Abstract
Objective:
Small animal radiotherapy research platforms such as XStrahl’s SARRP enable more precise irradiation of tumours and normal tissues in pre-clinical models of cancer. Using an orthotopic G7 glioblastoma xenograft model we studied the impact of four different radiotherapy plans on tumour and normal tissue dosimetry.
Methods:
Plans were created using four different approaches (single beam, parallel opposed pair, single plane arcs, couch rotation arcs) and dose volume histograms (DVH) for the tumour and the relevant organs at risk (OARs) (mouth, ipsilateral brain, contralateral brain, brain stem) were compared for a sample mouse subject. To evaluate the accuracy of delivery, treatment plans were recreated in solid-water phantoms and delivered to radiochromic film.
Results:
Favourable tumour dosimetry was achieved by all plans. DVH analysis showed that different plans could be used to spare specific OARs depending on the objectives of the study. The delivery accuracy of the various treatments was better than 2%/2mm (dose difference/distance to agreement) in terms of global γ analysis.
Conclusion:
Small animal radiotherapy research platforms are an exciting addition to the pre-clinical research environment. Such systems improve the conformality of irradiation of tumours and OARs while maintaining a high degree of accuracy and enable investigators to optimise experiments in terms of tumour coverage and inclusion or exclusion of relevant OARs.
Advances in knowledge:
This study confirms the utility of the SARRP in terms of the accuracy of plan delivery, and informs decisions on treatment planning to optimise the clinical relevance and scientific value of experiments.
Introduction
The recent development of image-guided small animal microirradiation systems creates opportunities for radiation researchers to conduct much more accurate pre-clinical evaluation of novel radiotherapy techniques or radiotherapy-drug combinations, and to undertake experiments that more reliably recapitulate clinical scenarios. A review of small animal radiotherapy research platforms published in 2011 by Verhaegen et al1 provides a comprehensive overview of the systems in general use and outlines the key developments in irradiation, imaging and treatment planning that have been made possible by these platforms.
Meaningful pre-clinical evaluation of novel radiotherapy-drug combinations requires thorough assessment of the impact of potential treatments on both the tumour and the relevant normal tissues [organs at risk (OARs)]. To maximise clinical relevance it is important that the relative radiation doses delivered to these structures recapitulate clinical dosimetry, and that the absolute doses delivered can be accurately measured. Our overall research programme aims to improve outcomes for patients with glioblastoma, the most common and most aggressive primary brain tumour, by using novel molecular targeted agents to enhance tumour responses to radiotherapy without exacerbating adverse effects on the surrounding brain. The aim of this study was to evaluate four different radiotherapy treatment plan approaches in terms of tumour and OAR dosimetry.
Investigation of CD1 nude mice bearing intracranial G7 glioblastoma xenografts was performed on the XStrahl Small Animal Radiation Research Platform (SARRP, XStrahl, Ltd, Surrey, UK), which was originally developed at Johns Hopkins University.2 The ability of the SARRP to accurately deliver conformal radiotherapy plans was investigated by performing dose volume histograms (DVH) and two-dimensional film-based analyses.
Methods and materials
All animal experiments were performed under the relevant UK Home Office Project Licence and carried out with ethical approval from the University of Glasgow under the Animal (Scientific Procedures) Act 1986 and the European Union directive 2010. Mice were maintained in individually ventilated cages with environmental enrichment; ARRIVE guidelines were followed.
Female CD1 nude mice were orthotopically injected with 1 × 105 G7 primary glioblastoma cells into the subventricular zone as previously described.3,4 Tumours were allowed to establish for 10–11 weeks at which time MRI was performed to confirm the presence and location of tumours.
The European Society for Radiotherapy and Oncology Advisory Committee on Radiation Oncology Practice has recently published a review5 of the technologies of small animal radiotherapy research, which gives guidance on the reporting of irradiations. The irradiations performed are described in accordance with these recommendations, which are listed in Table 2 of the guidelines.
