Abstract
Objective:
The study performs a comprehensive analysis of image metrics to objectively support the reduction of injected activity in pediatric oncology 18F-FDG PET/MR (18F-fludeoxyglucose PET/MR) examinations. Contrast-to-Noise Ratio (CNR), Normalized Noise (NN), tumor burden, and standardized uptake value (SUV) parameters stability were investigated to robustly define the acceptable reduced activity level that preserves the clinical utility of images, considering different PET applications.
Methods:
21 PET/MRI examinations performed on a 3-Tesla Biograph mMR scanner were analyzed. Tracer activity reduction was stimulated by decreasing the count statistics of the original list-mode data (3 MBq kg–1). In addition to the already studied SUV metrics and subjective scoring on lesion detectability, a thorough analysis of CNR, NN, Metabolic Tumor Volume (MTV), and Total Lesion Glycolysis (TLG) was performed.
Results:
SUVmax and SUVmean increased more than 5% only in 0.6 MBq kg–1 reconstructed images (+10% and +9%, respectively), while SUVpeak was almost unaffected (average variations < 2%). The quantified CNR, NN, MTV, and TLG behavior with the decrease of the injected activity clearly defines 1.5 MBq kg–1 as a threshold of activity after which the quality of the image degrades. Subjective and objective analyses yielded consistent results. All 56 lesions were detected until activity of 1.2 MBq kg–1, whereas five lesions were missed on the 0.6 MBq kg–1 image. Perceived image quality (IQ) decreased in Lower Tracer Activity (LTA) images but remained acceptable until 1.5 MBq kg–1.
Conclusion:
Results about the stability of image metrics beyond the semi-quantitative SUV parameters and subjective analysis, rigorously proves the feasibility of the reduction of injected activity to 1.5 MBqkg-1 for pediatric patients aged between 7 and 17 years.
Advances in knowledge:
This is the first report on the quantitative evaluation of the effect of activity reduction on image quality in pediatric PET/MR. The findings offer objective corroboration to the feasibility of a significant dose reduction without consequences on clinical image reading and tumor burden metrics.
Introduction
18F-FDG PET/CT (18F-fludeoxyglucose PET/CT) has become a standard procedure in many types of neoplasms in children.1–11 The combination of anatomical and metabolic imaging modalities provides accurate diagnostic information useful in initial staging, therapy monitoring, and follow-up of different pediatric diseases. Nevertheless, the introduction of integrated PET/MR scanners in clinical practice has raised interest in its use and benefit in pediatric patients. Published studies have confirmed its non-inferiority to PET/CT in many oncological applications.12–15
The main reasons in support of PET/MR use in children are the potential of MRI to complement PET metabolic information and the significant reduction in radiation exposure. The latter is of great interest in children because they are more radiosensitive than adults and thus prone at risk to develop secondary tumors during their lifetime.16–21 A dose reduction in PET/MR scanner is possible thanks to both the lack of the CT component and the feasibility to reduce the injected activity. Indeed, in PET/MR, because of the MR scanning duration, PET acquisition time can be set longer than the standard PET/CT.
It has been shown13 that this increased scan time makes feasible a proportional reduction of the injected activity while preserving lesion detectability and image quality (IQ). Nevertheless, although recent studies concerning adult patient PET examinations have proposed quantitative metrics for IQ analyses,22–25 only a subjective scoring system26,27 has been used in pediatric studies.13 This approach depends heavily on the observer's experience, and it is not easy to compare results in different settings.
Furthermore, given the relevance of PET in cancer management, including tumor detection, staging, volume identification for radiation treatment and treatment monitoring, it is advisable that other image parameters be studied in addition to subjective scoring and semiquantitative metrics13 to support the reduction of activity in clinical practice reliably.
Therefore, this study aimed to investigate the effect of administered activity reduction on the metrics related to image quality, lesions detectability, and tumor burden information in PET/MR examinations in pediatric oncology. The stability of these parameters can identify the activity level requested to guarantee the clinical utility of the images, as proposed by Armstrong and colleagues in a different context.28 For the sake of completeness, a subjective analysis was also carried out.
Two different segmentation methods were considered: the first mimicked the clinical situation in which only the reduced injected activity acquisition with low statistical counts is the available volume; the second used a fixed volume of interest (VOI) that is pasted into all acquisitions, resembling the situation in which a modality other than PET is used to define the VOIs.
Methods and materials
Data acquisition and reconstruction
Between March 2015 and December 2016, a trial was conducted at the Nuclear Medicine Department for clinical validation of 18F-FDG PET/MR through18F-FDG PET/CT comparison (Ethical Committee N. 2772P). 17 patients with an average age of 12.7 ± 3.1 (range: 7–17 years) who were diagnosed with solid cancers (11 Hodgkin’s lymphoma, 2 non-Hodgkin’s lymphoma, 4 Rhabdomyosarcomas) underwent an 18F-FDG PET/CT scan followed by PET/MR. A total of 21 PET/RM scans were included in this study, with 4 patients imaged twice.
Patients consisted of nine girls and eight boys. Patients data are listed in Table 1. Acquisitions were performed on a 3-Tesla clinical PET/MR hybrid scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany). Patients were injected with 3 MBq/kg of 18F-FDG,29 following national and international guidelines,30,31 taking into account the higher sensitivity of the solid-state PET detectors and the 22 cm axial field-of-view of the PET/MR scanner, compared to the PET/CT scanners used as a reference in the European association of nuclear medicine pediatric dosage card/dose calculator. All patients fasted at least 6 h before the examination and PET/MR. The transfer between the two scanners, which are in separate buildings, determined a longer uptake time for PET/MR acquisitions.
Table 1.
