Abstract
Yellow fever is a mosquito-borne viral hemorrhagic fever endemic to sub-Saharan Africa and South America associated with significant morbidity and mortality. The use of a live attenuated vaccine can prevent yellow fever, but vaccine-associated neurologic disease has been reported and is a safety concern. We present the case of a previously healthy 35-year-old active-duty man who received the yellow fever vaccine prior to deployment and subsequently developed progressive neurologic dysfunction consistent with transverse myelitis.
Keywords: Transverse myelitis, yellow fever, yellow fever vaccine–associated neurologic disease
Yellow fever (YF) is a mosquito-borne viral hemorrhagic fever endemic to sub-Saharan Africa and South America with significant morbidity and mortality. The World Health Organization estimates that 200,000 cases occur each year.1,2 YF can result in severe illness with hepatic dysfunction, renal failure, coagulopathy, and shock.3 Supportive care is the mainstay of therapy, because there is no specific treatment for YF. Primary prevention with the YF vaccine is pivotal in reducing its incidence. The Centers for Disease Control and Prevention recommends YF vaccination for travelers to and residents of endemic areas aged 9 months and older. The YF vaccine was developed in 1937, with over 600 million doses administered to date, and has been described as a safe vaccine with rare cases of serious adverse reactions.4 YF vaccine–associated neurologic disease has been reported by the Yellow Fever Working Group. Encephalitis, meningoencephalitis, Guillain-Barre syndrome, and acute disseminated encephalomyelitis are among the most frequently described neurologic complications. These complications account for 0.4 to 0.8 cases per 100,000 doses, as reported to the Vaccine Adverse Event Reporting System.5 We present the case of a 35-year-old active-duty man who received the YF vaccine prior to deployment and subsequently developed progressive neurologic dysfunction consistent with transverse myelitis.
CASE PRESENTATION
A 35-year-old active-duty man with no significant past medical history presented to the hospital with a 3-week history of progressively worsening urinary retention, sensory disturbances and weakness in both arms and legs, loss of balance, and difficulty ambulating. Examination revealed moderately decreased strength in the lower extremities, ascending sensory loss up to the level of the elbows and knees, and diffuse hyperreflexia without clonus. Within the last month he had received multiple predeployment vaccines (meningococcal, tetanus-diphtheria-pertussis, typhoid, and YF) and had a mild self-limited upper respiratory tract infection. Magnetic resonance imaging (MRI) of the brain showed nonspecific findings that may have been related to the patient’s clinical presentation (Figure 1). Multiple MRI examinations of the spine revealed longitudinally extensive transverse myelitis (Figure 2). An extensive workup for primary neurologic, infectious, and malignant etiologies was obtained. Pertinent results in cerebrospinal fluid (CSF) were lymphocytic pleocytosis (white blood cells 300 cells/mcL, lymphocytes 82%), elevated protein (147.24 mg/dL), qualitatively positive immunoglobulin M (IgM) antibodies to YF vaccine virus, and negative neuromyelitis optica-immunoglobulin G (NMO-IgG) antibodies (titer <1:1). The patient was started on high-dose intravenous corticosteroid therapy with suboptimal results. His treatment regimen was escalated to 5 days of plasmapheresis, which resulted in adequate improvement in symptoms. He was subsequently discharged to a neurologic rehabilitation center.
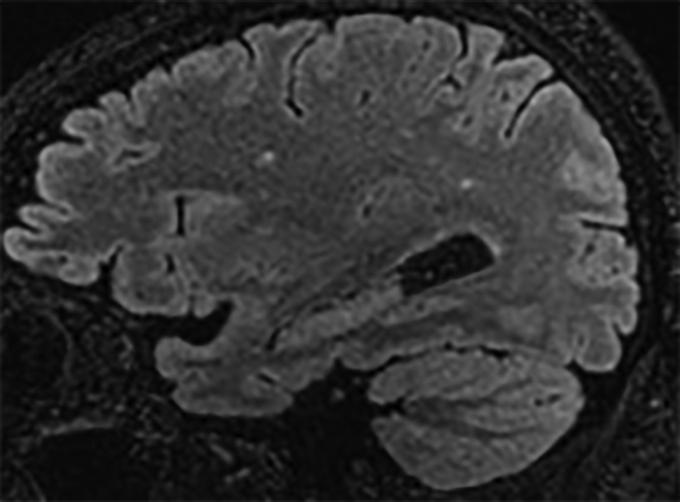
Figure 1.
A representative parasagittal volumetric T2 fluid-attenuated inversion recovery image of the brain demonstrating a few subcentimeter foci of signal abnormality confined to the white matter of the cerebral hemispheres. This is a nonspecific pattern that may be seen as a result of infection and inflammation (as well as prior trauma, migraine-related change, and primary demyelination). There is no evidence of meningitis.
