Abstract
With the advent of tyrosine kinase inhibitors, the identification of tumors with alterations in tyrosine kinase genes has become important to guide treatment. Lung adenocarcinomas harboring ALK translocations may be targeted with drugs such as crizotinib. We undertook a retrospective review of our institution’s pathology records from January 2015 through September 2017 and identified 10 lung adenocarcinomas with ALK rearrangements. We reviewed the histomorphologic features and immunohistochemical results from these 10 cases. Morphologic features included patterns such as acinar, papillary, micropapillary, and solid, as well as features such as cribriform, signet ring, and extracellular mucin. Acinar (including simple and cribriform) was the most common pattern, followed by papillary. Solid and signet ring features were the least common. These findings were consistent with prior histomorphologic studies of ALK-positive lung adenocarcinomas. Certain histomorphologic patterns are associated with ALK positivity. However, histomorphologic features are neither absolutely sensitive nor absolutely specific in suggesting ALK rearrangement. Thus, identification of lung adenocarcinomas that may benefit from treatment with tyrosine kinase inhibitors requires comprehensive molecular testing.
Keywords: ALK rearrangement, histomorphology, lung adenocarcinoma, tyrosine kinase inhibitors
Crizotinib was the first tyrosine kinase inhibitor approved by the US Food and Drug Administration for treatment of locally advanced and metastatic ALK-positive lung adenocarcinomas on the basis of superior progression-free survival and objective response rate when compared to chemotherapy—a finding that has been borne out by recent studies.1 Since crizotinib’s approval, three other ALK inhibitors have been approved: ceritinib, alectinib, and brigatinib. This targeted therapy is only applicable to cancers exhibiting the appropriate translocation, so detection of the translocation by fluorescence in situ hybridization (FISH) is a prerequisite for treatment. Because ALK rearrangement is relatively rare, comprising only 5.1% of lung adenocarcinomas in one large study,2 there have been efforts to identify morphologic features associated with ALK rearrangement to guide testing.
METHODS
Our institution routinely performs a LungSEQ genotyping panel on lung adenocarcinomas, which consists of targeted next-generation sequencing to identify mutations in nine known oncogenes (AKT1, BRAF, EGFR, ERBB2, KRAS, MAP2K1, NRAS, PIK3CA, and PTEN) and a reflex FISH panel designed to detect gene rearrangements in ALK, ROS1, and RET genes. The results of this panel are then reported in addenda to the original pathology reports. We performed a search in our electronic record of pathology reports from January 2015 through September 2017 to locate all cases of lung adenocarcinoma that underwent this panel of testing. We reviewed these reports to identify cases that were found to be positive for ALK rearrangements. The slides from these ALK-rearranged cases were pulled to identify the specific histomorphologic features present in each ALK-rearranged adenocarcinoma of the lung. We also reviewed the results of immunohistochemistry performed in support of the diagnoses in each case.
RESULTS
Between January 2015 and September 2017, we found 405 biopsy, fine-needle aspiration, and resection specimens of primary or metastatic lung adenocarcinoma diagnosed at our institution and subjected to the LungSEQ panel and reflex FISH. Ten of these cases were found to be positive for ALK gene rearrangement, representing approximately 2.5% of all cases sampled. The mean patient age for these specimens was 61 years. Age, specimen type, histomorphologic pattern, and immunohistochemical results are summarized in Table 1.
Table 1.
Summary of patient demographics, specimen type, histomorphologic patterns, and immunohistochemical findings
| Age | Specimen | Acinar | Crib | Papillary | Micropapillary | Solid | Signet ring cell | Extracellular mucin | TTF1 | Napsin-A |
|---|---|---|---|---|---|---|---|---|---|---|
| 38 | Left supraclavicular | 0 | 0 | 0 | 0 | + | 0 | 0 | + | + |
| 50 | RLL | 0 | 0 | + | 0 | + | 0 | 0 | + | NP |
| 54 | LLL | + | 0 | + | 0 | 0 | 0 | 0 | + | + |
| 59 | RLL | + | + | + | + | 0 | 0 | + | NP | NP |
| 60 | 4R LN | + | 0 | + | 0 | 0 | 0 | + | + | NP |
| 67 | 4R LN | + | + | 0 | 0 | 0 | + | + | + | NP |
| 68 | Sacrum | + | 0 | + | 0 | 0 | 0 | 0 | + | NP |
| 68 | LUL | + | + | 0 | 0 | 0 | + | + | + | + |
| 71 | 4R LN FNA | + | + | 0 | 0 | 0 | 0 | 0 | + | + |
| 74 | Aortic implant | + | + | 0 | 0 | 0 | 0 | 0 | + | + |
Crib indicates cribriform; FNA, fine needle aspiration; LLL, left lower lobe; LN, lymph node; LUL, left upper lobe; NP, not performed; RLL, right lower lobe.