Patients data
| Patient | Age at the time of PET/MR (y) | Weight (kg) | Height (cm) | Administered activity (MBq) |
Uptake
time (min) |
N° of lesions |
| 1 (M) | 12,8 | 47 | 1,63 | 144 | 128 | 4 |
| 2 (F) | 15,9 | 48 | 1,57 | 147 | 129 | 4 |
| 16,5 | 50 | 1,57 | 144 | 137 | 1 | |
| 3 (M) | 8,6 | 44 | 1,37 | 127 | 168 | 2 |
| 4 (M) | 12,0 | 42 | 1,50 | 119 | 152 | 4 |
| 5 (F) | 13,2 | 36 | 1,50 | 106 | 111 | 2 |
| 13,4 | 34 | 1,50 | 100 | 92 | 1 | |
| 6 (M) | 15,0 | 57 | 1,74 | 170 | 73 | 5 |
| 7 (F) | 10,4 | 35 | 1,38 | 99 | 168 | 3 |
| 10,7 | 33 | 1,41 | 98 | 214 | 2 | |
| 8 (F) | 16,6 | 40 | 1,68 | 118 | 140 | 1 |
| 9 (F) | 9,2 | 27 | 1,34 | 103 | 145 | 2 |
| 10,0 | 31 | 1,35 | 87 | 80 | 2 | |
| 10(F) | 16,1 | 58 | 1,65 | 174 | 120 | 5 |
| 11 (M) | 6,9 | 29 | 1,24 | 86 | 77 | 3 |
| 12 (F) | 15,1 | 75 | 1,68 | 225 | 178 | 3 |
| 13 (F) | 11,3 | 28 | 1,45 | 90 | 86 | 4 |
| 14 (M) | 9,8 | 52 | 1,50 | 156 | 77 | 1 |
| 15 (F) | 9,8 | 57 | 1,64 | 169 | 124 | 4 |
| 16 (M) | 16,9 | 68 | 1,67 | 203 | 66 | 1 |
| 17 (M) | 16,4 | 85 | 1,65 | 263 | 158 | 2 |
| Average±standard deviation | 12,7 ± 3,1 | 46 ± 16 | 1,52 ± 0,14 | 139 ± 48 | 125±40 | |
| Range | 6,9–16,9 | 27–85 | 1, 24–1,74 | 86–263 | 66–214 |
PET, positron emission tomography.
PET data were acquired in list mode with 5 min per bed position, and a total number of beds ranging from 4 to 6, depending on patient size. Corrections for random coincidences, daily normalization, dead-time losses, and scatter were applied. Attenuation correction was performed with MR Dixon sequence, using Siemens maximum-Likelihood activity and attenuation (MLAA) approach. Acquisitions were reconstructed with a three-dimensional Ordinary Poisson Ordered Subset Expectation Maximization algorithm with three iterations, 21 subsets, 172 × 172 matrix, a voxel size of 4.17 × 4.17 × 2.03 mm, and 4 mm gaussian filter, as recommended by the manufacturer. The MR protocol includes axial Turbo Spin Echo, axial T1 weighted Turbo Inversion Recovery Magnitude, axial Diffusion Weighted Imaging (b = 50/800 s mm–2 and b = 50/1000 s/mm2) and axial Half-Fourier Acquisition Single-Shot Turbo Spin Echo T2 weighted. Reduction of administered tracer activity from 3MBqkg-1 to 0.6 MBqkg-1 (3.0, 2.4, 1.8, 1.5, 1.2, 0.6 MBq kg–1) was simulated truncating the original list data at 4, 3, 2.5, 2, and 1 min for bed position.32 For each patient, a total of six acquisitions, including the original, were generated and reconstructed with Siemens e7-tools (a software package provided by the manufacturer of PET/MR system). In the following, FTA and LTA stand for the Full Tracer Activity and the Lower Tracer Activity images, respectively.
VOI definition
Two strategies have been adopted for lesion VOI segmentation: a) both for LTA and FTA images, a 40% threshold of the maximum standardized uptake value (SUVmax) was applied to define the relative VOI isocontour33 using the same segmentation bounding box; b) each VOI identified in the FTA images with the criteria described above was copied identically in all reconstructed LTA images. The first method has straightforward clinical significance, mimicking the actual situation in which only the reduced injected activity acquisition with low statistical counts is available. The second method, where a fixed VOI is pasted into all acquisitions, resembles the situation in which a modality other than PET is used to define the VOIs, as integrated MRI or a co-registered CT. It will be referred to as “fixed VOI” in the following text. No partial volume correction was applied, reproducing the standard clinical setting.
An expert nuclear medicine physician validated the contours, and the possible isolated spots originating in the segmentation process but not actually linked to the VOI were manually deleted.
Background regions (BKG) used in the following section were defined as the VOI outer shell of two voxels in size. In case of two or more close lesions, the overlap regions between background regions and VOIs were removed from background regions. In addition, a 10 cm2 circular region of interest (ROI) in the liver was delineated in the original activity coronal image (no focal liver uptakes were present for the considered patients) and copied to LTA images.
RayStation software (v. 5.0.2, RaySearch Laboratories, Stockholm, Sweden) was used for segmentation while all ROIs were analyzed with in-house made Python scripts.
Image metrics
Perceived IQ and the number of detectable lesions were evaluated qualitatively by two expert nuclear medicine physicians, first independently and then in consensus. Images were extracted randomly from the whole image dataset and examined in coronal, transversal, and sagittal views without any other anatomical images or medical report supports. IQ score ranged from 1 to 5 (insufficient/non-diagnostic, sufficient, moderate, good, excellent), focusing on anatomical structure resolution, noise, and ease in distinguishing lesions.
Semi-quantitative and quantitative metrics, selected as connected to the clinic are described in the following text:
Lesions SUV measurements: SUVmax, SUVmean, SUVpeak inside the VOIs, that are the most commonly used semi-quantitative parameters for evaluation of tracer uptake;
Images Normalized Noise (NN): in the liver ROI, image noise was calculated as the standard deviation divided by the SUVmean in the ROI, as a quantitative evaluation of image quality;34
-
Lesions Contrast-to-noise ratio (CNR) was computed for all images as an objective metric of lesion detectability considering image noise around the lesion as defined by Yan et al23 as
where SD is the standard deviation;
Lesion Metabolic Tumor Volume (MTV), defined as the volume inside the lesion VOI, has relevant implications for radiotherapy applications;
Total Lesion Glycolysis (TLG) was computed as the product of MTV times the SUVmean of the VOI. It offers a numerical synthesis of the global activity of disease, and it is related to the progression-free survival and overall survival.35–42
Each of the metrics was quantified in the VOIs defined by method a) in VOI definition paragraph, while SUVmean and CNR were also evaluated for fixed VOI (SUVmeanfixed VOI and CNRfixedVOI). Differences in metrics were expressed as the ratio between LTA and FTA values, assuming the latter as the ground truth for each metric. All the comparisons are made between intrapatients and intralesions. We assume as ground truth for each metric the values in the full-time acquisition; no interpatient comparisons were made.
