Objective:
To describe the main tomographic findings in patients with acute Q fever pneumonia.
Methods:
We analyzed high-resolution CT (HRCT) findings from six male patients (mean age, 22.6 years) with confirmed diagnoses of acute Q fever. Two chest radiologists analyzed the images and reached decisions by consensus. All patients presented fever, myalgia, prostation, headache, and dry cough. They also had common epidemiologic factors (recent travel for military service, where they had contact with sheep and capybara). Diagnoses were confirmed by the detection of C. burnetii DNA in clinical samples by polymerase chain reaction.
Results:
The predominant HRCT findings were areas of consolidation (100%) and nodules (66.6%) with halos of ground-glass opacity, predominantly with segmental and peripheral distributions. Lesions affected all lobes, and predominated in the left upper and lower lobes. Involvement of more than one lobe was observed in four patients. No pleural effusion or lymph node enlargement was found.
Conclusion:
The predominant HRCT findings in patients with acute Q fever pneumonia were bilateral, peripheral areas of consolidation and nodules with irregular contours and halos of ground-glass opacity.
Advances in knowledge:
Acute Q fever should be included in the differential diagnosis of lesions with the halo sign on HRCT.
Introduction
Q fever is a zoonotic disease caused by Coxiella burnetii, an obligate intracellular Gram-negative aerobic bacterium. The incubation period can extend up to 2–4 weeks after exposure, and the infection is usually self-limiting.1 The most common mode of transmission in humans is inhalation of infectious aerosols directly from birth fluids of infected animals or in dust contaminated with dried birth fluids or excreta.2 Although small wild rodents seem to be important reservoirs, the most commonly identified sources of human infection are farm animals such as cattle, goats, and sheep.3 Q fever has acute and chronic stages that correspond to two distinct antigenic phases of antibody response.2 Clinical symptoms develop in approximately half of infected persons, and the most common clinical manifestation of acute disease is a flu-like illness characterized by myalgia, headache, anorexia, asthenia, high fever and dry cough, with pneumonia and hepatitis occurring in more severe cases.2 Chronic disease is rare and reflects the persistence of the pathogen after primary infection. Typically, chronic Q fever is characterized by endocarditis in patients with pre-existing risk factors, such as valvular or vascular defects.2,4 Pulmonary involvement is possible in the acute and chronic forms.5–8
The diagnosis of Q fever is based on serologic, clinical, and radiologic criteria. Polymerase chain reaction (PCR) of whole blood or serum provides rapid results and can be used to diagnose acute Q fever in the first 2 weeks after symptom onset but before antibiotic administration. A negative acute titer does not rule out Q fever because most patients seroconvert by the third week of illness. After the 2 week post-onset period and when the date of illness onset is not known, serology is recommended for initial testing.2 The diagnosis of chronic Q fever requires demonstration of an increased Phase I immunoglobulin G antibody (≥1:1024) and an identifiable persistent infection (e.g. endocarditis).2,4,7 Doxycycline is the drug of choice for adults, children aged ≥8 years, and patients of any age with severe infection. Serologic monitoring is recommended following acute Q fever infection to assess possible progression to the chronic form of the disease.2 The purpose of this study was to describe high-resolution CT (HRCT) findings from six patients with microbiologic diagnosis of acute Q fever pneumonia.
methods AND MATERIALs
Our institutional review board approved this study and waived the requirement for informed patient consent. All data used in this study were anonymized. This study involved the retrospective analysis of HRCT images from six consecutive patients with confirmed acute Q fever, performed during a 2 week hospitalization period in December 2016. All patients participated in a 1 week military expedition in Ribeirão das Lages, a rural area in the State of Rio de Janeiro, Brazil. The military unit camped near a river and had contact with sheep and capybara.
The sample included six males with a mean age of 22.6 (range, 20–28) years. The clinical presentation was acute respiratory infection characterized by fever, dry cough, dyspnea, myalgia, asthenia, malaise, and anorexia, starting a few days after contact with these animals. Five of six patients had ill-defined opacities on chest X-ray. The other patient had normal chest X-ray findings. All six patients were hospitalized for 1–2 weeks, and underwent HRCT immediately after hospitalization (3–5 days after symptom onset). Based on the reported contact and clinical findings, Q fever was suspected. The diagnosis was confirmed by PCR, which detected C. burnetii DNA in blood samples. No other military personnel participating in the expedition became ill. All patients showed progressive improvement of the symptoms after the initiation of antibiotic treatment, becoming asymptomatic and finally being discharged. Control chest X-rays performed an average of 1 month after the onset of symptoms were normal.
