Abstract
Spinal haematoma is a rare occurrence, however has the potential to cause significant spinal injury and morbidity. MRI is the gold-standard of investigation, and urgent imaging is required for early diagnosis and treatment to ensure best patient outcomes. We present a pictorial review demonstrating the imaging features of spinal haematoma based on meningeal space assignment; epidural, subdural, subarachnoid, intramedullary and a combination of these locations. In this review, we summarise the literature and imaging findings of spinal haematoma on MRI. Particular imaging features which help to differentiate between haematoma in the different spinal meningeal compartments are discussed below.
INTRODUCTION
Spinal haematoma is rare occurrence, a 2003 metanalysis reports only 613 cases were published until 1996. Even if, as the authors suggest, this represents under reporting or under diagnosis, it is apparent that the overall incidence of spinal hematoma is low.
Spinal haematoma may occur spontaneously; "no definite etiological factor", or "trivial trauma only" was evident in the majority of these cases.1 Anticoagulation therapy is the most common association with spinal haematoma.2 The incidence of central nervous system haemorrhage associated with anticoagulation or coagulopathy is reported variably between 0.01 and 12%, however, given the low incidence of overall central nervous system haemorrhage in large studies of cardiology patient cohorts undergoing thrombolytic therapy the authors suggest the true incidence of spinal haematoma on anticoagulant therapy is likely low.1 Spinal anaesthetic procedures and lumbar puncture are also identified as precipitating events. The incidence of vertebral canal haematoma post-spinal anaesthesia in patients with normal coagulation is 0.85/100 000. Consensus guidelines from Anaesthesia 2013 acknowledge the “extent to which the risk of haemorrhagic complications is increased in patients with abnormalities of coagulation is unquantifiable, but likely to be small”.3 The incidence of spinal haematoma with cord compression in patients with spinal trauma is reported to be 1–1.7%. Less frequent aetiologies include vascular malformations, vasculopathies and tumours.1
Patients usually present acutely with abrupt onset of pain and variable degrees of paralysis and neurologic impairment. Less common presentations include radicular pain and cerebral signs such as headache, vomiting and visual disturbances. Cerebral symptoms are more common in subarachnoid spinal haemorrhage.1
MRI is the imaging of choice in the investigation of spinal haemorrhage.2 Spinal haematoma are described based on the location(s) of haematoma, including epidural, subdural, subarachnoid, intramedullary or a combination of these. Early recognition and diagnosis, including correct anatomic space assignment, is paramount for optimal management and best patient outcomes.
The epidural space is the most common location of spinal haemorrhage. Haematoma affecting multiple meningeal compartments accounts for 2.1% of spinal haematoma.1
Management of spinal haematoma is based on several factors including patient comorbidities, associated injuries and severity of neurological impairment. In the presence of cord compression, conservative management is usually reserved for patients presenting with very mild neurological deficit, those who undergo early progressive improvement, or where coagulopathy is present. If surgery is undertaken, time to surgery following symptom onset is an important prognostic factor, with best recovery rates in patients undergoing surgery within 12 h of symptom onset. The extent of neurological impairment and rapidity of onset also affects outcome. For all causes of spinal haemorrhage, Kreppel et al reported complete recovery in 39.6% of patients. Intramedullary haemorrhage is associated with more severe neurological sequalae and worse prognosis.1,4
Review of anatomy
An understanding of spinal anatomy is paramount to imaging interpretation and management of spinal haematoma. The spinal cord usually extends to the level of T12 or L1 in adults and terminates as the conus medullaris, spinal nerve roots then extend inferiorly as the cauda equina.5 The spinal cord is covered by three meningeal layers; the pia mater immediately adjacent to the cord, the arachnoid mater overlying the pia and dura mater as the most superficial layer. Between the arachnoid and pia mater is the subarachnoid space which is filled with cerebrospinal fluid (CSF). The dura is closely related to the arachnoid mater, with only a potential space (the subdural space) usually contained between these two layers (Figure 1). The spinal subdural space does not contain bridging veins, unlike the intracranial subdural space. This is the likely reason that epidural haemorrhage is more common than subdural haemorrhage in the spine, and the reverse true for intracranial haemorrhage. The space between the dura mater and the vertebral column is the epidural space. Connective tissue including plicae and meningovertebral ligaments divide the epidural space into anterior, lateral and posterior compartments.6 Contents of the epidural spaces may pass freely between the cavities despite septations.7
Figure 1.