Statistical analysis
In order to examine the stability of image parameters as the injected activity changes, variations in SUV measurements, NN, CNR, MTV, and TLG were analyzed utilizing box plots and Bland-Altman graphs. The pairwise paired Wilcoxon signed-rank test was used to highlight statistically significant differences for the CNR metric at various tracer activities. Because of non-symmetry of SUVmax, SUVpeak, SUVmean, MTV, and TLG data, the pairwise paired t-test was employed to evaluate log(SUVmax), log(SUVpeak), log(SUVmean), log(MTV), and log(TLG) data after checking the normality condition with Shapiro test. The Bonferroni-corrected p-value of 0.05 (5*0.01, two-tailed tests) or lower was considered statistically significant. All statistical analyses were performed with the software RStudio, v. 0.98.1103 (RStudio, Inc., Boston, MA).
Results
Subjective image quality analysis
The qualitative analysis showed that the perceived IQ decreased when reducing tracer activity, as expected (Figure 1 shows an example of original and low activity images). IQ scores for each activity level are displayed in Figure 2. Perceived IQ was acceptable until the activity of 1.5 MBq kg–1 (average score 4.7, 4.5, 3.9, and 3.4 respectively for 3, 2.4, 1.8, and 1.5 MBq kg–1). At a simulated activity of 1.2 MBq kg–1, the average score decreased to 2.9, and one patient image was assessed as not suitable for clinical use. Regarding lesion detectability, a total of 56 lesions were detected on all images from 3 MBqkg-1 to 1.2 MBq kg–1; five lesions were missed only at 0.6 MBq kg–1. No false positives were detected.
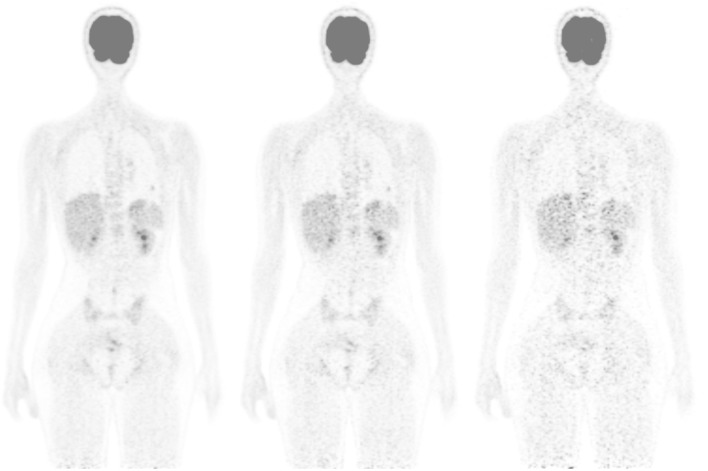
Figure 1.
Coronal view of a patient for standard injected activity of 3MBq kg–1 and simulated activity of 1.5 and 0.6 MBq kg–1 (from left to right). The subjective score was 5, 3, and 1, respectively. In the 0.6 MBq kg–1 image, the lung lesion was missed.
Figure 2.
Quality grade distributions for all tracer activity images. FTA, full tracer activity; LTA, lower tracer activity; SUV, standardized uptake value.
Objective image analysis
Results of the quantitative analyses of image metrics are reported in the following.
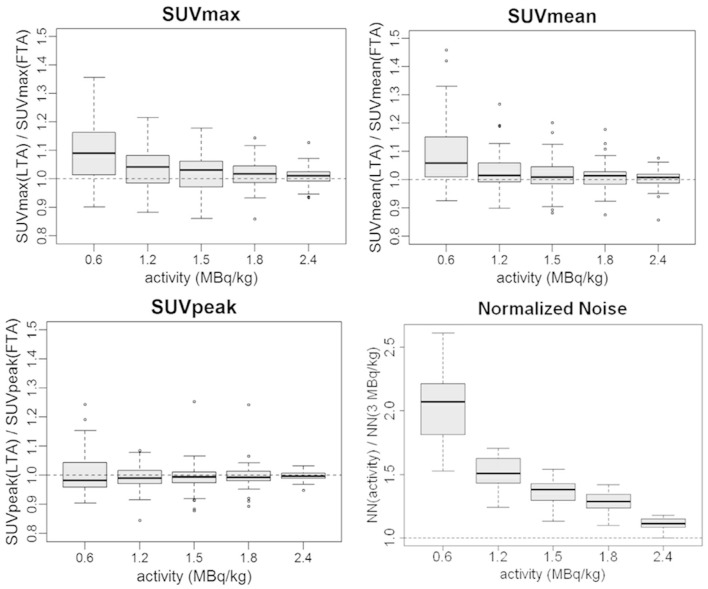
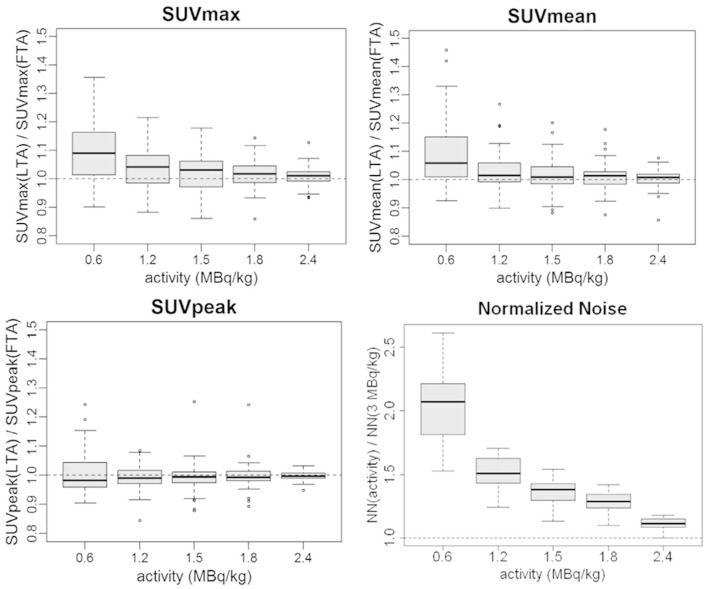
SUV measurements: SUV ratio statistics are reported in Figure 3 and Table 2. SUVmean variations remained below 3% until 1.2 MBq kg–1, while increased to +9% on average at 0.6 MBq kg–1. SUVmax variations were more relevant with an average increase of +10% in the 0.6 MBq kg–1 image. SUVpeak was the more stable metric with a deviation from full activity values inferior to 1% on average. All SUV metric ratios showed an increased spread at lower activity levels (Figure 3). SUV metrics changes were assessed with the pairwise paired t-test: for activity ≤1.2 MBq kg–1, statistically significant differences vs original tracer activity were found for SUVmean and SUVmax (Table 3) while SUVpeak differences were not statistically significant anywhere. Except for SUVpeak, SUV statistical results confirmed the subjective analyses.