Irradiations were performed using 200 kVp, 13 mA photons with a broad focal spot. Additional filtration of 0.5 mm copper was added in the beam path, giving a half-value layer of 1.0 mm of copper. For the 5 × 5 mm collimator used in this investigation, a dose-rate of approximately 150 cGy min–1 was achieved at isocentre for 1 cm depth in a full scatter phantom. The source to isocentre distance was 35 cm. All other details of irradiations are given elsewhere in this article.
Plan assessment
Four different approaches to irradiating a lateralised intracranial tumour were investigated for their benefits and disadvantages in terms of tumour coverage and OAR irradiation. The small size of the target volume and the mouse brain enabled us to consider simple beam arrangements as well as more complex, arc-based approaches. In terms of acute toxicity, the mouth is an important OAR, while ipsilateral and contralateral cerebral hemispheres and brainstem dosimetry are important determinants of subacute and late toxicity. For experiments focusing on tumour growth delay and mouse survival, tumour coverage and mouth dosimetry are prioritised, whereas brain and brainstem dosimetry are critical in experiments measuring subacute and late toxicities.
For a single mouse subject, three-dimensional non-deformable registration between MRI and cone beam CT (CBCT) imaging was performed (Figure 1a) to enable accurate delineation of the cerebral hemispheres, brainstem, mouth, eyes and tumour. Four different plans were generated for the subject, and calculated with prescription doses altered to provide tumour coverage with the 95% isodose. OAR tolerances for mouse brain are not defined and were not utilised, as this investigation was aimed at providing general principles for achievable reduction of OAR doses. Doses were prescribed to the isocentre. Cumulative DVH are shown with data relative to the prescription dose, as is most common in clinical treatment planning.
Figure 1.
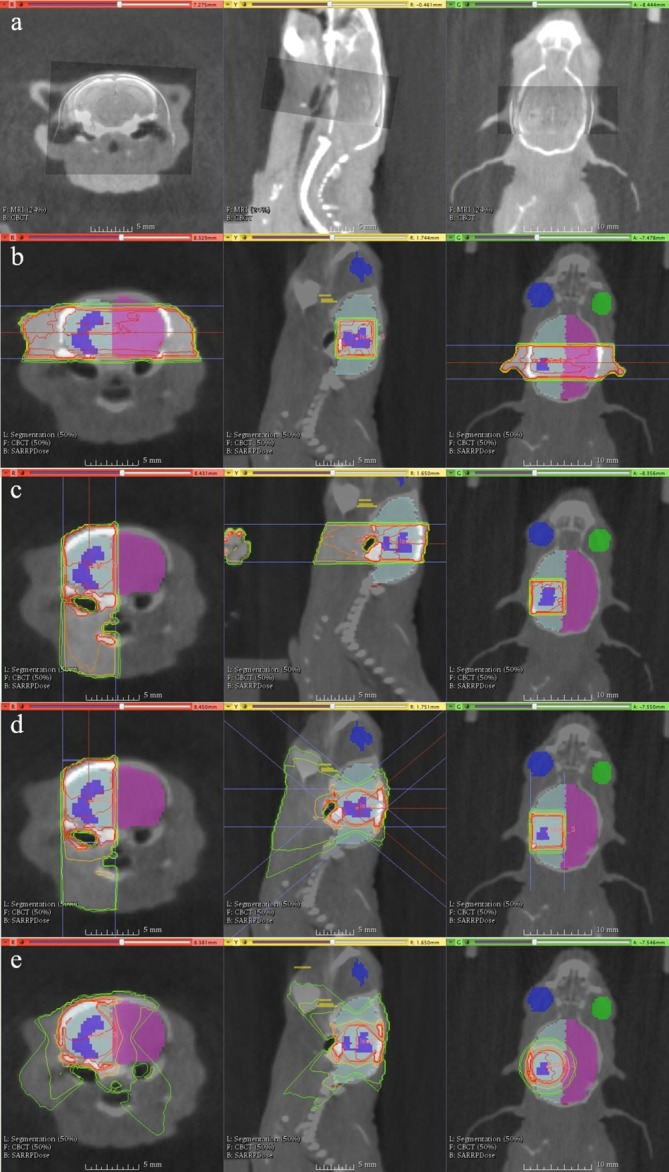
Contoured colours: tumour (purple), ispilateral brain (light blue), contralateral brain (magenta), left eye (blue), right eye (green), mouth (yellow, largely out of viewing plane), brainstem (not shown). The lower left legends describe the current datasets in view in Muriplan, which can currently only show a background and foreground dataset, alongside contours.(a) MRI-CBCT fusion for target volume delineation. (b) Parallel opposed pair: two opposed lateral beams positioned so that the central axis passes through the centre of the tumour. (c) Single beam – single hemisphere: a single beam aimed “top-down” so that the central axis passed through the centre of the tumour, thus sparing the contralateral cerebral hemisphere.(d) Single plane arcs (“TopArc”) – single hemisphere: an arc that travels from gantry 40 to 0 degrees at couch 90, and then from 0 to 40 degrees at couch −90 to form an arc over the tumour from above, with the aim of sparing the contralateral hemisphere of the brain while improving tumour conformity.(e) Couch rotation arcs (“ArcXX”): fixed gantry set up with a complete couch rotation designed to create a spherical type dose distribution. Gantry angles, XX, of 30, 45 and 60 degrees were used to modulate the sphericity of the distribution. In the example shown the gantry angle is 45 degrees. CBCT, cone beam CT.