Sagittal T1 (a) with annotations depicting the meningeal spaces and (b) unannotated. Axial T2 (c) with annotations depicting the spinal meningeal spaces and (d) unannotated.
Mr imaging
Imaging features of haematoma on MRI evolve over time (Table 1): due to the presence of intracellular oxyhaemoglobin, spinal haematoma in the hyperacute phase appear isointense on T1 and hyperintense on T2 weighted imaging. A rim of hypointensity surrounding the haematoma is variably seen.8,9 Intracellular deoxyhaemoglobin accounts for acute phase imaging characteristics; T2 signal intensity decreases and haematoma appear hypointense. T1 signal remains intermediate to long and haematoma, therefore appear hypo-/isointense.8,9 In the early subacute phase, T1 signal intensity increases, T2 shortens and haematoma appear T1 hyperintense and T2 hypointense. In the late subacute phase, extracellular methaemoglobin is formed and haematoma is visualized as both T1 and T2 hyperintense. Paramagnetic haemosiderin and ferritin accounts for the usual appearance of chronic haematoma which is hypointense on both T1 and T2 weighted imaging.9 T2*- Gradient Echo Imaging (T2* or GRE) and susceptibility weighted imaging are highly sensitive to paramagnetic substances and useful in the imaging of haemorrhage, which are predominantly hypointense on these sequences. Imaging in at least two planes including sagittal and axial is recommended, MR sequences may be tailored to the individual circumstance, however Lamertesse et al recommend including sagittal T1, T2 fast spin echo, T2*; and axial T2 fast spin echo, T2* for the assessment of patients with suspected acute spinal cord injury.2
Table 1.
Imaging features of haematoma on MRI over time
| Stage | Time | Component | T1 | T2 |
| Hyperacute | <24 h | Oxyhaemoglobin | Hypointense | Hyperintense |
| Acute | 1–3 days | Deoxyhaemoglobin | Isointense | Hypointense |
| Subacute–early | 3–7 days | Intracellular methaemoglobin | Hyperintense | Hypointense |
| Subacute–ate | 1–2 weeks | Extracellular methaemoglobin | Hyperintense | Hyperintense |
| Chronic | >2 weeks | Haemosiderin | Hypointense | Hypointense |
Haematoma by anatomic location
Epidural
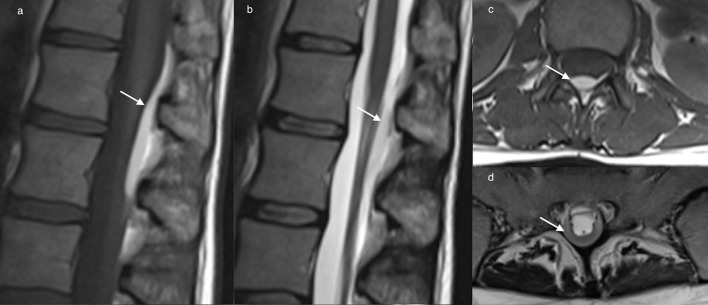
Haematoma may occur in the epidural space, it is usually described as being located predominantly posterior (Figures 2–4), anterior (Figure 5) or diffuse.10 Loss of the normal epidural fat signal in the posterior epidural space is a useful locational sign (Figure 4, solid white arrow). As the haematoma is contained by the dura, the contour adjacent to the canal is smooth. Bulging of the dura into the canal may be seen as a thin hypointense line on T2 weighted imaging (Figure 4, black arrow), displaced anteriorly by the haematoma (broken white arrow).
Figure 2.
Sagittal T1 (a) and T2 (b) weighted images and axial T1 (c) and T2 (d) weighted images of the lumbar spine depicting a posterior epidural haematoma (white arrows). Loss of the normal epidural fat signal in the posterior epidural space is a useful locational sign. Bulging of the dura into the canal may be seen as a smooth thin hypointense line displaced by the haematoma.
Figure 3.
T1sagittal (a) and axial (b) imaging demonstrates a right posterolateral epidural haematoma post trauma (black arrows), an associated vertebral body fracture is evident.
Figure 4.
Sagittal GRE image (a) and axial T2 weighted image (b) depicting a posterior epidural haematoma (broken white arrow) in the lumbar spine which obscures the normal epidural fat signal (solid white arrow). The dura is displaced anteriorly (black arrow). GRE, gradient echo.