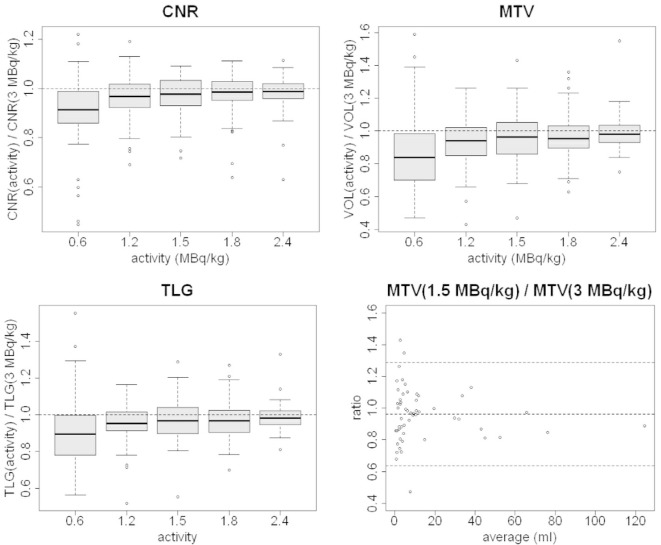
Normalized Noise: as predictable, the noise increased at lower tracer activities pointing out a worsening in IQ. The image degradation is clearly shown by the sharp increase in the boxplot graph at 0.6 MBq kg–1 (average values of the NN ratio 1.11, 1.28, 1.37, 1.51, 2.05 with LTA from 2.4 MBq kg–1 to 0.6 MBq kg–1), (Figure 3).
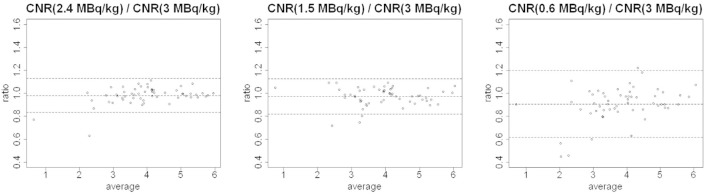
CNR: lesion Contrast-to-Noise Ratio has a limited variation on average when reducing tracer activity (Figure 4) except for 0.6 MBq kg–1 with an average change of −9%. Larger increases were detected for standard deviations at lower simulated activities (Table 2 and Figure 4). Lesion CNRs of FTA images showed variations not statistically significant from values in LTA images until 1.5 MBq kg–1 (Table 3).
Figure 3.
Boxplots of the ratios between LTA and FTA images of SUVmax, SUVmean, SUVpeak, and Normalized Noise. FTA, full tracer activity; LTA, lower tracer activity; SUV, standardized uptake value.
Table 2.
Average values ± standard deviations of SUVmax (activity), SUVmean (activity), CNR (activity), MTV (activity), TLG (activity), SUVmeanfixed VOI (activity) and CNRfixed VOI (activity) for simulated activity A = 0.6, 1.2, 1.5, 1.8, and 2.4 MBq kg–1 divided into corresponding values at original activity (3 MBq kg–1)
| A = 0.6 MBq kg–1 | A = 1.2 MBq kg–1 | A = 1.5 MBq kg–1 | A = 1.8 MBq kg–1 | A = 2.4 MBq kg–1 | |
| SUVmax(A)/SUVmax(FTA) | 1.10 ± 0.11 | 1.04 ± 0.07 | 1.02 ± 0.06 | 1.02 ± 0.05 | 1.01 ± 0.03 |
| SUVmean(A)/SUVmean(FTA) | 1.09 ± 0.12 | 1.03 ± 0.07 | 1.02 ± 0.06 | 1.01 ± 0.05 | 1.00 ± 0.03 |
| SUVpeak(A)/SUVpeak(FTA) | 1.01 ± 0.07 | 0.99 ± 0.04 | 0.99 ± 0.05 | 1.00 ± 0.05 | 1.00 ± 0.02 |
| CNR(A)/CNR(FTA) | 0.91 ± 0.15 | 0.96 ± 0.09 | 0.97 ± 0.08 | 0.97 ± 0.09 | 0.98 ± 0.08 |
| MTV(A)/MTV(FTA) | 0.86 ± 0.24 | 0.93 ± 0.15 | 0.96 ± 0.16 | 0.97 ± 0.14 | 0.99 ± 0.11 |
| TLG(A)/TLG(FTA) | 0.91 ± 0.19 | 0.95 ± 0.11 | 0.97 ± 0.12 | 0.97 ± 0.10 | 0.99 ± 0.08 |
| NN(A)/NN(FTA) | 2.05 ± 0.30 | 1.51 ± 0.14 | 1.37 ± 0.11 | 1.28 ± 0.09 | 1.11 ± 0.05 |
| SUVmeanfixed VOI(A)/SUVmean(FTA) | 0.98 ± 0.06 | 0.99 ± 0.03 | 0.99 ± 0.02 | 0.99 ± 0.02 | 1.00 ± 0.01 |
| CNR(A)fixed VOI/CNR(FTA) | 0.79 ± 0.15 | 0.90 ± 0.09 | 0.93 ± 0.08 | 0.95 ± 0.06 | 0.98 ± 0.03 |
CNR, contrast-to-noise ratio;FTA, full tracer activity ; MTV, metabolic tumor volume ; SUV, standardized uptake value; TLG, total lesion glycolysis.
