Abstract
In recent decades, stress response models of adolescent depression have gained attention, but it remains unclear why only certain adolescents are vulnerable to the depressogenic effects of stress while others are not. Building on evidence that affective and physiological responses to stress moderate the impact of stress exposure on depression, the current study examined whether the interaction between severity of interpersonal stress, subjective affective reactivity, and hypothalamic-pituitary-adrenal (HPA) axis reactivity to an acute, in-vivo psychosocial stressor prospectively predicted depressive symptoms nine months later. Hypotheses were examined with a clinically-oversampled group of 182 adolescent girls (aged 12–16) to ensure an examination of the widest possible range of risk. Self-report measures of affect and salivary cortisol samples were collected before and after an in-vivo stress task to assess affective reactivity and HPA axis reactivity, respectively. Severity of interpersonal stress between baseline and nine months was assessed using a semi-structured interview and was objectively coded for severity and content theme (i.e., interpersonal vs. non-interpersonal). Results indicate that experiences of severe interpersonally-themed stress predict elevated levels of depressive symptoms longitudinally only for adolescent girls with elevated affective reactivity to stress, and suggest that these deleterious effects of stress are most exacerbated for girls with elevated physiological responses to stress. Findings suggest that it may be critical to examine both affective and physiological stress responses when assessing risk for depression in adolescents.
Keywords: hypothalamic-pituitary-adrenal axis, adolescence, interpersonal stress, Positive affect, depression
Parallel increases in experiences of interpersonal stress and the prevalence of depressive symptoms during adolescence have stimulated greater attention to stress response models of adolescent depression. Experiences of severe stress have been associated consistently with major depressive disorder (Hammen, 2005; Kessler, 1997; Mazure, 1998; Monroe, Slavich, & Georgiades, 2008; Paykel, 2003; Tennant, 2002). Compared to healthy individuals, depressed patients are more than twice as likely to have experienced an instance of severe stress, and more than 80% of depressed individuals experience a severe stressor prior to a major depressive episode (Mazure, 1998).
Yet, not all types of severe stress seem to be equally relevant to the development of depression. Interpersonal stressors, defined as challenges that impact an individual’s relationships or stem from social interactions, predict depression more robustly than non-interpersonal stressors (e.g. O’Neill, Cohen, Tolpin, & Gunthert, 2004; Rudolph et al., 2000). This association may help account for an increased prevalence in depression among adolescent girls in particular. Over the course of adolescence, there is a marked increase in experiences of interpersonal stress (Ge, Lorenz, Conger, & Elder, 1994), with girls reporting more frequent and severe interpersonal stress than boys (Rudolph & Hammen, 1999; Shih, Eberhart, Hammen, & Brennan, 2006). During the same period, the prevalence of depression more than doubles from 4.5% to 10% (Avenevoli, Swendsen, He, Burstein, & Merikangas, 2015) and the gender difference in depression prevalence also magnifies with lifetime rates of 14.9% for adolescent girls and 7.3% for adolescent boys (Avenevoli et al., 2015).
While increased severity of interpersonal stress in adolescence may help explain the corresponding increase in depression, it remains unclear why certain adolescents succumb to the depressogenic effects of stress while others do not. To reduce the public health burden of depression, it is necessary to clarify how and for whom stress confers vulnerability for depression in adolescence. This study examined affective reactivity and HPA axis reactivity as moderators of the predictive association between interpersonal stress and the elevations in depressive symptoms over time.
Remarkably few prior studies have examined how heightened affective reactivity might moderate depression risk following experiences of interpersonal stress. One study among college students suggested that decreases in positive affect and increases in negative affect in response to daily interpersonal stressors were prospectively associated with depressive symptoms (O’Neill et al., 2004). In the context of higher-than-usual stress, individuals with remitted depression demonstrate greater decreases in positive affect and greater increases in depressed affect than healthy controls (O’Hara, Armeli, Boynton, & Tennen, 2014). (O’Hara et al., 2014). Changes in positive affect, in particular, may render individuals vulnerable to the depressogenic effects of stress. Greater variability in positive affect is associated with increased depression, regardless of overall positive affect levels (Gruber, Kogan, Quoidbach, & Mauss, 2013). For individuals with a history of depression, positive associations between stress and depression symptoms are diminished on days characterized by greater positive affect in the face of elevated stress (O’Hara et al., 2014). Overall, emerging results suggest that stress-responsive declines in positive affect may heighten the association between stress and depression. However, this hypothesis has been understudied in longitudinal research, and we could identify no prior prospective studies examining the moderating effects of affective reactivity on the relation between stress and depressive symptoms among youth. Consistent with prior research, we hypothesized that greater decreases in positive affect, and to a lesser extent greater increases in negative affect, following exposure to stress would exacerbate the association between experiences of severe interpersonal stress and depressive symptoms over time.
The relation between stress and depression may be further moderated by elevated hypothalamic-pituitary-adrenal (HPA) axis stress responses. In the face of stress, the HPA axis releases cortisol, which facilitates the mobilization of energy reserves and amplifies individual sensitivity to salient environmental cues in preparation for a behavioral reaction to potential threat. Although little longitudinal data among youth have been reported, several studies of adolescents have found positive associations between diurnal fluctuations in cortisol and elevated cortisol awakening responses and depressive symptoms (Adam et al., 2010; LeMoult, Ordaz, Kircanski, Singh, & Gotlib, 2015; Schuler et al., 2017). Fewer studies of adolescents have examined associations between cortisol reactivity to an acute stressor and depressive symptoms. These have found positive associations between cortisol reactivity and depressive symptoms (Hankin, Badanes, Abela, & Watamura, 2010; Rao, Hammen, Ortiz, Chen, & Poland, 2008), though one study found adolescents with moderate to severe depression have blunted cortisol responses compared to healthy controls (Harkness, Stewart, & Wynne-Edwards, 2011). In one longitudinal study with pre-teen children (M age = 9.46 years), results revealed that heightened anticipatory cortisol responses to an in-vivo social stressor moderated the association between elevated levels of interpersonal stress and depressive symptoms one year later (Rudolph, Troop-Gordon, & Granger, 2011). These initial findings suggest that HPA axis reactivity may be a relevant factor that moderates the association between the actual experience of severe interpersonal stress and depressive symptoms. Accordingly, we hypothesized that elevated HPA axis responses to an in-vivo stressor would magnify the association between severe interpersonal stress and later depressive symptoms within a sample of adolescent girls.