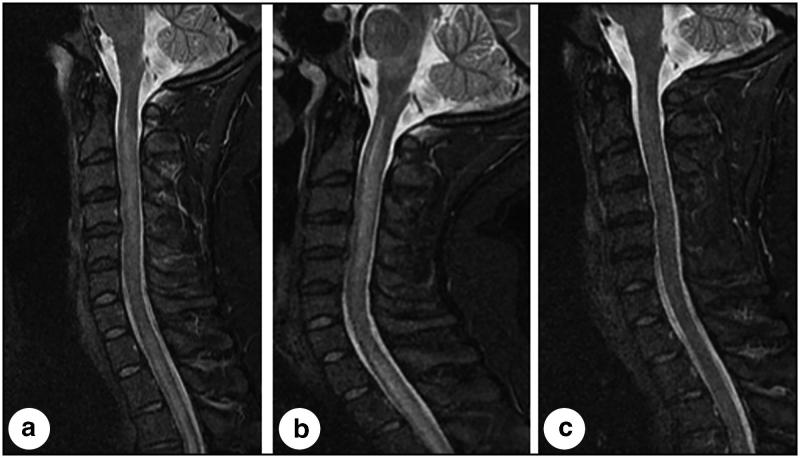
Figure 2.
Three midline sagittal short tau inversion recovery images of the cervical spine obtained throughout the clinical course of this patient. (a) At presentation, the image demonstrates longitudinally extensive signal abnormality in the spinal cord extending from the cervical-medullary junction into the thoracic spinal cord (not completely visualized). (b) Three days later, the cord became more edematous, resulting in further narrowing of the subarachnoid space, and signal abnormality became more evident in the brainstem, particularly the medulla. (c) Seventeen days after presentation, following corticosteroid and plasmapheresis therapy, and correlating with a profound clinical improvement, the image demonstrates nearly completely resolved signal abnormality and resolved edema in the brainstem and spinal cord.
DISCUSSION
Transverse myelitis associated with YF vaccination has been infrequently reported in the medical literature. A case report by Chaves et al2 described a 56-year-old man with urinary retention and lower-extremity paresthesia 45 days after YF vaccination. CSF testing was positive for YF-specific IgM. NMO-IgG testing was not performed. MRI imaging showed longitudinal myelitis. The patient exhibited spontaneous symptom improvement 5 days after admission. This case shared similarities in clinical presentation, CSF results, and MRI findings. They differed in the timing of symptom onset following YF vaccination, testing for NMO-IgG, and treatment course.
McMahon et al5 described 15 cases of YF vaccine–associated neurologic disease within 30 days of YF vaccination. They identified five cases of encephalitis and one case of acute disseminated encephalomyelitis that were confirmed by the presence of YF-specific IgM in CSF. Martins et al6 reported 67 cases of YF vaccine–associated neurologic disease. Meningoencephalitis with positive YF-specific IgM in CSF accounted for 55 of these cases. One case of transverse myelitis without confirmation of YF-specific IgM was also reported.
According to McMahon et al,5 the presence of YF-specific IgM in CSF with clinically compatible illness and recent YF vaccination is sufficient evidence for causality when no other apparent etiology is found. IgM does not readily cross the blood-brain barrier; thus, its presence in CSF suggests intrathecal antibody production in response to nervous system infection. This assumption is limited by a lack of published cases reporting YF-specific IgM in CSF of YF vaccine recipients without YF vaccine–associated neurologic disease. Additionally, tests for YF-specific IgM can cross-react with antibodies to other flaviviruses, which may yield a false-positive result.5
Current efforts to develop a new inactivated YF vaccine to reduce serious adverse events have shown good immunogenicity in preclinical and clinical studies.7,8 YF vaccination is not contraindicated in high-risk patients, because it remains the most important strategy of primary prevention against YF and the overall risk of developing adverse effects is low. YF has significant morbidity and mortality and without immunization large-scale epidemics might result. Early recognition of vaccine-associated complications is paramount for prompt treatment and prevention of long-term disability.
References
- 1.World Health Organization District Guidelines for Yellow Fever Surveillance (Publication no. WHO/EPI/GEN 98.09). Geneva, Switzerland: World Health Organization; 1998. Available at http://www.who.int/emc-documents/yellow_fever/whoepigen9809c.html. [Google Scholar]
- 2.Chaves M, Riccio P, Patrucco L, Rojas JI, Cristiano E. Longitudinal myelitis associated with yellow fever vaccination. J Neurovirol. 2009;15(4):348–350. doi: 10.1080/13550280903062805. [DOI] [PubMed] [Google Scholar]
- 3.Monath TP, Barrett AD. Pathogenesis and pathophysiology of yellow fever. Adv Virus Res. 2003;60:343–395. [DOI] [PubMed] [Google Scholar]
- 4.Cetron MS, Marfin AA, Julian KG, et al. . Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm Rep. 2002;51(RR-17):1–11. [PubMed] [Google Scholar]
- 5.McMahon AW, Eidex RB, Marfin AA, et al. ; Yellow Fever Working Group . Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine. 2007;25(10):1727–1734. doi: 10.1016/j.vaccine.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Martins RM, Pavão AL, de Oliveira PM, et al. . Adverse events following yellow fever immunization: report and analysis of 67 neurological cases in Brazil. Vaccine. 2014;32(49):6676–6682. doi: 10.1016/j.vaccine.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen LH, Hamer DH. Vaccination challenges in confronting the resurgent threat from yellow fever. JAMA. 2017;318(17):1651–1652. doi: 10.1001/jama.2017.14258. [DOI] [PubMed] [Google Scholar]
- 8.Monath TP, Fowler E, Johnson CT, et al. . An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364(14):1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]