Table 3.
p-values of the pairwise paired Wilcoxon test for CNR and the pairwise paired t-test for log(SUVmax), log(SUVmean), log(SUVpeak), log(MTV), and log(TLG)
| 0.6 MBq kg–1 | 1.2 MBq kg–1 | 1.5 MBq kg–1 | 1.8 MBq kg–1 | 2.4 MBq kg–1 | |
| SUVmax (FTA) | 2.7e-08* | 0.001* | 0.078 | 0.083 | 0.587 |
| SUVmean (FTA) | 2.9e-06* | 0.006* | 0.436 | 0.614 | 1 |
| SUVpeak (FTA) | 1 | 0.670 | 1 | 1 | 1 |
| CNR (FTA) | 4.4e-05* | 0.017* | 0.067 | 0.182 | 0.493 |
| MTV (FTA) | 1.6e-05 | 0.003* | 0.156 | 0.184 | 1 |
| TLG (FTA) | 4.3e-04* | 0.006* | 0.132 | 0.147 | 1 |
CNR, contrast-to-noise ratio;FTA, full tracer activity; MTV, metabolic tumor volume; SUV, standardized uptake value; TLG, total lesion glycolysis.
*values indicates p < 0.05.
Figure 4.
Bland-Altman plots of CNR (activity) / CNR (3 MBq kg–1) for activity = 2.4, 1.5 and 0.6 MBq kg–1. CNR, contrast-to-noise ratio.
The CNR worsening with reduced activity is mainly due to the increase of noise in lesion background as inferred by the formula.1 Indeed, the changes in lesion SUVmean (Figure 3) are inferior to 9% (Table 2); similar behavior is shown by the SUVmean in the lesion background (average values equal to 1.04, 1.01, 1.00, 1.00, and 1.01 for increasing tracer activity levels) while SD(background regions) shows greater changes (average values equal to 1.25, 1.10, 1.06, 1.05, and 1.03 increasing activity from 0.6 to 2.4 MBq kg–1).
MTV: lesion volumes deviate progressively from reference values defined on the full statistic image (Figure 5). MTV is the lesion parameter more affected by the reduction in statistical counts (Table 2): for the 0.6 MB kg–1 image, the average values of LTA to FTA ratio and the standard deviation (0.86 and 0.24 respectively) are substantially inferior/larger than the corresponding values for the other simulated injected activities (Table 2). A statistically significant difference was found for activity inferior to 1.2 MBq kg–1 vs the FTA (Table 3). From Bland-Altman plots analysis (Figure 5), it is apparent that small volumes (<5 ml) show larger relative errors when reducing activity. This is probably mainly due to the fact that for small lesions, small differences in segmentation could produce larger relative volume differences.
TLG: Total lesion glycolysis, being the product of SUVmean and MTV, changed less than other factors (Figure 5); this is due to the fact that, as shown in point c), SUVmean increases, reducing injected activity while the volume tends to decrease. The average values of TLG(LTA)/TLG(FTA) are reported in Table 2. For our data, TLG variations between lesions segmented on LTA images with respect to full count image were non-statistically significant until 1.5 MBqkg-1 (Table 3).
Figure 5.
Boxplots of the ratios between LTA and FTA images for CNR, MTV, and TLG and Bland-Altman plot of MTV(1.5 MBqkg-1)/MTV(3 MBqkg-1). CNR, contrast-to-noise ratio; FTA, full tracer activity; LTA, lower tracer activity; MTV, metabolic tumor volume; TLG, total lesion glycolysis.
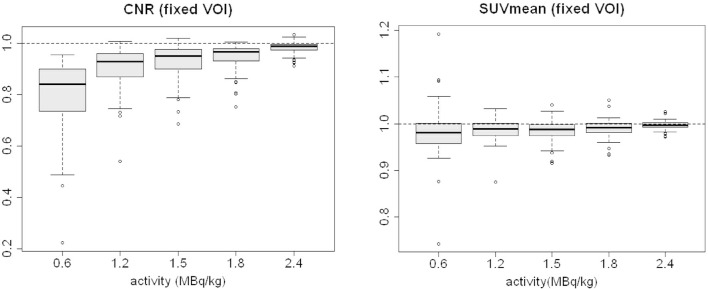
SUVmean and CNR were also calculated as SUVmeanfixed VOI and CNRfixedVOI.
The average value of SUVmean (variable VOI) has more significant variations in comparison to the average SUVmeanfixed VOI: average values of the SUVmeanfixedVOI ratios (LTA/FTA images) range from 1.0 to 0.98, while the maximum standard deviation is 0.06 for 0.6 MBq/kg (Table 2, Figure 6). The major increase in SUVmean (variable VOI) is driven by the increase in the SUVmax and by the use of a fixed threshold for VOI definition (Figure 5).
Figure 6.
Boxplots of the ratios between LTA and FTA images for SUVmeanfixedVOI and CNRfixed VOI. CNR, contrast-to-noise ratio; FTA, full tracer activity; LTA, lower tracer activity; SUV, standardized uptake value.
It is worth noting that CNRfixed VOI changes more than CNR when reducing activity (Figure 6). This is mainly due to the above-mentioned fact that SUVmean (variable VOI) has larger variations on average than SUVmeanfixedVOI (Figures 5 and 6) while background SUV statistics (mean and SD) display minor differences if computed in variable or fixed VOIs (not reported), thus resulting in higher values of CNR (Eq. 1 (undefined) ) compared to CNRfixed VOI.
Discussion
In the present study, a thorough analysis of objective image metrics was performed to investigate image stability concerning tracer activity reduction in PET/MRI pediatric patient, aged between 7 and 17 years, examinations. CNR, NN, semi-quantitative SUV metrics, MTV, and TLG as well as lesion detectability were evaluated.
This study demonstrates that from the perspective of the chosen metrics related to IQ, image noise, tumor burden information, metabolic volume, and lesion detectability, in agreement with subjective analysis assessment, the 1.5 MBqkg-1 LTA images maintain the same quantitative information of the FTA images without statistically significant differences. This confirms the possibility of halving the standard injected activity (3 MBqkg-1) without any significant clinical impact at least in this group of patients (7–17 years). This has evident benefits on radiation exposure, which is of particular concern in pediatric patients, as repeatedly observed.17–20
From the subjective point of view, IQ corresponding to a simulated injected activity of 1.2 MBq kg–1 is evaluated unsuitable for diagnosis, although there are no undetected lesions. The perceived quality gets worse at 0.6 MBq kg–1 where five lesions are missed. Results agree with Gatidis et al,13 in which authors showed that a dose reduction from 3.2 MBq kg–1 to 1.5 MBq kg–1 is possible.