While the majority of studies have associated elevated cortisol reactivity with depressive symptoms in adolescents, there remains some inconsistency in findings (see Guerry & Hastings, 2011 for review). The apparent inconsistency in the relation between HPA axis functioning and depression may stem from the tendency to examine the HPA axis in isolation. Few studies have examined HPA axis effects on depressive symptoms in the context of elevated real-life stress exposure. Fewer still have examined the interaction of HPA axis stress reactivity with the affective stress response, despite evidence that affective responses to stress may moderate and be moderated by physiological arousal (Ursin & Eriksen, 2004). This study will address these limitations by prospectively examining how elevated HPA axis responses to an in-vivo psychosocial stressor may interact with affective reactivity to modify the relation between naturally occurring life stress and depression. Our primary hypothesis pertaining to the HPA axis was informed by recent theories, such as the Adaptive Calibration Model proposed by Del Giudice, Ellis, and Shirtcliff (2011), suggesting that elevated HPA axis responses may enhance sensitivity to social information. Under conditions of severe interpersonal stress and low positive affect, elevated HPA axis responses may serve to increase feelings of social evaluation and rejection, exacerbating the association between interpersonal stress and depressive symptoms. Thus, we hypothesized a three-way interaction in a longitudinal study of adolescent girls’ depressive symptoms, such that elevated cortisol reactivity would increase the deleterious effects of elevated affective responses to severe interpersonal (but not non-interpersonal) stress on later depressive symptoms.
Our study addressed several limitations of prior work by examining girls at the critical vulnerability period associated with depressive symptoms in adolescence and using an oversampling procedure of clinically-referred youth to ensure an examination of the widest possible range of depression risk. This study also addressed methodological limitations of prior work by utilizing an in vivo stress paradigm to examine affective and HPA axis reactivity and semi-structured interviews and objective severity ratings to stringently examine the intensity of stressful interpersonal and non-interpersonal experiences.
Method
Participants
Recruited participants included 182 female adolescents between the ages of 12 and 16 (Mage = 14.46 years, SD = 1.33). Participants were recruited from a wide range of community and clinical placements, including inpatient psychiatric units, outpatient mental health agencies, high schools, and the local community via flyers, radio, and mass e-mail advertisements. Inclusion criteria for the study included (a) female gender, (b) baseline age between 12 and 16, (c) caregiver available to participate, and (d) mental health concerns (e.g. mood and adjustment disorders, substance use, disruptive behavior disorders) in the prior two years. A qualifying history of mental health concerns was determined based on parent report of their adolescent’s prior diagnosis or treatment, or a brief screening interview (KSADS) administered by a trained researcher. Adolescents were excluded for current psychosis, intellectual disability, and pervasive developmental disorders. Approximately 60% of participants identified as Caucasian, 26% as African-American, 1% as Hispanic or Latinx-American, 1% as Asian-American, and 12% identified as multi-racial or belonging to another group. At baseline, approximately half of the adolescents lived with two parents or caregivers, while the remainder reported living in a single-parent household. Approximately 47% reported current medication use, including antidepressants, stimulants, antipsychotics, antihistamines, antibiotics, anxiolytics, and anticonvulsants.
Data were collected at baseline on a sample of 220 girls. A total of 199 (90.5%) of these participants were available for follow-up phone call assessment of depressive symptoms 9 months later at Time 2, and 146 (73.4%) of these participants were available for the separate Life Stress Interview follow-up phone call assessment at Time 2. Data for one or more constructs included in the study analyses were missing for an additional 17 participants. No significant differences were revealed for any of the constructs measured in this study between adolescents with and without complete data. Additionally, Little’s MCAR test revealed a nonsignificant coefficient, χ2 (46) = 55.51, p = .159. Data thus were imputed using an expectation maximization procedure, allowing all analyses to include the full sample of adolescents who participated at least once in the study. Analyses reported below were re-conducted using only available data, yielding the same pattern of significant results.
Preliminary analyses revealed that adolescents who reported taking oral contraceptives at baseline (n = 38) had significantly blunted cortisol responses to the stress task (M = −.04, SD = .17) compared to adolescents who were not taking oral contraceptives (M = .10, SD = .22), t(220) = 4.40, p <.001, d = .71, even after controlling for baseline levels of depression and experiences of interpersonal stress, consistent with prior work (Bouma, Riese, Ormel, Verhulst, & Oldehinkel, 2009; Kirschbaum, Pirke, & Hellhammer, 1995). Thus, adolescents who reported using oral contraceptives were excluded from further analyses; the final sample size for all analyses was 182.
Procedure
Participants attended the baseline visit with a caregiver. Upon arrival, trained research assistants explained the content of the laboratory visits to adolescents and their caregivers before asking them to complete a consent form. During the baseline visit, participants completed a series of self-report questionnaires (e.g. demographics, depressive symptoms). Approximately three hours after arrival, participants watched an emotionally neutral film clip before providing an initial saliva sample to ensure that cortisol levels reflected a resting baseline of hypothalamic-pituitary-adrenal axis functioning. Participants then underwent a modified Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993, see below) and provided additional saliva samples.
Nine months after the baseline visit, participants completed a questionnaire by phone to assess depressive symptoms (MFQ; Costello & Angold, 1988, see below). On a separate call, also approximately nine months after the baseline visit, a different trained researcher conducted a semi-structured phone interview (LSI; Rudolph & Flynn, 2007, see below) to assess experiences of stress. All procedures were approved by the human subjects committee of the University of North Carolina at Chapel Hill.
Measures
Depressive Symptoms.