The HRCT scans were performed using helical acquisition and reconstructed with 1–2 mm slice thickness and 1–2 mm intervals using a high-spatial-frequency reconstruction algorithm. The acquisition time was 0.5–1 s per rotation, peak voltage was 120 kVp, modulated tube current was 100–400 mA, pitch was 1, and matrix was 512 × 512 pixels (MDTC Brilliance 64; Philips, Chicago, IL). Two chest radiologists with more than 15 years of experience independently reviewed the images using mediastinal (width, 350–450 HU; level, 10–20 HU) and lung (width, 1200–1600 HU; level, 2500–2700 HU) window settings, and reached final decisions regarding the findings by consensus. The radiologists were blinded to patient demographics, clinical data, and final diagnoses. Parenchymal abnormalities were defined according to the recommendations of the Fleischner Society glossary of terms for thoracic imaging.9 The HRCT scans were assessed to determine the presence, distribution, and extent of the following findings: air–space consolidation, ground-glass opacity, halo sign, and nodules. The axial distribution of lesions in the lung parenchyma was classified as central, peripheral, or diffuse. The distribution was considered to be peripheral when abnormalities were predominant in the outer third of the lung periphery, and central when abnormalities were predominant in the inner two-thirds of the transverse plane. The location of abnormalities was recorded in relation to six lobes, considering the lingula as a lobe. Lesions were defined as lobar when one or more lobes were involved, segmental when one or more segments were involved, and patchy when a subsegment of one or more segments was involved.
Results
The main HRCT patterns consisted of consolidations and nodules with halos of ground-glass opacity. Consolidations were observed in six (100%) patients, and nodules were observed in four (66.6%) patients. Four (66.6%) patients presented association of nodules and consolidation, and two (33.3%) patients had only consolidations. Consolidations had halos of ground-glass opacity in six (100%) cases (Figure 1). Four (66.6%) patients had nodules with halos of ground-glass opacity. Thus, all patients presented lesions with the halo sign (Figures 2 and 3).
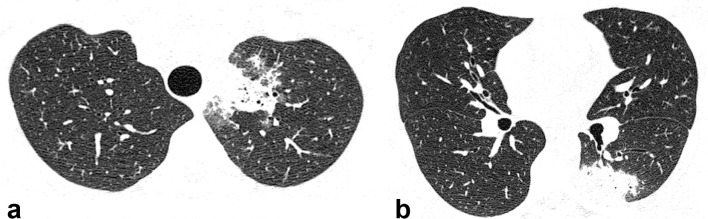
Figure 1.
Axial HRCT images from a 20-year-old male with Q fever showing peripheral consolidations in the left upper (a) and left lower (b) lobes. HRCT, high-resolution CT.
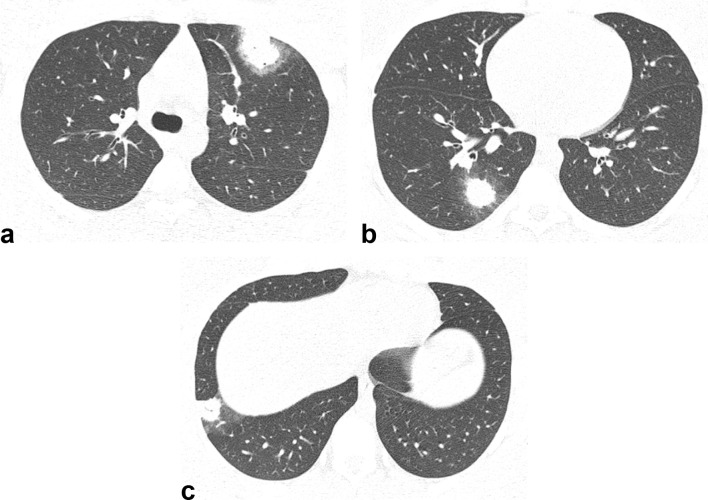
Figure 2.
Axial HRCT images from a 22-year-old male with Q fever showing peripheral nodules in the left upper lobe (a) and right lower lobe (b, c). Note that the nodules are surrounded by halos of ground-glass opacity (halo sign). HRCT, high-resolution CT.
Figure 3.