In agreement, changes in SUVs measurements (max, peak, and mean) were not statistically significant down to 1.2 MBq kg–1 with relative differences from FTA below 4% for all three parameters. This is of relevance in clinical practice, because the SUVs are the most commonly used semiquantitative parameter for analysis of tracer uptake, as reported by the EANM guidelines.30
The maximum SUV is routinely used in clinical studies. SUVmax is a single-voxel measure in a particular lesion with highest maximum uptake. As a consequence of that, it is expected that this parameter is unstable because of its dependence on image noise and statistical fluctuations. Indeed, SUVmax was the value most affected by tracer activity reduction, showing a consistent trend with the recently published data of Seith et al24 on adult patients.
On the contrary, SUVmean and SUVpeak are expected to be more stable parameters as they are averaged as on different pixel values. Indeed, SUVpeak was not sensitive to the activity reduction, showing a maximum variation of 1% and no significant statistical differences. SUV mean is obtained as the average of pixel values inside a volume; in this work, we used a 40% threshold of the SUVmax. As a consequence of that, SUVmean variations are inherently connected to SUVmax variations, but less evident.
As expected, SUVmean shows less variation when calculated in fixed VOIs than in variable VOIs (−2% vs. +9%) because it is not affected by the SUVmax variations.
The sharp increase of NN shown in the boxplot graph at 0.6 MBqkg-1, makes evident the increased image noise at LTA, already highlighted by the decreased score in the perceived image quality. Despite image granularity increase in PET images, no false positive lesions were detected.
CNR, which depends on both the counts in the lesion and in the surrounding background, is considered one of the most closely related metrics to lesion detectability.23 In support of this, our results give a lower CNR (0.76 ± 0.27) for the five lesions missed at 0.6 MBq/kg than all the other lesions (0.91 ± 0.15).
Although the CNR ratio has a clear dependence on statistical counts, the median values remain comparable to full activity images until tracer activity equal to 1.5MBqkg-1, as demonstrated through Bland-Altman plots and paired Wilcoxon tests and it starts to decrease when the noise rises.
CNR shows the same trend even when using fixed ROIs, although with this method it was reduced up to - 21% from FTA to LTA (−9% with the repeated thresholding method).
Tumor burden parameters, MTV and TLG, are gaining interest because of their proven correlation to the progression-free survival and overall survival.35–41 MTV assessment is also of primary interest for FDG PET/CT-based tumor delineations in radiotherapy planning. Both metrics show no statistically significant differences until 1.5 MBq kg–1.
In 18F-FDG PET/CT, several authors investigated the possibility of lowering the injected activity.
Cheng et al43 explored the achievable value of the reduced injected activity keeping the validity in comparing SUVs between studies. With SUVmean and SUVMax as a comparing metrics, a reduction of one-third of a standard activity (550MBq) was suggested.
Alessio et al32 showed that for pediatric (>22 Kg) 18F-FDG PET/CT examinations the acquisition time can be reduced from 5 min/field of view to 3 min/field of view while maintaining the diagnostic utility. IQ were assessed via subjective evaluation and lesion detectability. Recently, Armstrong et al28 investigated the impact of PET detectors technological innovations in reducing the injected activity while maintaining the consistency of IQ and concluded that a significant activity reduction is feasible. Through the evaluation of the stability of the Liver SNR, mediastinum SUVmean, and lesion SUVmax a reduction from 400 MBq to 280 MBq was proven for patients < 100 Kg. For pediatric18F-FDG PET/MR examination Gatidis et al,13 checking for the SUV stability and subjective evaluation, showed the feasibility to halve the injected dose (1.5 MBq kg–1).
Even if previous studies have discussed the possibility to reduce the injected activity, this was done analyzing the stability of only a few metrics, although clinically significant. The relevance of this study lies on demonstrating that several IQ metrics, related to the clinical use of PET, are consistent in identifying the lowered value of the injected activity and therefore in demonstrating the feasibility of the reduction whatever the clinical application of PET. To our knowledge, a rigorous quantitative analysis of CNR, NN, MTV, and TLG variations in PET/MR examinations of pediatric patients is presented here for the first time. The metrics behavior confirms that activity reduction to 1.5 MBq kg–1 has no significant impact on the clinical utility of PET/MR images and strengthening the PET/MR dosage recommendations.
Equally, the results support an alternative shortened acquisition protocol (keeping the injected activity fixed), that may be useful, for instance, when patient compliance is reduced, and sedation is risky. In such a situation, acquisition time can be safely reduced to 2.5 min/bed position without any loss in PET clinical performance.
Nevertheless, some limitations are present in our study. Reduced tracer activity was simulated truncating list mode data. This could introduce limited bias due to temporal changes of radionuclide internal distribution considering fractions of bed time instead of the whole-time interval (5 min) and due to patient movement, that can affect the tracer spatial distribution.
Our study population shows a long uptake time (range: 66–214 min). The reason for this variable uptake time is that all studies were acquired after a standard PET/CT scan during the clinical validation protocol of the PET/MR scanner, and transfer time could vary from patient to patient. Additionally, four patients were imaged twice. Nevertheless, these points are not relevant to our conclusions because all the metric comparisons were made intrapatients without interpatient comparisons. We assume as ground truth for each metric the values in the full-time acquisition.
We investigate the behavior of the metric using a 40% threshold of the SUVmax to delineate lesions noted as the usual clinical standard. Further analysis should be carried out regarding the effect of the segmentation method on different image metrics.
The sample size should be enlarged including lower ages and stratifying with regard to lesion volume and cancer type. A future study, based on a larger population, could address this topic and explore the acquisition protocols indicated for different clinical situations, offering an objective base for the available choices.
Conclusion
In the present study, results on metrics related to image quality, lesion detectability, tumor burden information beyond the semi-quantitative SUV parameters, and subjective analysis rigorously proved the feasibility of the reduction of the injected activity to 1.5 MBq kg–1 in pediatric oncology 18F-FDG PET/MR examinations preserving the clinical utility of images. Indeed, all the metrics were stable showing variations not statistically significant until the administered activity of 1.5 MBq kg–1. This result, obtained in a population between 7 and 17 years, combined with the lack of ionizing radiation from the CT component, makes PET/MR even more attractive for pediatric imaging.