Depressive symptoms were assessed with the Mood and Feelings Questionnaire (MFQ; Costello & Angold, 1988), a 33-item self-report measure of depressive symptoms in children and adolescents between the ages of 8 and 18. Participants were asked how true (0 = not true, 1 = sometimes true, 2 = mostly true) each statement about depressive symptoms (e.g. “I felt miserable or unhappy”) had been for them in the previous two weeks. Data were analyzed using a mean score of all items, with higher mean scores indicating more depressive symptoms. The MFQ had high internal consistency across time points (Cronbach’s α = .95 for both baseline and nine months).
Life Stress.
The Youth Life Stress Interview (LSI; Rudolph & Flynn, 2007), an adapted version of the Child Episodic Life Stress Interview (Rudolph & Hammen, 1999) was used to assess the severity, frequency, and type of participants’ experiences of stress during the nine months between baseline and follow-up. This semi-structured interview was conducted by extensively trained researchers who used probes to gather detailed factual information about the precise timing and contextual features of stressful experiences relating to school and academics, behavioral problems, family relationships, peer relationships, and romantic relationships. The interviewer then provided a detailed narrative of each event and its surrounding context to a team of 3–6 expert raters. These raters used a consensus process to assign an objective stress rating on a 5-point scale to represent how the event would impact a typical adolescent under the same circumstances, with higher scores representing greater stressfulness. Information such as the adolescent’s subjective experience of the stressor were masked to prevent biases in objective ratings. Consensus ratings were also used to categorize each event as interpersonal (affecting an adolescent’s relationship or involving an interaction between the adolescent and another individual) or noninterpersonal. Using these data, a mean interpersonal stress severity score was then calculated for each participant. Mean interpersonal stress severity scores were used rather than sum scores to prevent artificial inflation of stress levels due to differences in number of events reported. As individuals with more depressive symptoms tend to report greater numbers of stressful events, the use of mean scores further limits the potential confounding effect of current psychopathology on the measurement of interpersonal stress (Hammen, 2006). Events rated 1 (no negative impact/stress) were excluded. To assess reliability, two independent teams of raters double coded 30% of participant interviews, yielding high reliability for both ratings of episodic stress impact (intraclass correlation coefficient = .95) and interpersonal vs. noninterpersonal event content (Cohen’s K = .92).
Affective Response.
Affect was measured at baseline (approximately 2 hours after arrival to the lab, 50 minutes prior to the stress task) and immediately post-stress task with a modified version of the Positive and Negative Affect Schedule for Children (PANAS-C; Laurent et al., 1999). The original PANAS-C is a 27-item self-report measure assessing negative and positive dimensions of affect in children and adolescents. Participants are asked to rate their present identification with a list of feelings using a scale ranging from not at all (0) to extremely (100). In the modified version used for this study, 12 items were taken from the Negative Affect scale of the PANAS-C (Frightened, Nervous, Afraid, Scared, Mad, Miserable, Gloomy, Lonely, Ashamed, Sad, Guilty, Disgusted) for the purpose of brevity, and two additional items (Annoyed and Angry) were added to better capture what Watson and Clark describe as the “hostility” dimension of affect (Watson & Clark, 1999). Three items were taken from the Positive Affect scale of the PANAS-C to capture basic positive affect (Calm, Happy, Joyful). Positive and Negative Affect scales were kept independent rather than collapsed into a single measure of affective response, as there is substantial evidence to suggest that positive and negative affect are orthogonal constructs rather than ends of a continuum (e.g., Kercher, 1992). Composite scores were created for the Positive and Negative Affect subscales, based on the mean of the item scores, with higher scores representing greater self-reported positive and negative affect respectively. Both factors demonstrated good internal consistency (Cronbach’s α = .81 for positive affect, .86 for negative affect). Positive and negative affective reactivity were computed by subtracting baseline PANAS scores from corresponding post-stress task PANAS scores.
HPA Axis Response.
The TSST is a social-evaluative stress task that significantly increases salivary cortisol output across diverse populations (Gunnar, Talge, & Herrera, 2009). Approximately three hours after arrival in the laboratory, participants watched an emotionally neutral film clip to ensure that baseline cortisol reflected HPA-axis activity at rest. Participants were then instructed to spend one minute preparing a three-minute audition speech about why they should be selected to star in a fictional reality show about teens’ ability to form friendships. After the preparation period, an undergraduate male judge entered and instructed the participant to give the speech while facing a video camera and a screen displaying their live image. Participants were informed that the judge could not answer questions and would be evaluating their audition throughout. Judges were trained to refrain from providing feedback of any kind, but instructed to prompt the participant to continue if she ceased before the three-minute limit. All TSST procedures took place in the afternoon (M = 2:30 pm) to limit variability due to normative diurnal fluctuations in cortisol (Dickerson & Kemeny, 2004).
To measure HPA axis responses to stress, cortisol samples were collected using salivettes immediately prior to the stress task to capture cortisol levels during the end of the baseline video and 20 minutes after the conclusion of the TSST to capture peak cortisol levels. Cortisol reactivity was computed by subtracting pre-Trier cortisol levels from peak cortisol levels. Saliva samples were frozen and stored at −25 C before being transported on dry ice to the Behavioral Endocrinology Laboratory at Pennsylvania State University (Salimetrics, PA) for analysis. Each saliva sample was assayed for cortisol with a 510-k cleared high-sensitivity enzyme immunoassay with a sensitivity range of 0.007 ug/dl to 1.2 ug/dl. All values were log transformed to correct for skew prior to analyses. For a subset of participants (n=30), baseline cortisol was collected using passive drool rather than salivettes. For these participants, baseline cortisol was treated as missing to ensure consistency across cortisol assessments. Missing cortisol values were handled using full information maximum likelihood method on M-plus.