In vivo studies of this type are generally designed using previous experience of the intracranial tumour model, so that irradiation is performed when tumours are of a certain, pre-defined size. It would be extremely unusual to irradiate a tumour that was much larger than the tumours used in this study because mice would become symptomatic. If a study were to be performed that required the larger collimator size, we would recommend additional evaluation of the different planning solutions. For the volume of tumour in this study (0.015 cm3), it was found that in all scenarios a 5 × 5 mm collimator provided adequate tumour coverage while minimising irradiation of normal tissues.
The four plans are described below and illustrated in Figure 1b–e.
Plan deliverability and accuracy
To determine the deliverability of the various treatments, and the geometric and dosimetric accuracy of the delivery, plans were delivered to Gafchromic EBT3 (Ashland Advanced Materials, NJ) film in solid water. The use of Gafchromic EBT3 is comprehensively covered in the literature.6 Dose distributions on the films were compared with planned doses through two-dimensional γ analysis7 using MATLAB (MATLAB 2016b, The Mathworks, Natick, 2016) with the function CalcGamma (Copyright 2015, University of Wisconsin Board of Regents) as developed by M. Geurts, released under the GNU GPL v. 3.0 License.8 Global γ analysis was calculated, with analysis performed for doses greater than 20% of the prescription dose.
Initially a dose response curve for the Gafchromic film was generated using a two-colour channel optical density-based calibration, by irradiating the film to known doses. The Farmer type chamber (NE2571) was irradiated following the AAPM TG61 dosimetry recommendations,9 after which the film was substituted into a similar set up where the film was positioned at the effective point of measurement of the chamber. The calibration was performed for doses between 0 and 480 cGy, and accuracy of the calibration curve was found to be within ±2%.
Plans of 100 cGy dose prescription at the isocentre were then delivered to 5 × 5 cm squares of Gafchromic film, stacked in the centre within four blocks of 5 × 5 × 0.5 cm of solid water build-up, provided with the depth-dose calibration kit from XStrahl. CBCT scans of the entire set up were performed upon which the isocentre was placed at the level of the film, segmentation was performed to have all material in the field of view as water and the beam geometries were created. The CBCT dose to the film was seen as negligible as the dose for a 360 degree CBCT was determined to be around 0.04 cGy in a centre of a 1 cm cube (designed to mimic the approximate size of a mouse head).
Image registration was performed by putting markers on the edges of the film that were visible on the scanned film and on the CBCT image. Using a rigid registration (translation and rotation only) the delivered and planned doses were compared, and γ analysis performed over a range of dose difference and distance to agreement criteria to determine the accuracy of the system. An analysis is said to “pass” if 95% of points in the dataset have a γ value of less than unity. Image analysis was performed using absolute doses and thus proves calculation accuracy in terms of dose distribution and absolute dose delivery.