Footnotes
Acknowledgements: We thank Judson Jones and Bjoern Jakoby (Siemens Healthcare) for precious assistance with setting up E7-tools reconstruction software
Contributor Information
Pietro Zucchetta, Email: pietro.zucchetta@unipd.it.
Marco Branchini, Email: branchini.marco@gmail.com.
Alessandra Zorz, Email: alessandra.zorz@iov.veneto.it.
Valentina Bodanza, Email: bodanza.valentina@gmail.com.
Diego Cecchin, Email: diego.cecchin@unipd.it.
Marta Paiusco, Email: marta.paiusco@iov.veneto.it.
Franco Bui, Email: franco.bui@unipd.it.
REFERENCES
- 1.Kluge R, Kurch L, Montravers F, Mauz-Körholz C. FDG PET/CT in children and adolescents with lymphoma. Pediatr Radiol 2013; 43: 406–17. doi: 10.1007/s00247-012-2559-z [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Li X, Li F, Ouyang Q, Yu T. Evolving role of 18F-FDG-PET/CT for the body tumor and metastases in pediatrics. Eur J Radiol 2010; 75: 329–35. doi: 10.1016/j.ejrad.2010.05.039 [DOI] [PubMed] [Google Scholar]
- 3.Kaste SC. PET-CT in children: where is it appropriate? Pediatr Radiol 2011; 41(Suppl 2): 509–13. doi: 10.1007/s00247-011-2096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricard F, Cimarelli S, Deshayes E, Mognetti T, Thiesse P, Giammarile F. Additional Benefit of F-18 FDG PET/CT in the staging and follow-up of pediatric rhabdomyosarcoma. Clin Nucl Med 2011; 36: 672–7. doi: 10.1097/RLU.0b013e318217ae2e [DOI] [PubMed] [Google Scholar]
- 5.Franzius C. FDG-PET/CT in pediatric solid tumors. Q J Nucl Med Mol Imaging 2010; 54: 401–10. [PubMed] [Google Scholar]
- 6.Rhodes MM, Delbeke D, Whitlock JA, Martin W, Kuttesch JF, Frangoul HA, et al. Utility of FDG-PET/CT in follow-up of children treated for hodgkin and non-hodgkin lymphoma. J Pediatr Hematol Oncol 2006; 28: 300–6. doi: 10.1097/01.mph.0000212912.37512.b1 [DOI] [PubMed] [Google Scholar]
- 7.Kluge R, Kurch L, Georgi T, Metzger M. Current role of FDG-PET in pediatric hodgkin's lymphoma. Semin Nucl Med 2017; 47: 242–57. doi: 10.1053/j.semnuclmed.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 8.Harrison DJ, Parisi MT, Shulkin BL. The Role of 18F-FDG-PET/CT in Pediatric Sarcoma. Semin Nucl Med 2017; 47: 229–41. doi: 10.1053/j.semnuclmed.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 9.McCarville MB, Federico SM, Bishop MW, Pappo AS, Shulkin BL. Assessment of chemotherapy response in ewing sarcoma. Radiology 2016; 281: 647–9. doi: 10.1148/radiol.2016160903 [DOI] [PubMed] [Google Scholar]
- 10.Lapa P, Marques M, Costa G, Iagaru A, Pedroso de Lima J. Assessment of skeletal tumour burden on 18F-NaF PET/CT using a new quantitative method. Nucl Med Commun 2017; 38: 325–32. doi: 10.1097/MNM.0000000000000654 [DOI] [PubMed] [Google Scholar]
- 11.Juhász C, Bosnyák E. PET and SPECT studies in children with hemispheric low-grade gliomas. Childs Nerv Syst 2016; 32: 1823–32. doi: 10.1007/s00381-016-3125-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schäfer JF, Gatidis S, Schmidt H, Gückel B, Bezrukov I, Pfannenberg CA, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology 2014; 273: 220–31. doi: 10.1148/radiol.14131732 [DOI] [PubMed] [Google Scholar]
- 13.Gatidis S, Schmidt H, la Fougère C, Nikolaou K, Schwenzer NF, Schäfer JF. Defining optimal tracer activities in pediatric oncologic whole-body 18F-FDG-PET/MRI. Eur J Nucl Med Mol Imaging 2016; 43: 2283–9. doi: 10.1007/s00259-016-3503-5 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch FW, Sattler B, Sorge I, Kurch L, Viehweger A, Ritter L, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol 2013; 43: 860–75. doi: 10.1007/s00247-012-2570-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyer T, Hacker M, Goh V. PET/MRI-knocking on the doors of the rich and famous. Br J Radiol 2017; 90: 20170347. doi: 10.1259/bjr.20170347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parisi MT, Bermo MS, Alessio AM, Sharp SE, Gelfand MJ, Shulkin BL. Optimization of Pediatric PET/CT. Semin Nucl Med 2017; 47: 258–74. doi: 10.1053/j.semnuclmed.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Brody AS, Frush DP, Huda W, Brent RL, .American Academy of Pediatrics Section on Radiology . Radiation risk to children from computed tomography. Pediatrics 2007; 120: 677–82. doi: 10.1542/peds.2007-1910 [DOI] [PubMed] [Google Scholar]
- 18.Nievelstein RA, Quarles van Ufford HM, Kwee TC, Bierings MB, Ludwig I, Beek FJ, et al. Radiation exposure and mortality risk from CT and PET imaging of patients with malignant lymphoma. Eur Radiol 2012; 22: 1946–54. doi: 10.1007/s00330-012-2447-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 2013; 167: 700–7. doi: 10.1001/jamapediatrics.2013.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001; 176: 289–96. doi: 10.2214/ajr.176.2.1760289 [DOI] [PubMed] [Google Scholar]
- 21. BEIR VII PHASE II Health risks from exposures to low levels of ionizing radiation : Washington, DC: The British Institute of Radiology.; 2006. [Google Scholar]
- 22.Schaefferkoetter JD, Yan J, Townsend DW, Conti M. Initial assessment of image quality for low-dose PET: evaluation of lesion detectability. Phys Med Biol 2015; 60: 5543–56. doi: 10.1088/0031-9155/60/14/5543 [DOI] [PubMed] [Google Scholar]