Figure 5.
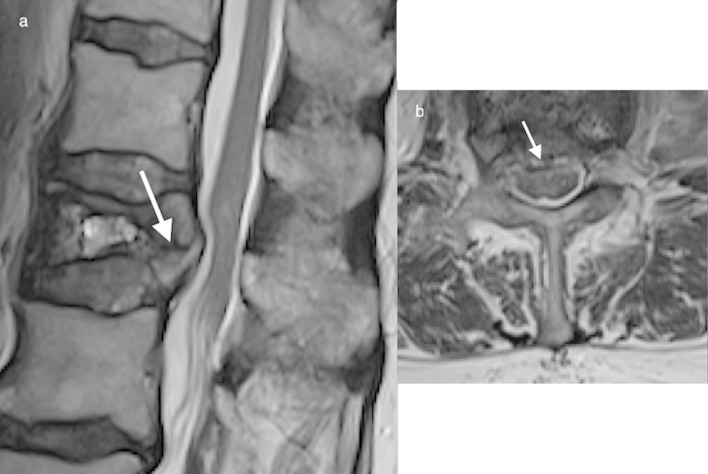
Sagittal T2 (a) demonstrates a small (3 mm) anterior epidural haematoma (white arrow), a thin rim of haematoma is evident on axial imaging (b). A T12 burst fracture is present.
Subdural
A subdural haematoma occurs in the potential space between the dura and arachnoid mater. The dura may be identified as a dark line on T2 imaging which limits the haematoma posteriorly and separates the haematoma from the epidural fat. Identifying the presence of normal epidural fat is helpful to differentiate a subdural from an epidural haematoma. The inner confines of a subdural haematoma are often concave where residual CSF fluid is visualized. The inner contour of a subdural haematoma may be irregular, as opposed to the usual smooth contour of an epidural haematoma.11 The haematoma causes mass effect on the cord (Figure 6). This helps to differentiate haematoma from CSF flow voids in this case, which can artifactually produce low signal, and may confound interpretation. Haematoma is also persistent on multiple sequences, including GRE, useful to reduce time-of-flight artefact due to its short echo time. A subdural haematoma which is large enough to obliterate the canal may increase difficulty in differentiating between subdural and subarachnoid haemorrhage (SAH), or establishing the presence of multicompartmental haematoma (Figure 7). Careful evaluation for signs of each compartmental haemorrhage is imperative, such as presence of normal epidural fat and absence of the fluid fluid levels of SAH.
Figure 6.
Axial (a, b) and sagittal (c) T2 imaging demonstrates subdural haematoma extending from C7 -T5 posteriorly, surrounding the cord at the T1 level (a). The haematoma is persistent on multiple sequences, including GRE, and causes mass effect on the cord (b), differentiating it from CSF flow artefact. Normal epidural fat is helpful to establish the presence of subdural haematoma. The inner contour of a subdural hematoma may be irregular, and is often concave.
Figure 7.

Sagittal T1 (a) and T2 (b) weighted images of the thoracolumbar spine, and axial T2 (c) and T1 (d) weighted images of the thoracolumbar spine depicting a a large posterolateral subdural haematoma causing spinal cord compression.
Subarachnoid
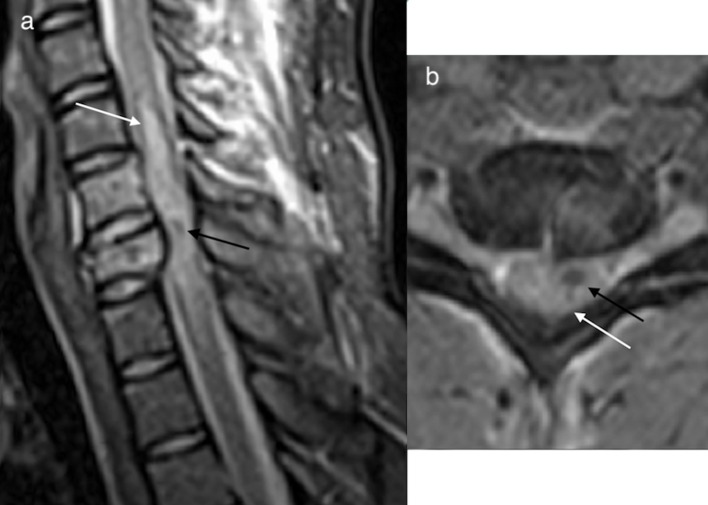
Due to the movement of CSF, SAH usually spreads longitudinally rather than becoming contained focally.4,10 A distinguishing feature of a SAH is the presence of fluid levels (Figure 8). SAH may layer within the distal thecal sac (Figure 9). As in subdural haemorrhage there is preservation of the epidural fat signal, and the normal dural demarcation is present.