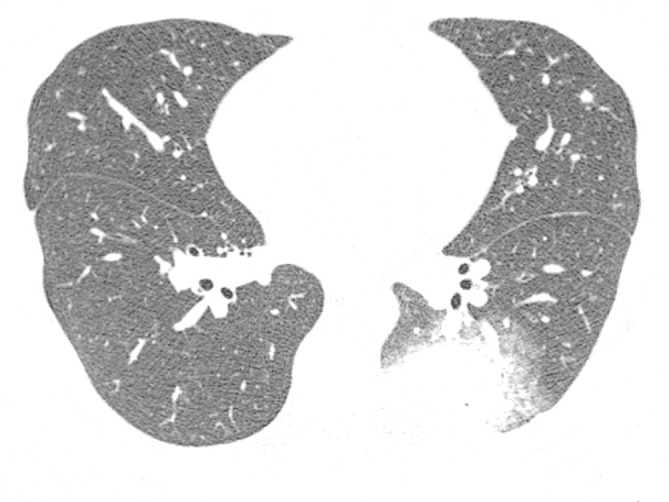
Axial HRCT image from a 28-year-old male with Q fever showing a nodule with irregular contours and a halo of ground-glass opacity in the left lower lobe. HRCT, high-resolution CT.
HRCT scans from the six patients showed a total of 16 lesions comprising 8 (50%) consolidations and 8 (50%) nodules. All 16 (100%) lesions had irregular contours. 14 (87.5%) lesions presented halos of ground-glass opacity: 8 (57.2%) were consolidations and 6 (42.8%) were nodules. All eight (100%) consolidations showed halos of ground-glass opacity. Six of the eight (75%) nodules presented the halo sign. Peripheral distribution of lesions was observed in all (100%) cases. 14 (87.5%) lesions were defined as segmental and 2 (12.5%) were characterized as patchy. Multilobar distribution occurred in four (66.6%) patients. Six (37.5%) lesions were located in the right lower lobe, three (18.7%) were in the left upper lobe, three (18.7%) were in the left lower lobe, two (12.5%) were in the lingula, one (6.3%) was in the middle lobe, and one (6.3%) was in the right upper lobe. No pleural effusion or lymph node enlargement was found.
Discussion
Pneumonia is one of the primary clinical manifestations of acute Q fever.2 Q fever pneumonia can range from mild to severe, and numerous patients have extrapulmonary manifestations, including severe headache, myalgia, and arthralgia.2,5,6,8,10 The onset of symptoms can be gradual or abrupt, with variable severity. Typically, pneumonia in acute Q fever infection results in a dry to productive cough, pleuritic chest pain, and focal or bilateral infiltrates that are visible on chest radiographs.11 Although the mortality rate is <2% in patients with acute Q fever, up to 50% of patients were hospitalized in an outbreak in the Netherlands.2 Acute respiratory failure and acute respiratory distress syndrome are rare manifestations of Q fever.6,11 The majority of patients with acute Q fever present abnormalities on chest radiographs,2 although the findings are non-specific. The most common abnormalities are pulmonary infiltrates and single or multiple consolidations, with segmental or lobar distribution, involving the upper or lower lobes. In the early stages of the disease, chest radiographs might be normal.11–16 Importantly, Q fever cannot be differentiated from other causes of community-acquired pneumonia based solely on radiographic findings.6
HRCT is rarely required for the diagnosis of this disease, which may explain the paucity of studies concerning the tomographic findings of acute Q fever.7,8,17 Nevertheless, in selected cases in which radiographic findings are confusing or coexistent disease is suspected, chest CT may be requested.17 The HRCT features of acute Q fever pneumonia consist of airspace consolidation or nodules, which may be associated with a halo of ground-glass opacity, in a segmental, patchy, or lobar distribution.8,17
All cases in our series had been in contact with sheep and capybara, presumably C. burnetii reservoirs, on a military trip. Coxiella burnetii DNA was identified by PCR of blood samples. HRCT showed a total of 16 lesions in this population. The predominant HRCT findings were bilateral peripheral consolidations and nodules with irregular contours and halos of ground-glass opacity. All six patients presented consolidations with halos of ground-glass opacity, and four patients had nodules with such halos. Thus, both consolidations and nodules were present in four patients. In five patients, all lesions had halos of ground-glass opacity. Multilobar distribution was found in four patients. The right lower lobe was the most frequently involved location in our sample (six lesions in three patients). All 16 lesions presented peripheral distribution.