Results
Plan assessment
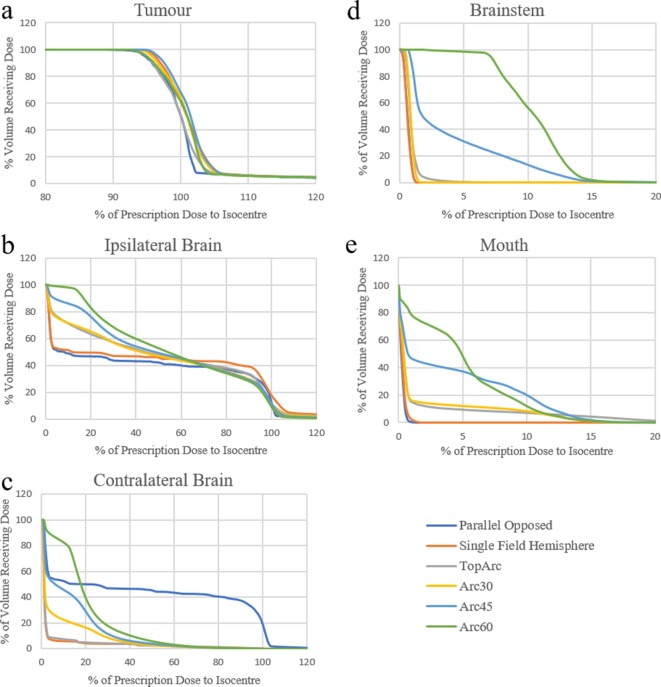
The different plan types were evaluated by exporting DVHs and comparing dose-volume parameters for tumour and OARs. Figure 2a shows there to be no appreciable difference in tumour coverage between the plans. When considering dose to the ipsilateral brain, all arc-based plans generated a “low-dose bath” (Figure 2b), consistent with clinical experience using volumetric modulated arc treatment. This effect was also observed in the contralateral brain when couch rotation arc plans were used (Figure 2c). In contrast, static beams generated a more stepped dose to the ipsilateral brain, with smaller volumes receiving lower doses. The single field technique delivered higher doses (>60%) to a slightly larger volume of the ipsilateral brain.
Figure 2.
Tumour DVH for the six different treatment plans: (a) tumour, (b) ipsiliateral brain, (c) contralateral brain, (d) brainstem and (e) mouth. DVH, dose volume histograms.
Contralateral brain dosimetry was most affected by plan type (Figure 2c). As anticipated, sparing of the contralateral brain was maximised by the single field and TopArc plans, which were explicitly designed for this purpose. For couch rotation arc plans, the shallower the gantry angle (from vertical) the better the sparing of the opposite cerebral hemisphere. Parallel opposed fields delivered high doses to the contralateral hemisphere.
The only plan types to deliver significant dose (>5%) to the brainstem were the Arc60 and Arc45 plans (Figure 2d), which delivered maximum point doses of approximately 14% of the prescription dose (note scale of x-axis). Tumour location and murine brain anatomy render it very unlikely that the brainstem would receive a significant dose in studies of this type.
The mouth, similarly to the brainstem, only received dose from plans with a large superior-inferior component of beam delivery (Figure 2e). The highest mouth doses (around 10–15% of prescription) were delivered by all of the arc-based plans (both single plane and couch rotation). No significant doses were delivered to the eyes (data not shown).
Rather than present Dmax the maximum dose to a 0.001 cm3 volume is presented with the other DVH parameters as it is a reasonable assumption of the smallest clinically relevant volume irradiated.
It can be seen from Table 1 that all plans achieved both D_95% and V_95% values of 95% or greater, apart from the parallel opposed pair arrangement. Better results were generally achieved with more complex beam arrangements, as there were more options for optimisation.
Table 1.