Figure 8.

Sagittal T1 (a) and T2 (b) weighted images of the thoracolumbar spine, and axial T2 (c) weighted image of the lumbar spine depicting an extensive subarachnoid haematoma, fluid–fluid levels are a distinguishing feature of SAH. SAH usually spreads rather than becoming contained focally and may layer within the distal thecal sac. SAH,subarachnoid haemorrhage.
Figure 9.
T2sagittal (a) and axial (b, c) demonstrates subdural and SAH in the lumbar spine post-trauma; a 3 mm subdural haematoma is seen anteriorly (white arrows) and fluid–fluid levels indicate SAH settled posteriorly (black arrows). SAH,subarachnoid haemorrhage.
Intramedullary
Intramedullary haemorrhage (haematomyelia) most often occurs where a patient has been subject to trauma.1 The haemorrhage tends to occur at the point of maximal impact and usually involves the central grey mater, occurring as a result of haemorrhagic necrosis of the cord.12 Axial T2* imaging is recommended for identification of intramedullary haemorrhage, which may be seen as a foci of hypointense signal on T2*. Intramedullary haemorrhage (Figures 10 and 11 black arrows) is frequently accompanied by cord oedema, (Figures 10 and 11 white arrows) seen as hyperintensity on T2 sequences, with loss of the grey white matter differentiation.4,12
Figure 10.
Sagittal T2 STIR image (a) and axial T2 GRE image (b) of the cervical spine demonstrating extensive cord oedema (white arrow) with intramedullary haemorrhage (black arrow) which is often seen in the presence of intramedullary haemorrhage. Intramedullary haemorrhage is seen as a foci of hypointense signal usually within the central grey matter of the cord. GRE,gradient echo; STIR, short tau inversion-recovery.
Figure 11.
Axial T2 (a) demonstrates cord swelling and oedema (white arrow) and axial GRE (b) shows intramedullary haemorrhage (black arrow). GRE,gradient echo.
MultiCOMPARTMENTAL SPINAL HAEMATOMA
Spinal haemorrhage affecting multiple spinal compartments may cause diagnostic uncertainty. Sequentially identifying the features of each compartment and systematic analysis of each imaging feature is key to establish which and how many and which compartments are involved. Figure 12 demonstrates thoracic subdural haematoma anteriorly and subarachnoid haematoma settling at the level of the sacrum, sustained by a 37-year-old patient involved in a biking accident.
Figure 12.
Sagittal (a) and axial T1 (b) images of the thoracic spine demonstrating subdural haematoma anteriorly (black arrow). Sagittal T1 (c) and T2 (d) images of the lumbosacral spine, demonstrating SAH settling at the level of the sacrum (white arrows). SAH,subarachnoid haemorrhage.
Figure 13 demonstrates a cervicothoracic MRI of a patient post-high speed motor vehicle accident with anterior and posterior epidural haematoma (broken white arrows) in the lower cervical spine, intramedullary haemorrhage (solid white arrows) and oedema (black arrow). Follow-up imaging of the same patient 1 day subsequently, post-anterior decompression C5–C6 anterior cervical discectomy and fusion (Figure 14), demonstrates increased cord oedema (black arrow) and intramedullary haemorrhage (white arrows).
Figure 13.
Epidural haematoma extends throughout the epidural space (anterior, lateral, posterior) (broken white arrows). Cord oedema (black arrow) is seen on T2 imaging, intramedullary haemorrhage is evident on axial T2 (b) and GRE (c) (solid white arrows).
Figure 14.
Follow-up imaging (same patient as Figure 13), post-C5–C6 anterior discectomy & fusion. Sagittal STIR (a), T2 (b), axial T2 (c) and GRE (d) demonstrate increased cord swelling and oedema (black arrow), and intramedullary haemorrhage (white arrow). Pre-vertebral oedema and haematoma is also evident. GRE,gradient echo.