Data Analytic Plan
Given the small sample size used in these analyses, several diagnostic tests were conducted to explore the integrity of the analyses. Regression diagnostics were conducted to ensure that no single case exerted undue influence on parameter estimates and to confirm the appropriateness of the proposed model for the data. There was no evidence to suggest that any case had undue influence on parameter estimates; all |DFFIT| statistics and all |DFBetas| were less than 1. Moreover, all VIF values were below 2 and all tolerance values were above the cutoff of 0.2, suggesting no violations of multicollinearity assumptions. The assumption of normality of residuals was examined using a P-P plot and a graph of residuals by percentile. The residuals appeared to be fairly normally distributed, with the exception of Time 2 depressive symptoms. To account for this heteroscedasticity in the dependent variable, all analyses were computed using Huber-White robust standard errors.
Preliminary analyses included descriptive statistics and correlations among all study variables (see Table 1).
Table 1.
Bivariate Associations Among Primary Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time 1 | ||||||||||
| 1. Depressive Symptoms | - | |||||||||
| 2. Baseline Positive Affect | −.15* | - | ||||||||
| 3. A Positive Affect | −.01 | −.42*** | - | |||||||
| 4. Baseline Negative Affect | .28*** | −31*** | .06 | - | ||||||
| 5. A Negative Affect | .15* | .09 | −.39*** | .06 | - | |||||
| 6. Baseline Cortisol | .01 | −.05 | −.03 | .01 | .05 | - | ||||
| 7. Cortisol 20 min post-task | −.02 | .02 | −.21** | .04 | .11 | .41*** | - | |||
| 8. Mean Interpersonal Stress | 34*** | −.01 | .14* | −.12 | .11 | −.01 | −23*** | - | ||
| 9. Mean Noninterpersonal Stress | 25*** | −.05 | .12 | .05 | .22** | .08 | −.08 | .32*** | - | |
| Time 2 | ||||||||||
| 10. Depressive Symptoms | .43*** | −.15* | −.03 | .26*** | .05 | .08 | .10 | .32*** | .12 | - |
| Means | .51 | 63.51 | −33.12 | 5.63 | 11.20 | .13 | .17 | 2.61 | 2.42 | .411 |
| Standard Deviations | .39 | 24.42 | 24.58 | 6.89 | 12.86 | .07 | .09 | .35 | .48 | .33 |
Raw cortisol values (ug/dl) were used for means and for bivariate correlations.
p < .05;
p < .01;
p < .001
A three-way interaction effect was hypothesized between average stressor severity, affective reactivity, and HPA axis dysregulation as a prospective predictor of depressive symptoms. Four hierarchical multiple regression analyses were conducted with adolescents’ Time 2 depressive symptoms as the dependent variable, with positive and negative affective reactivity as well as interpersonal and noninterpersonal stress severity examined in separate models (see Table 2). All predictor variables were centered prior to computing interaction terms, and centered variables were used in the regression analyses. After controlling for adolescents’ baseline depressive symptoms in an initial step, HPA axis covariates (corticosteroid use, psychotropic medication use, and time between waking and saliva collection1), as well as constituent variables used to compute deviation scores (i.e., baseline affect and baseline cortisol levels) were entered in the second step. The three main effects were centered and also entered in the second step: interpersonal stress severity, (positive or negative) affective reactivity, and cortisol reactivity. All two-way interactions between the three primary variables of interest were entered in the third step, and a three-way interaction was entered in the fourth step.
Table 2.
Longitudinal Prediction of Depressive Symptoms by Interpersonal Stress Severity, Change in Positive Affect, and Cortisol Reactivity
| Time 2 Depressive Symptoms (MFQ) | |||
|---|---|---|---|
| Step Statistics | Final Statistics | ||
| Predictors | ΔR2 | b (se b) | b (se b) |
| Step 1 | 20*** | ||
| Time 1 MFQ | .39 (.06)*** | .28 (.06)*** | |
| Step 2 | .08* | ||
| Mean Interpersonal Stress | .26 (.09)** | .22 (.08)** | |
| Baseline Positive Affect | .00 (.00) | .00 (.00) | |
| Δ Positive Affect | .00 (.00) | .00 (.00) | |
| Baseline Cortisol | .12 (.11) | .14 (.11) | |
| Δ Cortisol | .14 (.11) | .19 (.10) | |
| Corticosteroid Use | .00 (.09) | −.01 (.09) | |
| Psychotropic Medication Use | .03 (.04) | .05 (.04) | |
| Step 3 | .03 | ||
| Δ Positive Affect × Mean Interpersonal Stress | −.01 (.00)* | −.01 (.00)** | |
| Δ Cortisol × A Positive Affect | −.01 (.00) | −.01 (.00) | |
| Δ Cortisol × Mean Interpersonal Stress | −.12 (.34) | −.39 (.38) | |
| Step 4 | .02* | ||
| Δ Cortisol × Δ Positive Affect × Mean Interpersonal Stress | −.03 (.01)* | ||
| Total R2 | .32* | ||
p < .05;
p < .01;
p < .001;
MFQ = Depressive Symptoms
Results
Means and standard deviations for the primary variables of interest are presented in Table 1. There was a significant decrease in positive affect (M (SD) baseline = 63.51 (24.42); M (SD) post-TSST = 28.97 (27.61)), paired t(181) = 17.69, p < .001, d = 1.31, a significant increase in negative affect (M (SD) baseline = 5.63 (6.89); M (SD) post-TSST = 17.16 (16.02)), paired t(181) = −11.04, p < .001, d = .82, and a significant increase in salivary cortisol, paired t(181) = −5.95, p < .001, d = .44, in response to the acute stressor task.
Pearson correlations were conducted to examine bivariate associations among primary variables (see Table 1). Interpersonal stress severity was positively correlated with depressive symptoms at both time points, as expected. Cortisol levels post-TSST were not associated with negative affective reactivity, but were modestly negatively associated with positive affective reactivity, such that individuals who experienced a greater HPA axis response to the TSST also reported a larger decrease in positive affect following the task. There was a moderate level of stability for depressive symptoms over time. Mean peak cortisol levels in our sample were equivalent to those elicited by the TSST and modified TSST in other studies of adolescents (e.g. Bouma et al., 2009; Stroud et al., 2009); 64% of participants in our sample exhibited an increase in cortisol in response to the TSST.