Few studies have presented HRCT findings from patients with acute Q fever.7,8,17 Voloudaki et al17 described tomographic findings from 12 patients. HRCT revealed airspace consolidations in all cases, with lobar, segmental, patchy, and combined distributions. One patient presented nodular lesions with halos of ground-glass opacity. The authors found lesions in all lobes. The right lower lobe was the most frequently affected. Involvement of more than one lobe was detected by HRCT in seven patients, with no sparing of or predilection for any particular lobe.
Several differences between our study and that of Voloudaki et al17 should be noted. Their patients were diagnosed during a period about 8 years. All of our cases occurred in the same period, related to a microepidemic in a military camp. The predominant pattern observed in all cases described by Voloudaki et al17 was airspace consolidation, with only one patient presenting nodular lesions. Lesions with halos of ground-glass opacity were observed in all (100%) cases in our study, but in only 1 of 12 (8.33%) cases examined by Voloudaki et al.17 This difference can probably be explained by the long time interval (>20 years) between the two studies. Voloudaki et al17 performed CT examinations with older technology, using 8–10 mm sections. In five patients, additional thinner (5 mm) sections were obtained. We used 1–2 mm slice thicknesses, which have greater sensitivity for the evaluation of ground-glass opacity. Voloudaki et al17 observed pleural effusion in three (25%) and lymph node enlargement in four (33.3%) of their patients. None of our patients had lymph node enlargement or pleural effusion.
Gikas et al18 showed that segmental pneumonia was observed as a unilateral single area of opacity in 38 (72%) patients, and was located more frequently in the upper lobes. The left upper lobe was most frequently involved (in 31% of cases). The authors found no correlation between the extent of pulmonary involvement and the course of the disease.
Several pathologic findings have been reported to account for the presence of ground-glass opacity surrounding pulmonary lesions on HRCT; main findings are tumor cells, inflammatory infiltrates, and, most commonly, alveolar hemorrhage.19 Coxiella burnetii infection was believed to be a rare and exceptional cause of the halo sign.8,17 However, 100% of patients in our series presented consolidations with halos of ground-glass opacity, and 66.6% had nodules with the halo sign. To date, the histopathological correlation of the halo sign in Q fever has not been described. According to HRCT data from a few previous studies,8,16 and especially due to the great frequency of lesions with the halo sign found in our study, Q fever should be considered in the differential diagnosis of lesions with the halo sign in appropriate clinical and epidemiologic contexts.
No specific tomographic pattern allows the differentiation of Q fever from the spectrum of other infectious diseases associated with an airspace consolidation pattern on HRCT, or from diseases that cause lesions with the halo sign.8,17 Lesions with peripheral distribution, such as those of thromboembolism and infarction, also must be considered in the differential diagnosis of Q fever.
Our study has some limitations, including the small sample and lack of histopathological confirmation of the tomographic features. Although our sample was small, the HRCT patterns observed in this study are consistent with previous reports of HRCT findings in patients with Q fever. To our knowledge, this study documents the highest reported frequency of lesions with the halo sign, detected by HRCT, in patients with acute Q fever. Further studies are necessary to draw conclusions about the pathologic cause of the halo sign.
In conclusion, the most common HRCT findings in our series of patients with Q fever were consolidations and nodules with halos of ground-glass opacity, predominantly with segmental and peripheral distributions. In addition, the lesions affected all lobes, and were located most frequently in the lower and left upper lobes. In the appropriate clinical setting, these findings have diagnostic value. Moreover, our findings contribute to the inclusion of acute Q fever in the differential diagnosis of lesions with the halo sign on HRCT.
Contributor Information
Felipe Mussi von Ranke, Email: feliperanke@yahoo.com.br.
Fernanda Miraldi Clemente Pessoa, Email: fernandamiraldi@hotmail.com.
Felipe Batista Afonso, Email: felipebafonso@hotmail.com.
Josiani Bastos Gomes, Email: josi@engelnet.com.br.
Danielle Provençano Borghi, Email: danielle.borghi@uol.com.br.
Alessandro Severo Alves de Melo, Email: alesevero@gmail.com.
Edson Marchiori, Email: edmarchiori@gmail.com.