Discussion
Spinal haemorrhage with acute neurological impairment is considered a surgical emergency. Urgent MRI is necessary to establish the nature and extent of the haemorrhage. It is important to establish the specific meningeal space or spaces within which the haematoma is contained, as location impacts upon management and surgical strategy, and may also give an indication of the aetiology when none is clinically apparent. Identification of normal anatomic structures such as epidural fat and the dural line, and specific features of haematoma within the meningeal compartments, such as fluid–fluid levels, or concavity and outline of the haematoma are important to evaluate.
Differentiating spinal haematoma from inflammatory or neoplastic lesions can be challenging. The clinical history and contrast-enhanced imaging may help to differentiate pathologies. Malignancy such as infiltrative lymphoma or metastasis will enhance, further identifiers such as a pathologic fracture or lymphadenopathy may be visible. Necrotic areas of tumour often have marginal enhancement, also may also be seen in patients with spinal abscess (Figure 15).10 Patients with spinal abscess may show clinically infectious signs, and may have associated features such as diskitis. It is possible, but uncommon for the margins of a haematoma to enhance in the hyperacute phase.13 Contrast imaging may identify underlying intramedullary lesions such as haemorrhagic ependymoma which enhance avidly. Spinal cavernoma may be difficult to differentiate from intramedullary haemorrhage, however cavernoma are rarely associated with oedema unless there has been a recent bleed. Furthermore, intramedullary haemorrhage is most frequently associated with extensive oedema and usually evidence of significant trauma.4,5,14 Spinal angiography to evaluate for small vascular lesion such as an AVM if the history or imaging does not identify an aetiology is recommended.10
Figure 15.
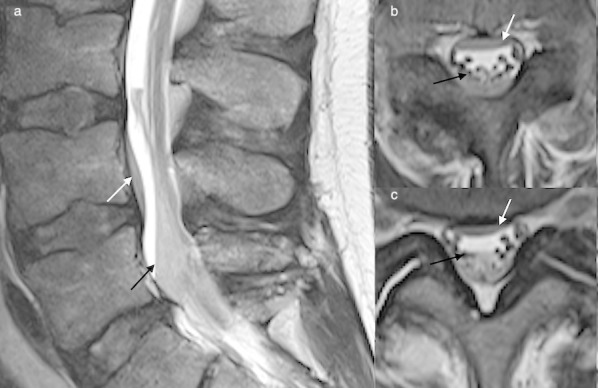
Sagittal T1 (a), STIR (b) and axial T2 (c) demonstrate a posterior epidural abscess (black arrows) secondary to facet joint septic arthritis. The dura is displaced anteriorly (white arrows). Diffuse contrast enhancement and the clinical presentation (subacute presentation, pyrexia) helped to differentiate abscess from haematoma in this case. STIR, short tauinversion-recovery.
Most authors agree that surgical decompression should be performed as soon as possible for cord compression due to spinal haematoma, however it is recommended to improve coagulation parameters preoperatively if coagulopathy is present.1 In patients with an acute intramedullary haemorrhage secondary to an underlying lesion, waiting several weeks prior to resection may facilitate surgery.15 Patients with spinal haematoma but no, mild or improving neurological deficits, may be managed conservatively. Subarachnoid haemorrhage may also be managed conservatively, if investigations into aetiology reveal no underlying lesion. If conservative management is pursued in spinal haematoma, close clinical and MRI follow-up is recommended.
Imaging findings may be used for prognostication purposes; it is reported that in traumatic spinal injury, intramedullary haemorrhage, cord transection, higher cord lesions, cord swelling and oedema, and longer length of involved cord correlates with poorer neurological outcomes.2,4
Conclusion
Spinal haematoma is a rare occurrence, that can cause acute neurological disturbance. Urgent MRI is imperative when spinal haematoma is a clinical concern, as early recognition and correct meningeal space assignment is important to ensure optimum management and best patient outcomes. MRI can establish the presence, location and extent of the haematoma, along with cord compression and concurrent injuries. Epidural haematoma is the most common location of haematoma in the spine. Management is based on aetiology and the clinical scenario, particularly extent of neurological disability and presence or absence of cord compression. MRI is also an important tool for follow up of patients who have been managed conservatively.
Contributor Information
Heather Kate Moriarty, Email: heather.moriarty@gmail.com.