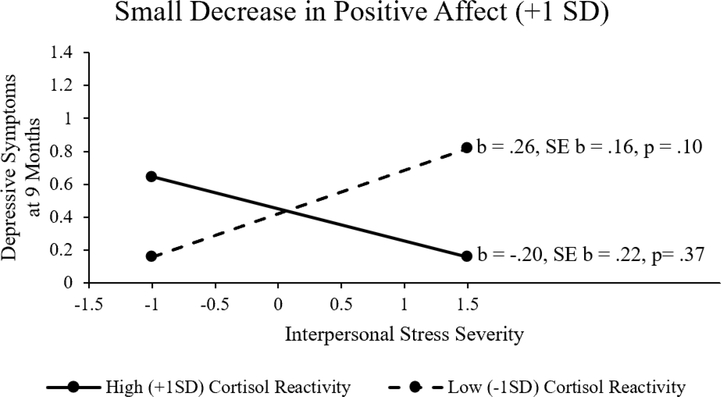
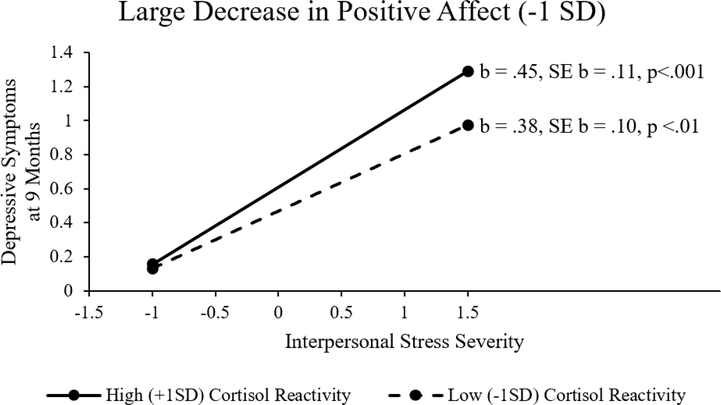
The regression model for the prediction of Time 2 depressive symptoms from interpersonal stress severity, positive affective reactivity and cortisol reactivity is presented in Table 2. There was a significant two-way interaction of interpersonal stress severity and positive affective reactivity that was further moderated by a significant three-way interaction of interpersonal stress severity, positive affective reactivity, and cortisol reactivity. As shown in Table 2, at average levels of positive affect change and cortisol change, the impact of interpersonal stress on symptoms was significant (b = .22, p = .008). To decompose the three-way interaction, the predictive association between interpersonal stress severity and Time 2 depressive symptoms were examined at both low (+1 SD, reflecting little change in positive affect) and high (−1 SD, reflecting larger decreases in positive affect) levels of positive affective reactivity, and within each of those, at low (−1 SD, reflecting little HPA axis response) and high (+1 SD, reflecting strong HPA axis response) levels of cortisol reactivity. When there was very little positive affective reactivity (+1 SD), interpersonal stress severity did not significantly predict Time 2 depressive symptoms at either high (+ 1 SD cortisol reactivity, b = −.20, SE b = .22, p= .37) or low (−1 SD, b = .26, SE b = .16, p = .10) cortisol reactivity (see Figure 1). When there were large declines in positive affective reactivity (−1 SD), interpersonal stress severity was more strongly related to depressive symptoms at nine months for high levels of cortisol reactivity (b = .45, SE b = .11, p<.001) than for low levels of cortisol reactivity (b = .38, SE b = .10, p <.01) (see Figure 2). Thus, the maintenance of positive affect appeared to buffer the effect of interpersonal stress on depression, regardless of HPA response, while large decreases in positive affect exacerbated the effect of interpersonal stress on depression, particularly when compounded with strong HPA responses as reflected in more highly elevated cortisol reactivity.
Figure 1.
Mean plot illustrating the interaction of interpersonal stress severity and cortisol reactivity in the context of small decreases in positive affect following acute stress.
Figure 2.
Mean plot illustrating the interaction of interpersonal stress severity and cortisol reactivity in the context of large decreases in positive affect following acute stress.
Contrary to expectation, neither the two-way interaction between interpersonal stress severity and cortisol reactivity nor the interaction among interpersonal stress, negative affective reactivity, and cortisol reactivity significantly predicted depressive symptoms at nine months. In addition, no significant results were revealed for noninterpersonal stress severity.
Discussion
This study fills a critical gap by using a prospective longitudinal design to examine how the experience of severe interpersonal stress interacts with elevated affective and physiological responses to an in-vivo psychosocial stressor to confer risk for depression. To date, only one other study has prospectively examined how affective responses to stress predict depressive symptoms, and no research has examined this relation in youth. Furthermore, most prior studies associating HPA axis functioning with depression have examined diurnal cortisol secretion and the cortisol awakening response. Limited research of HPA axis reactivity has focused on acute cortisol responses in isolation, without assessing real-life stress exposure, despite evidence that the adaptiveness of HPA axis responses are context-dependent. Our results indicate that adolescent girls who experience severe interpersonal stress and large declines in positive affect following stress are most likely to experience elevated levels of depressive symptoms longitudinally and that this effect is most pronounced among those with elevated HPA axis responses to stress.
In particular, girls who experienced a high average severity of interpersonal stress had the greatest severity of depressive symptoms nine months later, but only if they also experienced large decreases in positive affect (i.e. calmness, happiness, and joyfulness) in response to psychosocial stress. Among these girls, those who experienced the largest increases in cortisol in response to psychosocial stress demonstrated the greatest depressive symptoms at nine months. Notably, no effect was found for changes in negative affect in response to stress. Additionally, consistent with prior literature (e.g. Sheets & Craighead, 2014), experiences of noninterpersonal stress did not predict changes in depressed mood, indicating the impact of social stressors in particular in the etiology of depression.