REFERENCES
- 1.Marrie TJ. Coxiella burnetii pneumonia. Eur Respir J 2003; 21: 713–9. doi: 10.1183/09031936.03.00099703 [DOI] [PubMed] [Google Scholar]
- 2.Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, et al. Diagnosis and management of Q fever--United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep 2013; 62(RR-03): 1–23. [PubMed] [Google Scholar]
- 3.Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol 1998; 36: 1823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson AD, Nicholson WL, Kruszon-Moran D, Candee AJ, Loftis AD, Patterson NE, et al. Seroprevalence of Q Fever in the United States, 2003–2004. The American Journal of Tropical Medicine and Hygiene 2009; 81: 691–4. doi: 10.4269/ajtmh.2009.09-0168 [DOI] [PubMed] [Google Scholar]
- 5.Raoult D, Marrie TJ, Mege JL. Natural history and pathophysiology of Q fever. The Lancet Infectious Diseases 2005; 5: 219–26. doi: 10.1016/S1473-3099(05)70052-9 [DOI] [PubMed] [Google Scholar]
- 6.Parker NR, Barralet JH, Bell AM. Q fever. The Lancet 2006; 367: 679–88. doi: 10.1016/S0140-6736(06)68266-4 [DOI] [PubMed] [Google Scholar]
- 7.Polo MF, Mastrandrea S, Santoru L, Arcadu A, Masala G, Marras V, et al. Pulmonary inflammatory pseudotumor due to Coxiella burnetii. Case report and literature review. Microbes Infect 2015; 17(11-12): 795–8. doi: 10.1016/j.micinf.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zang GQ, Tang ZH, Yu YS. The halo sign of Q fever pneumonia. Braz J Infect Dis 2015; 19: 220–1. doi: 10.1016/j.bjid.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 10.Madariaga MG. Q fever wildlife reservoir. Emerg Infect Dis 2005; 11: 776–7. doi: 10.3201/eid1105.041272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartzell JD, Peng SW, Wood-Morris RN, Sarmiento DM, Collen JF, Robben PM, et al. Atypical Q fever in US soldiers. Emerg Infect Dis 2007; 13: 1247–9. doi: 10.3201/eid1308.070218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glücker LG, Munk J. Roentgenologic pulmonary manifestations of Queensland fever. J Fac Radiol 1952; 3: 186–92. doi: 10.1016/S0368-2242(52)80004-1 [DOI] [PubMed] [Google Scholar]
- 13.Schimmer B, Dijkstra F, Vellema P, Schneeberger PM, Hackert V, ter Schegget R, et al. Sustained intensive transmission of Q fever in the south of the Netherlands, 2009. Eurosurveillance 2009; 14: 3. doi: 10.2807/ese.14.19.19210-en [DOI] [PubMed] [Google Scholar]
- 14.Pickworth FE, el-Soussi M, Wells IP, McGavin CR, Reilly S. The radiological appearances of 'Q' fever pneumonia. Clin Radiol 1991; 44: 150–3. doi: 10.1016/S0009-9260(05)80857-8 [DOI] [PubMed] [Google Scholar]
- 15.Smith DL, Wellings R, Walker C, Ayres JG, Burge PS. The chest X ray in Q-fever: a report on 69 cases from the 1989 West Midlands outbreak. Br J Radiol 1991; 64: 1101–8. doi: 10.1259/0007-1285-64-768-1101 [DOI] [PubMed] [Google Scholar]
- 16.Caron F, Meurice JC, Ingrand P, Bourgoin A, Masson P, Roblot P, et al. Acute Q fever pneumonia: a review of 80 hospitalized patients. Chest 1998; 114: 808–13. [DOI] [PubMed] [Google Scholar]
- 17.Voloudaki AE, Kofteridis DP, Tritou IN, Gourtsoyiannis NC, Tselentis YJ, Gikas AI. Q fever pneumonia: CT findings. Radiology 2000; 215: 880–3. doi: 10.1148/radiology.215.3.r00jn21880 [DOI] [PubMed] [Google Scholar]
- 18.Gikas A, Kofteridis D, Bouros D, Voloudaki A, Tselentis Y, Tsaparas N. Q fever pneumonia: appearance on chest radiographs. Radiology 1999; 210: 339–43. doi: 10.1148/radiology.210.2.r99fe20339 [DOI] [PubMed] [Google Scholar]
- 19.Alves GR, Marchiori E, Irion K, Nin CS, Watte G, Pasqualotto AC, et al. The halo sign: HRCT findings in 85 patients. J Bras Pneumol 2016; 42: 435–9. doi: 10.1590/s1806-37562015000000029 [DOI] [PMC free article] [PubMed] [Google Scholar]