Four of the specimens were from primary lung tumors, with two lobectomies, one wedge resection, and one biopsy. Two cases were biopsies of mediastinal lymph nodes, and one case was a fine-needle aspiration from a mediastinal lymph node. The remaining three cases were biopsies of various metastatic sites, including aortic implant, sacrum, and supraclavicular region. The site of origin of all metastases was confirmed by immunohistochemical positivity for TTF1. Three of the primary tumors were also found to be TTF1 positive. The identity of one of the primary tumors was not confirmed by immunohistochemistry, because the histology and clinical context were deemed sufficient for diagnosis. Napsin-A testing was also performed on a subset of cases and was positive in three of the metastases and two of the primary tumors (Table 1).
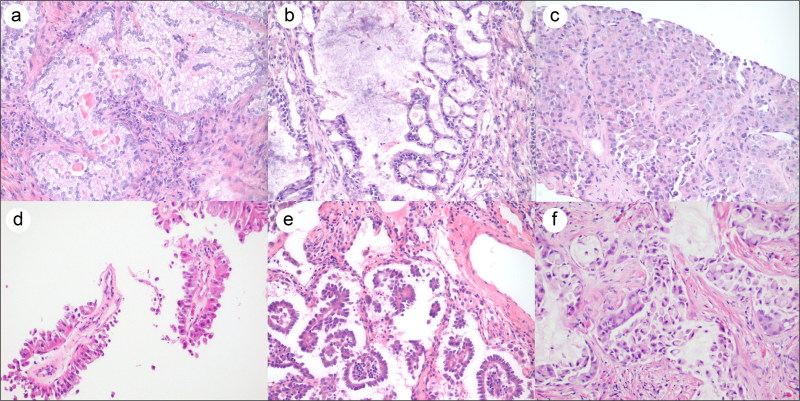
As has been done in previous studies, tumor patterns were classified according to the World Health Organization’s 2015 adenocarcinoma classification—that is, acinar, papillary, micropapillary, solid3—with additional classification by features such as signet ring (which was previously a separate classification and is treated in the new classification as a feature), cribriform (a subset of acinar with prognostic importance4), and extracellular mucin (noted to be more common in ALK-positive cancers in previous studies).2 The identified patterns are illustrated in Figure 1. By this categorization, there were eight cases of acinar pattern, including five with cribriform features. There were also five cases of papillary, one micropapillary, two solid, two with signet ring features, and four with extracellular mucin.
Figure 1.
Representative photomicrographs of the histologic patterns observed in the ALK rearranged adenocarcinomas: (a) acinar with mucin production; (b) cribriform acinar; (c) solid; (d) papillary; (e) micropapillary; (f) signet ring. Hematoxylin and eosin, ×200.
DISCUSSION
A subset of lung adenocarcinomas has been shown to harbor chromosomal translocations that involve the anaplastic lymphoma receptor tyrosine kinase (ALK) gene, resulting in fusion with the echinoderm microtubule-associated protein-like 4 (EML4) gene. This fusion protein spontaneously dimerizes, leading to unregulated activation of the tyrosine kinase domain and downstream activation of signaling pathways promoting proliferation and survival of tumor cells.5 This pathophysiologic mechanism is targeted by tyrosine kinase inhibitors such as crizotinib. There has been interest in identifying cases likely to harbor ALK translocations by histomorphology in order to better triage molecular testing. Toward this end, Nishino et al developed a scoring system to predict the likelihood of an ALK rearrangement. This group found that signet ring, micropapillary, papillary, and solid patterns were most predictive and gave them according weights in the scoring system.6 Pan et al, in contrast, did not find an association with micropapillary and papillary morphology but confirmed the association with solid and signet ring patterns and found additional associations with cribriform pattern and extracellular mucin.2
Our findings support the previously noted associations. Only two of our cases would not have been predicted to have high likelihood of carrying ALK rearrangements per the Nishino scoring system, and these two would have been suggested by the noted association between cribriform pattern and ALK rearrangement in Pan et al’s study. However, it is also notable that no absolute association between any of these features and ALK rearrangement has been observed and that our small set of ALK-rearranged lung adenocarcinomas includes almost the full spectrum of lung cancer morphologies. Thus, morphologic features are neither absolutely sensitive nor absolutely specific in suggesting ALK rearrangement, and universal screening remains the only certain way of capturing all potentially treatable ALK-positive lung cancers.
References
- 1.Nishio M, Kim DW, Wu YL, et al. Crizotinib versus chemotherapy in Asian patients with ALK-positive advanced non-small cell lung cancer. Cancer Res Treat. 2018;50(3):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84(2):121–126. doi: 10.1016/j.lungcan.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Nicholson AG, et al. WHO panel . The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 4.Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2014;27(5):690–700. doi: 10.1038/modpathol.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 6.Nishino M, Klepeis VE, Yeap BY, et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod Pathol. 2012;25(11):1462–1472. doi: 10.1038/modpathol.2012.109. [DOI] [PubMed] [Google Scholar]