These findings highlight the importance of examining affective as well as physiological responses to stress to determine who is at greatest risk for depression. These findings are consistent with the broaden-and-build theory posited by Fredrickson (2001), which posits that the maintenance of positive affect under conditions of stress facilitates adaptive coping in part through the attenuation of physiological stress responses (Tugade & Fredrickson, 2004). This study builds on cross-sectional literature associating positive affective variability with depressive symptoms (Gruber et al., 2013), and studies finding that those with a history of depression demonstrate larger decreases in positive affect on high stress days compared to healthy controls (O’Hara et al., 2014). Susceptibility to fluctuations in positive affect may leave individuals less capable of flexibly and adaptively responding to stress, while the maintenance of positive emotion under conditions of stress may contribute to effective emotional regulation and the subsequent downregulation of physiological stress responses. One prior study found that decreases in positive affect in response to interpersonal stress were associated with greater endorsement of disengagement and elevated substance use as strategies for coping with stress, while changes in negative affect were not (O’Neill et al., 2004). Given these associations, large decreases in positive affect in response to stress may contribute to the use of maladaptive coping strategies, which may prevent adaptive responses to interpersonal stress. The conclusions that can be drawn from this study about the mechanisms by which affective responses contribute to elevated risk for depression are limited by the fact that coping strategies were not measured. Further research should examine how affective responses to stress might facilitate the use of adaptive and maladaptive coping strategies to ameliorate or exacerbate the negative effects of stress.
This study is among the first to identify the interactive effect of interpersonal stress, positive affective reactivity, and cortisol reactivity as a novel vulnerability factor for depressive symptoms. The simple two-way interaction between interpersonal stress and cortisol was nonsignificant, perhaps because multiple patterns of cortisol response may be deleterious depending on other aspects of the stress response, such as affect, and between-person variables, such as early trauma. Prior research has found that early trauma alters the relations between cortisol reactivity and depression (e.g. Suzuki, Poon, Papadopoulos, Kumari, & Cleare, 2014); future research should examine how trauma history may alter the interaction between affective and HPA axis responses to stress. In our sample, for individuals with large declines in positive affect in response to stress, elevated HPA axis responses magnified the association between severe interpersonal stress and depression. In the presence of declining positive emotion and more limited coping resources, enhanced sensitivity to interpersonal cues facilitated by elevated cortisol levels may increase feelings of social judgment and rejection to prolong the negative effects of stress beyond the acute event, in keeping with the cortisol effects proposed by the Adaptive Calibration Model (Del Giudice et al., 2011). These results highlight the importance of examining multiple aspects of the stress response together to understand what factors render certain individuals vulnerable to the depressogenic effects of stress. Furthermore, prior research has associated other aspects of the acute cortisol response, such as delayed cortisol recovery, with internalizing symptoms (Shapero, McClung, Bangasser, Abramson, & Alloy, 2017; Stewart, Mazurka, Bond, Wynne-Edwards, & Harkness, 2013). Future research should examine how these other aspects of the HPA axis response may interact with affective responses and experiences of interpersonal stress.
Positive affective reactivity and cortisol reactivity may also confer risk for depression via interactions with other stress-responsive systems. A study by Moons, Eisenberger, and Taylor (2010) found that different affective responses to stress were associated with different profiles of physiological response, with some affective responses corresponding to increases in cortisol but not increases in proinflammatory cytokines, and vice versa. Given research demonstrating that stress may lead to depression via changes in inflammatory processes (e.g. Pariante & Lightman, 2008; Slavich & Irwin, 2014), affect may be essential to determine the conditions under which physiological changes are relevant to the longitudinal prediction of depression. While this study did not measure immune markers, these results underscore the importance of examining affective responses as moderators of physiological stress responses. Future research should explore how affective responses, HPA axis responses, and immune responses to stress might dynamically interact to confer risk for depression.
Contrary to expectation, we did not find negative affective reactivity to moderate the relations between stress and later depressive symptoms. Prior research examining the association between negative affective reactivity and depression has been largely cross-sectional and mixed, with some studies finding no difference in negative affective reactivity between depressed individuals and healthy controls (Croes, Merz, & Netter, 1993; Gotthardt et al., 1995; Morris, Rao, Wang, & Garber, 2014; O’Grady, Tennen, & Armeli, 2010), some finding increased negative affective reactivity in depressed individuals (Husky, Mazure, Maciejewski, & Swendsen, 2009; van Winkel et al., 2015; Young, Lopez, Murphy-Weinberg, Watson, & Akil, 2000), and some finding reduced negative affective reactivity in depressed individuals (Peeters, Nicolson, Berkhof, Delespaul, & deVries, 2003). The null findings for negative affect may be due in part to the fact that there was less variance in negative affective reactivity than positive affective reactivity, making an effect more challenging to detect. Additionally, given the correlation between baseline negative affect and later depressive symptoms, these analyses may have lacked adequate power to detect an association between changes in negative affect and changes in depressive symptoms. Further research is needed to clarify whether there are differences in negative affective reactivity for depressed individuals, and whether differences in negative affective reactivity represent a risk factor for depressive symptoms.
Our finding that interpersonal stressors rather than noninterpersonal stressors predicted subsequent depression highlights the relevance of negative social interactions in the etiology of depression, and underscores the importance of interpersonal experiences during adolescence in particular. Numerous studies have demonstrated that experiences of interpersonal stress, such as social loss and rejection, predict depression more than noninterpersonal stressful experiences (Hammen, 2005; Slavich, O’Donovan, Epel, & Kemeny, 2010; Slavich, Thornton, Torres, Monroe, & Gotlib, 2009). It is unclear from these findings whether the increasing importance of interpersonal experiences during adolescence might alter the impact of interpersonal stressors on adolescent mental health; future research should assess whether these effects differ with age across adolescence. Additionally, as there is evidence to suggest that girls experience more interpersonal stress than boys and report greater emotional responses to this stress (Rudolph, 2002), future research should examine whether there are gender differences in the interactive contribution of interpersonal stress, affective stress responses, and physiological stress responses to depressive symptoms.