Roisin O Cearbhaill, Email: roisinocearbhaill@gmail.com.
Peter D Moriarty, Email: petem1988@gmail.com.
Emma Stanley, Email: estanley@mater.ie.
Leo P Lawler, Email: llawler@mater.ie.
Eoin C Kavanagh, Email: ekavanagh@mater.ie.
REFERENCES
- 1.Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 2003; 26: 1–49. doi: 10.1007/s10143-002-0224-y [DOI] [PubMed] [Google Scholar]
- 2.Lammertse D, Dungan D, Dreisbach J, Falci S, Flanders A, Marino R, et al. Neuroimaging in traumatic spinal cord injury: An evidence-based review for clinical practice and research. J Spinal Cord Med 2007; 30: 205–14. doi: 10.1080/10790268.2007.11753928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrop-GriffithsW CT, Gill H, Hill D, Ingram M, Makris M, Malhotra S, et al. Regional anaesthesia and patients with abnormalities of coagulation : the association of anaesthetists of great britain & ireland the obstetric anaesthetists' association regional anaesthesia UK. Anaesthesia 2013; 68: 966–72. [DOI] [PubMed] [Google Scholar]
- 4.Flanders AE, Spettell CM, Friedman DP, Marino RJ, Herbison GJ. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. Am. J. Neuroradiol 1999; 20: 926–34. [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzo A, Marcoux J, Radhakrishna M, Pelletier J, Goulet B. The Role of Magnetic Resonance Imaging in the Management of Acute Spinal Cord Injury. J Neurotrauma 2011; 28: 1401–11. doi: 10.1089/neu.2009.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jagla G, Walocha J, Rajda K, Dobrogowski J, Wordliczek J. Anatomical aspects of epidural and spinal analgesia. Adv. Pall. Med 2009; 8: 135–46. [Google Scholar]
- 7.Plaisant O, Sarrazin JL, Cosnard G, Schill H, Gillot C. The lumbar anterior epidural cavity: The posterior longitudinal ligament, the anterior ligaments of the dura mater and the anterior internal vertebral venous plexus. Cells Tissues Organs 1996; 155: 274–81. doi: 10.1159/000147816 [DOI] [PubMed] [Google Scholar]
- 8.Sharma R, Gaillard F. Ageing blood on MRI. 2017. Available from: https://radiopaedia.org/articles/ageing-blood-on-mri [Updated 2017, cited 2018 January 25].
- 9.Braun P, Kazmi K, Nogués-Meléndez P, Mas-Estellés F, Aparici-Robles F. MRI findings in spinal subdural and epidural hematomas. Eur J Radiol 2007; 64: 119–25. doi: 10.1016/j.ejrad.2007.02.014 [DOI] [PubMed] [Google Scholar]
- 10.Holtås S, Heiling M, Lönntoft M. Spontaneous spinal epidural hematoma: findings at MR imaging and clinical correlation. Radiology 1996; 199: 409–13. doi: 10.1148/radiology.199.2.8668786 [DOI] [PubMed] [Google Scholar]
- 11.Mashiko R, Noguchi S, Uemura K, Takada T, Matsumura A. Lumbosacral subdural hematoma. Neurol Med Chir 2006; 46: 258–61. doi: 10.2176/nmc.46.258 [DOI] [PubMed] [Google Scholar]
- 12.Looby S, Flanders A, Trauma S. Spine Trauma. Radiol Clin North Am 2011; 49: 129–63. doi: 10.1016/j.rcl.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 13.Chang FC, Lirng JF, Chen SS, Luo CB, Guo WY, Teng MM, Mu M, et al. Contrast enhancement patterns of acute spinal epidural hematomas: a report of two cases. AJNR Am J Neuroradiol 2003; 24: 366–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon I, Jung WS, Suh SH, Chung T-S, Cho Y-E, Ahn SJ. MR imaging features that distinguish spinal cavernous angioma from hemorrhagic ependymoma and serial MRI changes in cavernous angioma. J Neurooncol 2016; 130: 229–36. doi: 10.1007/s11060-016-2239-1 [DOI] [PubMed] [Google Scholar]
- 15.Jallo GI, Freed D, Zareck M, Epstein F, Kothbauer KF. Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus 2006; 21: 1–6. doi: 10.3171/foc.2006.21.1.11 [DOI] [PubMed] [Google Scholar]