- 23.Yan J, Schaefferkoette J, Conti M, Townsend D. A method to assess image quality for Low-dose PET: analysis of SNR, CNR, bias and image noise. Cancer Imaging 2016; 16: 26. doi: 10.1186/s40644-016-0086-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seith F, Schmidt H, Kunz J, Küstner T, Gatidis S, Nikolaou K, et al. Simulation of Tracer Dose Reduction in 18F-FDG PET/MRI: Effects on Oncologic Reading, Image Quality, and Artifacts. J Nucl Med 2017; 58: 1699–705. doi: 10.2967/jnumed.116.184440 [DOI] [PubMed] [Google Scholar]
- 25.Schaefferkoetter JD, Yan J, Sjöholm T, Townsend DW, Conti M, Tam JK, et al. Quantitative accuracy and lesion detectability of low-dose 18F-FDG PET for lung cancer screening. J Nucl Med 2017; 58: 399–405. doi: 10.2967/jnumed.116.177592 [DOI] [PubMed] [Google Scholar]
- 26.Brown C, Dempsey MF, Gillen G, Elliott AT. Investigation of 18F-FDG 3D mode PET image quality versus acquisition time. Nucl Med Commun 2010; 31: 254–9. doi: 10.1097/MNM.0b013e3283355c5d [DOI] [PubMed] [Google Scholar]
- 27.Geismar JH, Stolzmann P, Sah BR, Burger IA, Seifert B, Delso G, et al. Intra-individual comparison of PET/CT with different body weight-adapted FDG dosage regimens. Acta Radiol Open 2015; 4: 204798161456007. doi: 10.1177/2047981614560076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong IS, James JM, Williams HA, Kelly MD, Matthews JC. The assessment of time-of-flight on image quality and quantification with reduced administered activity and scan times in 18F-FDG PET. Nucl Med Commun 2015; 36: 728–37. doi: 10.1097/MNM.0000000000000305 [DOI] [PubMed] [Google Scholar]
- 29.Stauss J, Franzius C, Pfluger T, Juergens KU, Biassoni L, Begent J, et al. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging 2008; 35: 1581–8. doi: 10.1007/s00259-008-0826-x [DOI] [PubMed] [Google Scholar]
- 30.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W. European association of nuclear medicine (EANM).FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42: 328–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Associazione Italiana di Medicina Nucleare ed Imagin Molecolare (AIMN) Raccomandazioni Procedurali per l’imaging oncologico con 18F-FDG PET/TC, Vrs. 03/2012; 2012. [Google Scholar]
- 32.Alessio AM, Sammer M, Phillips GS, Manchanda V, Mohr BC, Parisi MT, et al. Evaluation of optimal acquisition duration or injected activity for pediatric 18F-FDG PET/CT. J Nucl Med 2011; 52: 1028–34. doi: 10.2967/jnumed.110.086579 [DOI] [PubMed] [Google Scholar]
- 33.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999; 2: 159–71. doi: 10.1016/S1095-0397(99)00016-3 [DOI] [PubMed] [Google Scholar]
- 34.Aklan B, Oehmigen M, Beiderwellen K, Ruhlmann M, Paulus DH, Jakoby BW, et al. Impact of Point-Spread Function Modeling on PET Image Quality in Integrated PET/MR Hybrid Imaging. J Nucl Med 2016; 57: 78–84. doi: 10.2967/jnumed.115.154757 [DOI] [PubMed] [Google Scholar]
- 35.Manohar K, Mittal BR, Bhattacharya A, Malhotra P, Varma S. Prognostic value of quantitative parameters derived on initial staging 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with high-grade non-Hodgkin's lymphoma. Nucl Med Commun 2012; 33: 974–81. doi: 10.1097/MNM.0b013e32835673ec [DOI] [PubMed] [Google Scholar]
- 36.Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imag 2012; 39: 27–38. doi: 10.1007/s00259-011-1934-6 [DOI] [PubMed] [Google Scholar]
- 37.Muralidharan V, Kwok M, Lee ST, Lau L, Scott AM, Christophi C, et al. Prognostic ability of 18F-FDG PET/CT in the assessment of colorectal liver metastases. J Nucl Med 2012; 53: 1345–51. doi: 10.2967/jnumed.112.102749 [DOI] [PubMed] [Google Scholar]
- 38.Chen HH, Chiu NT, Su WC, Guo HR, Lee BF. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology 2012; 264: 559–66. doi: 10.1148/radiol.12111148 [DOI] [PubMed] [Google Scholar]
- 39.Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee SH, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer 2013; 119: 1195–202. doi: 10.1002/cncr.27855 [DOI] [PubMed] [Google Scholar]
- 40.Liu CJ, Lu MY, Liu YL, Ko CL, Ko KY, Tzen KY, et al. Risk stratification of pediatric patients with neuroblastoma using volumetric parameters of 18F-FDG and 18F-DOPA PET/CT. Clin Nucl Med 2017; 42: e142–e148. doi: 10.1097/RLU.0000000000001529 [DOI] [PubMed] [Google Scholar]
- 41.Andersen KF, Fuglo HM, Rasmussen SH, Petersen MM, Loft A. Volume-based F-18 FDG PET/CT imaging markers provide supplemental prognostic information to histologic grading in patients with high-grade bone or soft tissue sarcoma. Medicine 2015; 94: e2319. doi: 10.1097/MD.0000000000002319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S, Zaidi H, Salavati A, Hess S, Carlsen PF, Alavi A. FDG PET/CT methodology for evaluation of treatment response in lymphoma: from "graded visual analysis" and "semiquantitative SUVmax" to global disease burden assessment. Eur J Nucl Med Mol Imaging 2014; 41: 2158–60. doi: 10.1007/s00259-014-2826-3 [DOI] [PubMed] [Google Scholar]
- 43.Cheng DW, Ersahin D, Staib LH, Della Latta D, Giorgetti A, d'Errico F. Using SUV as a guide to 18F-FDG dose reduction. J Nucl Med 2014; 55: 1998–2002. doi: 10.2967/jnumed.114.140129 [DOI] [PubMed] [Google Scholar]