Continued study of the impact of interpersonal stressors on later depressive symptoms would benefit from the inclusion of more comprehensive assessment approaches. While the rigorous coding of the Youth Life Stress Interview provides objective consensus ratings of the stressfulness of each reported life event, it is still possible that participants’ reporting of the events might have been somewhat influenced by their depressive symptoms at the nine-month follow-up. The highly structured interview was conducted during a phone call several days apart from the assessment used to determine depressive symptom severity at nine months to facilitate the independence of these reports; however, it should be noted that the proximity between these two calls prevented interviewers from ensuring their complete independence. In order to further limit the effects of any potential reporting bias due to mood symptoms at nine months, all analyses were conducted using mean stress severity scores rather than sum scores. Analyses therefore exclusively utilized objective rater-assigned severity scores, preventing any potential artificial inflation of scores due to over-reporting of stressful events by depressed individuals. While this approach eliminates subjective bias due to the differences in the reporting of stressors, this approach does not allow for a fine-grained examination of the relative impact of single, severe stressful events as compared to the experience of numerous severely stressful events intermixed with more moderate stressors. Future research should examine how different profiles of stressful experiences may interact with affect and physiology to confer risk for depression. Furthermore, future research would benefit from the inclusion of experience sampling methods to allow for additional fine-grained examination of interpersonally stressful experiences. The inclusion of experience sampling methods would allow for an examination of affective responses to stressful experiences in daily life. While it is likely that the Trier Social Stress Test does not generalize to every experience of interpersonal stress that adolescents experience, the use of an in-vivo psychosocial stress task to elicit physiological and affective stress responses and prospectively predict longitudinal outcomes represents a novel addition to the adolescent depression literature, and a marked improvement in methodological rigor over cross-sectional and self-report designs. Future research should examine whether these associations hold in predicting more short-term changes in depressive symptoms, and whether this interaction remains predictive over years.
Overall, the results of this study offer compelling evidence to suggest that the interplay of subjective and physiological stress responses to interpersonal stressors predicts the development of depressive symptoms in adolescent girls who are exposed to severe interpersonal stressors. These findings highlight the importance of examining affective and HPA axis reactivity together as interactive components of the stress response, in the context of actual experiences of stress, rather than considering the three in isolation. While clinical work may eventually use biomarkers to assess risk for depression, this research highlights the importance of placing such biomarkers in the context of subjective emotional experiences.
Acknowledgements:
Thanks to Michael Arthur, M.S. SLP, Daphne Cole, Kathryn Fox, Michael Giordano, Karen Guan, Shahar Gur, Brian Lattner, M.S., Alyssa Poblete, Leigh Spivey, and Julia ter Haar for help with data collection and to all of the adolescents who participated in this project. This work was supported in part by a grant from the National Institute of Mental Health (R01-MH085505) to Mitchell J. Prinstein and Matthew K. Nock.
Footnotes
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Compliance with Ethical Standards
Ethical Approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
The effect of cortisol timing was not significant and removed from final analyses for model parsimony.
References
- Adam EK, Doane LD, Zinbarg R, Mineka S, Craske M, & Griffith JW (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35(6), 921–931. 10.1016/j.psyneuen.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenevoli S, Swendsen J, He J-P, Burstein M, & Merikangas KR (2015). Major depression in the national comorbidity survey–adolescent supplement: Prevalence, correlates, and treatment. Journal of the American Academy of Child & Adolescent Psychiatry, 54(1), 37–44.e2. 10.1016/j.jaac.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma EMC, Riese H, Ormel J, Verhulst FC, & Oldehinkel AJ (2009). Adolescents’ cortisol responses to awakening and social stress; Effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology, 34(6), 884–893. 10.1016/j.psyneuen.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Costello EJ, & Angold A (1988). Scales to assess child and adolescent depression: Checklists, screens, and nets. Journal of the American Academy of Child & Adolescent Psychiatry, 27(6), 726–737. 10.1097/00004583-198811000-00011 [DOI] [PubMed] [Google Scholar]
- Croes S, Merz P, & Netter P (1993). Cortisol reaction in success and failure condition in endogenous depressed patients and controls. Psychoneuroendocrinology, 18(1), 23–35. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35(7), 1562–1592. 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology. The American Psychologist, 56(3), 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, & et al. (1994). Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology, 30(4), 467–483. 10.1037/0012-1649.30.4.467 [DOI] [Google Scholar]
- Gotthardt U, Schweiger U, Fahrenberg J, Lauer CJ, Holsboer F, & Heuser I (1995). Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. The American Journal of Physiology, 268(4 Pt 2), R865–873. [DOI] [PubMed] [Google Scholar]
- Gruber J, Kogan A, Quoidbach J, & Mauss IB (2013). Happiness is best kept stable: Positive emotion variability is associated with poorer psychological health. Emotion, 13(1), 1–6. 10.1037/a0030262 [DOI] [PubMed] [Google Scholar]
- Guerry JD, & Hastings PD (2011). In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Family Psychology Review, 14(2), 135–160. 10.1007/s10567-011-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, & Herrera A (2009). Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34(7), 953–967. 10.1016/j.psyneuen.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (2005). Stress and depression. Annual Review of Clinical Psychology, 1(1), 293–319. 10.1146/annurev.clinpsy.1.102803.143938 [DOI] [PubMed] [Google Scholar]
- Hammen C (2006). Stress generation in depression: Reflections on origins, research, and future directions. Journal of Clinical Psychology, 62(9), 1065–1082. 10.1002/jclp.20293 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, & Watamura SE (2010). Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry, 68(5), 484–490. 10.1016/j.biopsych.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, & Wynne-Edwards KE (2011). Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology, 36(2), 173–181. 10.1016/j.psyneuen.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Husky MM, Mazure CM, Maciejewski PK, & Swendsen JD (2009). Past depression and gender interact to influence emotional reactivity to daily life stress. Cognitive Therapy and Research, 33(3), 264–271. 10.1007/s10608-008-9212-z [DOI] [Google Scholar]
