(4): p. 1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carney JA, Young WF, and Stratakis CA, Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. Am J Surg Pathol, 2011. 35(9): p. 1311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruggieri P, et al. , Malignancies in fibrous dysplasia. Cancer, 1994. 73(5): p. 1411–24. [DOI] [PubMed] [Google Scholar]
- 62.Majoor BC, et al. , Increased Risk of Breast Cancer at a Young Age in Women with Fibrous Dysplasia. J Bone Miner Res, 2018. 33(1): p. 84–90. [DOI] [PubMed] [Google Scholar]
- 63.Collins MT, et al. , Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab, 2003. 88(9): p. 4413–7. [DOI] [PubMed] [Google Scholar]
- 64.Gaujoux S, et al. , Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab, 2014. 99(1): p. E97–101. [DOI] [PubMed] [Google Scholar]
- 65.Parvanescu A, et al. , Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: : GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg, 2014. 149(8): p. 858–62. [DOI] [PubMed] [Google Scholar]
- 66.Paul SM, et al. , Disease severity and functional factors associated with walking performance in polyostotic fibrous dysplasia. Bone, 2014. 60: p. 41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Castro LF, et al. , Activation of RANK/RANKL/OPG Pathway Is Involved in the Pathophysiology of Fibrous Dysplasia and Associated With Disease Burden. J Bone Miner Res, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chapurlat RD, et al. , Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone, 2004. 35(1): p. 235–42. [DOI] [PubMed] [Google Scholar]
- 69.Plotkin H, et al. , Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab, 2003. 88(10): p. 4569–75. [DOI] [PubMed] [Google Scholar]
- 70.Majoor BC, et al. , Outcome of Long-Term Bisphosphonate Therapy in McCune-Albright Syndrome and Polyostotic Fibrous Dysplasia. J Bone Miner Res, 2017. 32(2): p. 264–276. [DOI] [PubMed] [Google Scholar]
- 71.Parisi MS, Oliveri B, and Mautalen CA, Effect of intravenous pamidronate on bone markers and local bone mineral density in fibrous dysplasia. Bone, 2003. 33(4): p. 582–8. [DOI] [PubMed] [Google Scholar]
- 72.Matarazzo P, et al. , Pamidronate treatment in bone fibrous dysplasia in children and adolescents with McCune-Albright syndrome. J Pediatr Endocrinol Metab, 2002. 15 Suppl 3: p. 929–37. [PubMed] [Google Scholar]
- 73.Florenzano P, et al. , Age-Related Changes and Effects of Bisphosphonates on Bone Turnover and Disease Progression in Fibrous Dysplasia of Bone. J Bone Miner Res, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boyce AM, et al. , A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab, 2014. 99(11): p. 4133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boyce AM, et al. , Fibrous Dysplasia/McCune-Albright Syndrome, in GeneReviews((R)), Adam MP, et al. , Editors. 1993, University of Washington, Seattle University of Washington, Seattle: GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.: Seattle (WA). [Google Scholar]
- 76.Metwally T, et al. , Fibrous Dysplasia and Medication-Related Osteonecrosis of the Jaw. J Oral Maxillofac Surg, 2016. 74(10): p. 1983–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boyce AM, et al. , Denosumab treatment for fibrous dysplasia. J Bone Miner Res, 2012. 27(7): p. 1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ganda K and Seibel MJ, Rapid biochemical response to denosumab in fibrous dysplasia of bone: report of two cases. Osteoporos Int, 2014. 25(2): p. 777–82. [DOI] [PubMed] [Google Scholar]
- 79.Benhamou J, Gensburger D, and Chapurlat R, Transient improvement of severe pain from fibrous dysplasia of bone with denosumab treatment. Joint Bone Spine, 2014. 81(6): p. 549–50. [DOI] [PubMed] [Google Scholar]
- 80.Stanton RP, et al. , The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis, 2012. 7 Suppl 1: p. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Majoor BC, et al. , What Is the Role of Allogeneic Cortical Strut Grafts in the Treatment of Fibrous Dysplasia of the Proximal Femur? Clin Orthop Relat Res, 2017. 475(3): p. 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leet AI, et al. , Bone-Grafting in Polyostotic Fibrous Dysplasia. J Bone Joint Surg Am, 2016. 98(3): p. 211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boyce AM, et al. , Surgical Management of Polyostotic Craniofacial Fibrous Dysplasia: Long-Term Outcomes and Predictors for Postoperative Regrowth. Plast Reconstr Surg, 2016. 137(6): p. 1833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Estrada A, et al. , Long-term outcomes of letrozole treatment for precocious puberty in girls with McCune-Albright syndrome. Eur J Endocrinol, 2016. 175(5): p. 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eugster EA, et al. , Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J Pediatr, 2003. 143(1): p. 60–6. [DOI] [PubMed] [Google Scholar]
- 86.Salenave S, et al. , Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab, 2014: p. jc20133826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vortmeyer AO, et al. , Somatic GNAS Mutation Causes Widespread and Diffuse Pituitary Disease in Acromegalic Patients with McCune-Albright Syndrome. J Clin Endocrinol Metab, 2012. 97(7): p. 2404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu F, et al. , A case of McCune-Albright syndrome associated with pituitary GH adenoma: therapeutic process and autopsy. J Pediatr Endocrinol Metab, 2011. 24(5–6): p. 283–7. [DOI] [PubMed] [Google Scholar]
- 89.Hansen MR and Moffat JC, Osteosarcoma of the Skull Base after Radiation Therapy in a Patient with McCune-Albright Syndrome: Case Report. Skull Base, 2003. 13(2): p. 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carpenter TO, et al. , A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res, 2011. 26(7): p. 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chapurlat RD, et al. , Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis, 2012. 7 Suppl 1: p. S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Boysson H, et al. , Tocilizumab in the treatment of a polyostotic variant of fibrous dysplasia of bone. Rheumatology (Oxford), 2015. 54(9): p. 1747–9. [DOI] [PubMed] [Google Scholar]
- 93.Bhattacharyya N, et al. , A high throughput screening assay system for the identification of small molecule inhibitors of gsp. PLoS One, 2014. 9(3): p. e90766. [DOI] [PMC free article] [PubMed] [Google Scholar]