DVH parameters for the six investigated plans, in terms of the mean dose (D_mean), the maximum dose to 0.001 cm3 (D_max_0.001 cm3), the dose to 95% of the volume (D_95%) and the volume receiving 95% of the prescription dose (V_95%)
| Plan type | D_mean | D_max_0.001 cm3 | D_95% | V_95% |
| Parallel opposed | 101.4% | 105.8% | 94.7% | 93.8% |
| Single hemisphere | 103.1% | 107.5% | 97.3% | 99.2% |
| TopArc | 101.9% | 105.8% | 95.1% | 95.2% |
| Arc30 | 102.7% | 106.3% | 96.3% | 98.1% |
| Arc45 | 103.2% | 106.7% | 96.7% | 99.8% |
| Arc60 | 102.4% | 106.0% | 95.0% | 95.0% |
DVH, dose volume histograms ;
Plan deliverability and accuracy
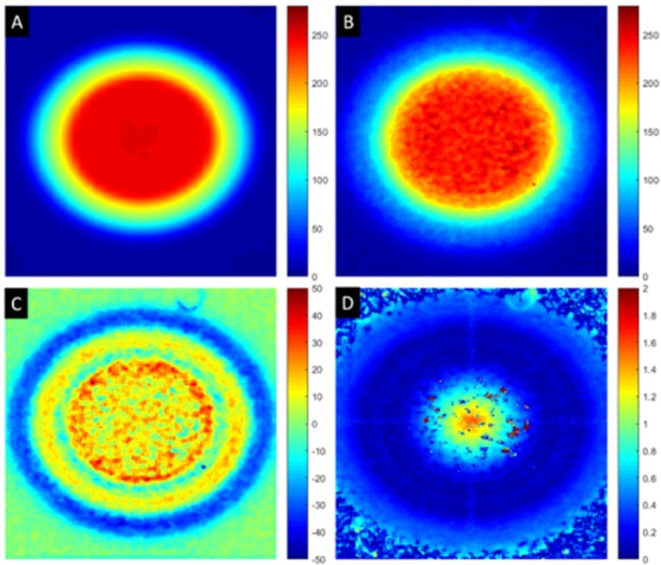
The γ criteria were tightened until pass rates dropped below 95%. For the single beam and parallel opposed pair cases, a single beam was used. The ability of the SARRP to position the gantry and couch has been well-established, and local testing has found positioning errors to be less than 0.25 mm. For the couch rotation arc type plans, only the 45 degree case was tested. The best γ criteria achieved for the single beam case was 2%/1.5 mm, for the couch arc rotation plan was 2%/0.75 mm or 1%/1.25 mm and for the TopArc plan was 2%/1 mm. Differences in the distance to agreement criteria are likely to be generated from inaccuracies in the image registration stage, which will be an area for future development. Shown in (Figure 3) are the results for the Arc 45 couch rotation plan.
Figure 3.
Couch rotation 45 arc plan results. (A) planned dose from Muriplan (cGy), (B) measured dose on Gafchromic film (cGy), (C) difference image, planned–measured (cGy) and (D) γ map for 1.0%/1.5 mm (no units).
Plans were created in 20–25 min including registration of MRI and CBCT images, segmentation of tissue types, voluming of OARs and arranging beams. It is anticipated that plan creation times may be reduced to 15–20 min given sufficient user experience. Irradiation times were 1–2 min for simpler beam arrangements, and up to 4 min for the most complex (“TopArc”) plan.
The majority of points failing were in the high-dose region of the beam and present as very small areas. While the film response and the conversion from optical density to dose will have noise and uncertainty contributions, no smoothing was applied to the measured data. As a test-case, a 10 × 10 pixel median filter was applied to the data and minimal gains (<0.5%) were seen in the pass rate for the same γ criteria. The areas where γ was failing were similar for all plan types.