- Kercher K (1992). Assessing subjective well-being in the old-old: The PANAS as a measure of orthogonal dimensions of positive and negative affect. Research on Aging, 14(2), 131–168. 10.1177/0164027592142001 [DOI] [Google Scholar]
- Kessler RC (1997). The effects of stressful life events on depression. Annual Review of Psychology, 48, 191–214. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, & Hellhammer DH (1995). Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology, 20(5), 509–514. 10.1016/0306-4530(94)00078-O [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The “Trier Social Stress Test” – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE Jr., Rudolph KD, Potter KI, Lambert S, … Gathright T (1999). A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment, 11(3), 326–338. 10.1037/1040-3590.11.3.326 [DOI] [Google Scholar]
- LeMoult J, Ordaz SJ, Kircanski K, Singh MK, & Gotlib IH (2015). Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. Journal of Abnormal Psychology, 124(4), 850–859. 10.1037/abn0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM (1998). Life stressors as risk factors in depression. Clinical Psychology: Science and Practice, 5(3), 291–313. 10.1111/j.1468-2850.1998.tb00151.x [DOI] [Google Scholar]
- Monroe SM, Slavich GM, & Georgiades K (2008). The social environment and life stress in depression In Gotlib IH & Hammen CL, Handbook of Depression, Second Edition. Guilford Press. [Google Scholar]
- Moons WG, Eisenberger NI, & Taylor SE (2010). Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity, 24(2), 215–219. 10.1016/j.bbi.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U, Wang L, & Garber J (2014). Cortisol reactivity to experimentally manipulated psychosocial stress in young adults at varied risk for depression. Depression and Anxiety, 31(1), 44–52. 10.1002/da.22125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady MA, Tennen H, & Armeli S (2010). Depression history, depression vulnerability and the experience of everyday negative events. Journal of Social and Clinical Psychology, 29(9), 949–974. 10.1521/jscp.2010.29.9.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara RE, Armeli S, Boynton MH, & Tennen H (2014). Emotional stress-reactivity and positive affect among college students: the role of depression history. Emotion (Washington, D.C.), 14(1), 193–202. 10.1037/a0034217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill SC, Cohen LH, Tolpin LH, & Gunthert KC (2004). Affective reactivity to daily interpersonal stressors as a prospective predictor of depressive symptoms. Journal of Social and Clinical Psychology, 23(2), 172–194. 10.1521/jscp.23.2.172.31015 [DOI] [Google Scholar]
- Pariante CM, & Lightman SL (2008). The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences, 31(9), 464–468. 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Paykel ES (2003). Life events and affective disorders. Acta Psychiatrica Scandinavica, 108, 61–66. 10.1034/j.1600-0447.108.s418.13.x [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J, Delespaul P, & deVries M (2003). Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology, 112(2), 203–211. 10.1037/0021-843X.112.2.203 [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen L-A, & Poland RE (2008). Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry, 64(6), 521–526. 10.1016/j.biopsych.2008.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD (2002). Gender differences in emotional responses to interpersonal stress during adolescence. Journal of Adolescent Health, 30(4 SUPPL. 1), 3–13. 10.1016/S1054-139X(01)00383-4 [DOI] [PubMed] [Google Scholar]
- Rudolph KD, & Flynn M (2007). Childhood adversity and youth depression: Influence of gender and pubertal status. Development and Psychopathology, 19(2), 497–521. 10.1017/S0954579407070241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, & Hammen C (1999). Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development, 70(3), 660–677. 10.1111/1467-8624.00048 [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, & Daley SE (2000). Toward an interpersonal life-stress model of depression: the developmental context of stress generation. Development and Psychopathology, 12(2), 215–234. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, & Granger DA (2011). Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology, 214(1), 209–219. 10.1007/s00213-010-1879-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler KL, Ruggero CJ, Goldstein BL, Perlman G, Klein DN, & Kotov R (2017). Diurnal cortisol interacts with stressful events to prospectively predict depressive symptoms in adolescent girls. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine, 61(6), 767–772. 10.1016/j.jadohealth.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapero BG, McClung G, Bangasser DA, Abramson LY, & Alloy LB (2017). Interaction of biological stress recovery and cognitive vulnerability for depression in adolescence. Journal of Youth and Adolescence, 46(1), 91–103. 10.1007/s10964-016-0451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JH, Eberhart NK, Hammen CL, & Brennan PA (2006). Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 35(1), 103–115. 10.1207/s15374424jccp3501_9 [DOI] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, & Kemeny ME (2010). Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience & Biobehavioral Reviews, 35(1), 39–45. 10.1016/j.neubiorev.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, & Gotlib IH (2009). Targeted rejection predicts hastened onset of major depression. Journal of Social and Clinical Psychology, 28(2), 223–243. 10.1521/jscp.2009.28.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, & Harkness KL (2013). Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology, 41(7), 1015–1026. 10.1007/s10802-013-9740-1 [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, & Niaura R (2009). Stress response and the adolescent transition: performance versus peer rejection stressors. Development and Psychopathology, 21(1), 47–68. 10.1017/S0954579409000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Poon L, Papadopoulos AS, Kumari V, & Cleare AJ (2014). Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology, 50, 289–299. 10.1016/j.psyneuen.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Tennant C (2002). Life events, stress and depression: A review of recent findings. Australian and New Zealand Journal of Psychiatry, 36(2), 173–182. 10.1046/j.1440-1614.2002.01007.x [DOI] [PubMed] [Google Scholar]
- Ursin H, & Eriksen HR (2004). The cognitive activation theory of stress. Psychoneuroendocrinology, 29(5), 567–592. 10.1016/S0306-4530(03)00091-X [DOI] [PubMed] [Google Scholar]
- van Winkel M, Nicolson NA, Wichers M, Viechtbauer W, Myin-Germeys I, & Peeters F (2015). Daily life stress reactivity in remitted versus non-remitted depressed individuals. European Psychiatry: The Journal of the Association of European Psychiatrists, 30(4), 441–447. 10.1016/j.eurpsy.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1999). The PANAS-X: Manual for the Positive and Negative Affect Schedule - Expanded form. Department of Psychological & Brain Sciences Publications. Retrieved from http://ir.uiowa.edu/psychology_pubs/11 [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, & Akil H (2000). Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 23(4), 411–418. 10.1016/S0893133X(00)00129-9 [DOI] [PubMed] [Google Scholar]