Discussion
This study shows that the SARRP enables a variety of different radiotherapy techniques to be used in studies of orthotopic brain tumour models, all of which achieve excellent tumour coverage. Plan selection is therefore primarily dictated by the objectives and requirements of the study in terms of normal tissue dosimetry. Almost complete sparing of the contralateral hemisphere can be achieved by the use of single beam or “TopArc” plans, of which the single beam also achieves maximum sparing of the mouth. For studies incorporating brain toxicity endpoints, arc-based techniques recapitulate the clinical scenario most effectively, and the Muriplan software on the SARRP enables accurate measurement of doses and DVHs for OARs. There is very little data to inform OAR tolerances for mouse models in general and the mouse brain in particular. Most studies have used whole brain irradiation and we are not aware of any published work in this area that has formally evaluated different methods of focal irradiation of orthotopic brain tumours. Pre-clinical evaluation of radiotherapy-drug combinations will be enhanced by accurate measurement of OAR dosimetry, and will facilitate accurate comparison of behavioural, histopathological and molecular sequelae of radiotherapy in the presence and absence of the investigational drug.
The relatively complex, arc-based treatment plans described in this paper were shown to be deliverable and highly accurate. This level of accuracy is important in the context of the small dimensions of both the intracranial tumours and the mouse brain. While excellent accuracy was generally achieved using the SARRP, the system in Glasgow currently requires the use of fixed collimators of various sizes which restricts conformality to some extent. Cho et al10 have now developed a variable collimator system for the SARRP which enables the user to more closely conform to tumour shape and size as well as to OAR geometry if required.
Due to machine time restrictions, repeat measurements could not be performed for the same plan type on different films, with an aim to reduce uncertainty. Gafchromic film, however, has been well-established as a reliable dose measurement method, and routine quality assurance tests give confidence in the reproducibility of set up and stability of dosimetry.
Conclusions
The ability of the SARRP to deliver accurate doses to small volumes is vital in providing confidence and clinical relevance in pre-clinical radiation studies. For relatively complex plan types the SARRP can deliver accurate doses while sparing OARs, enabling longer-term efficacy and toxicity studies.
Contributor Information
Alasdair Rutherford, Email: rutherford2212@gmail.com.
Katrina Stevenson, Email: Katrina.Stevenson@glasgow.ac.uk.
Amanda Tulk, Email: AmandaTulk@xstrahl.com.
Anthony J Chalmers, Email: Anthony.Chalmers@glasgow.ac.uk.
REFERENCES
- 1.Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol 2011; 56: R55–R83. doi: 10.1088/0031-9155/56/12/R01 [DOI] [PubMed] [Google Scholar]
- 2.Wong J, Armour E, Kazanzides P, Iordachita I, Tryggestad E, Deng H, et al. . High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys 2008; 71: 1591–9. doi: 10.1016/j.ijrobp.2008.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed SU, Carruthers R, Gilmour L, Yildirim S, Watts C, Chalmers AJ. Inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res 2015; 75: 4416–28. doi: 10.1158/0008-5472.CAN-14-3790 [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Roman N, Stevenson K, Gilmour L, Hamilton G, Chalmers AJ. A novel 3D human glioblastoma cell culture system for modeling drug and radiation responses. Neuro Oncol 2017; 19: 229–41. doi: 10.1093/neuonc/now164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhaegen F, Dubois L, Gianolini S, Hill MA, Karger CP, Lauber K, et al. . ESTRO ACROP: Technology for precision small animal radiotherapy research: Optimal use and challenges. Radiother Oncol 2018; 126: 471–8. doi: 10.1016/j.radonc.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 6.Sorriaux J, Kacperek A, Rossomme S, Lee JA, Bertrand D, Vynckier S, et al. . Evaluation of Gafchromic® EBT3 films characteristics in therapy photon, electron and proton beams. Phys Med 2013; 29: 599–606. doi: 10.1016/j.ejmp.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 7.Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys 1998; 25: 656–61. doi: 10.1118/1.598248 [DOI] [PubMed] [Google Scholar]
- 8.Geurts M. CalcGamma - gamma analysis implementation for MATLAB. 2015.
- 9.Ma CM, Coffey CW, DeWerd LA, Liu C, Nath R, Seltzer SM, et al. . AAPM protocol for 40-300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Med Phys 2001; 28: 868–93. doi: 10.1118/1.1374247 [DOI] [PubMed] [Google Scholar]
- 10.Cho NB. Dose painting with a variable collimator with the small animal radiation research platform (SARRP). 2014. Available from: http://hdl.handle.net/10380/3476.