Abstract
Airway eosinophils are increased in asthma and are especially abundant around airway nerves. Nerves control bronchoconstiction and in asthma, airway hyperreactivity (where airways contract excessively to inhaled stimuli) develops when eosinophils alter both parasympathetic and sensory nerve function. Eosinophils release major basic protein, which is an antagonist of inhibitory M2 muscarinic receptors on parasympathetic nerves. Loss of M2 receptor inhibition potentiates parasympathetic nerve-mediated bronchoconstriction. Eosinophils also increase sensory nerve responsiveness by lowering neurons’ activation threshold, stimulating nerve growth, and altering neuropeptide expression. Since sensory nerves activate parasympathetic nerves via a central neuronal reflex, eosinophils’ effects on both sensory and parasympathetic nerves potentiate bronchoconstriction. This review explores recent insights into mechanisms and effects of eosinophil and airway nerve interactions in asthma.
Keywords: asthma, eosinophil, major basic protein, parasympathetic nerve, sensory nerve
1 |. INTRODUCTION
While the association of eosinophils and asthma is well established, the importance of interactions between eosinophils and airway nerves has only recently been appreciated. Airways are densely innervated by sensory and parasympathetic nerves that regulate airway tone and provoke bronchoconstriction (Fig. 1).1–7 In humans with asthma and in animal models, eosinophils localize to nerves and alter nerve function.8–11 As a result, airway hyperreactivity develops, where airways contract excessively in response to inhaled stimuli.12 This review explores key mediators and mechanisms that govern interactions between eosinophils and airway nerves in asthma and in models of airway hyperreactivity induced by antigen exposure, respiratory virus infection, and ozone inhalation. The implications of these interactions for clinical asthma are also discussed.
FIGURE 1. Sensory and parasympathetic nerves form a complex network in the airways.
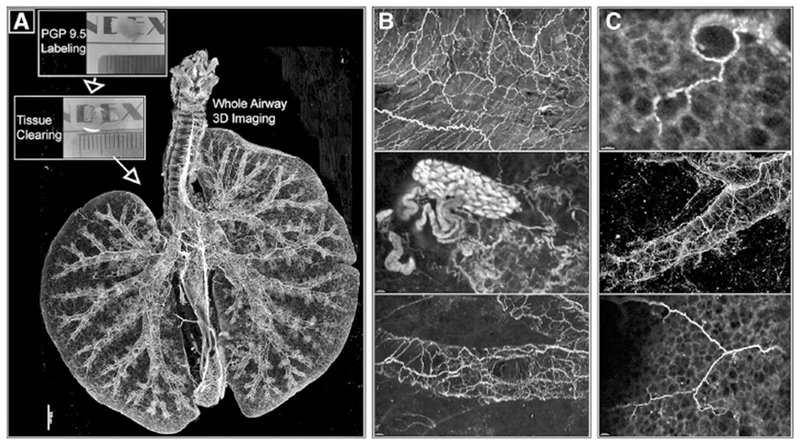
(A) Optically cleared mouse lungs labeled with neuronal marker PGP9.5. Images obtained by confocal microscopy. (B and C) Magnified images of nerves along airways and a cluster of nerve cell bodies (ganglia; B middle image). Reprinted with permission of the American Thoracic Society. Copyright © 2017 American Thoracic Society, from Scott et al.7
1.1 |. Sensory and parasympathetic nerves control airway reflexes
Airway sensory nerves detect stretch (mechanoreceptors) and chemical stimuli (chemoreceptors) and relay information along axons contained within the vagus nerves to the central nervous system via their cell bodies in the nodose and jugular ganglia at the base of the skull.13,14 The vagus nerves also contain parasympathetic nerve fibers. Parasympathetic nerves travel from the brain to the airways and provide the dominant autonomic control of bronchoconstriction. Parasympathetic nerves induce bronchoconstriction by releasing acetylcholine (ACh), which activates M3 muscarinic receptors on airway smooth muscle to stimulate contraction.5 ACh simultaneously activates prejunctional inhibitory M2 muscarinic receptors that reduce ACh release and limit bronchoconstriction, thus serving as an auto-inhibitory feedback mechanism (Fig. 2).3,4,15
FIGURE 2. Eosinophils cause airway hyperreactivity by altering sensory and parasympathetic nerve function.
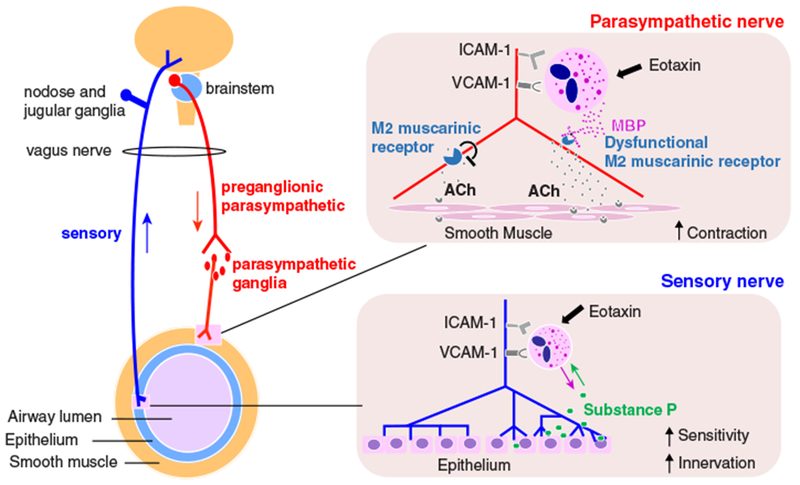
Parasympathetic nerves release ACh that activates M3 muscarinic receptors to induce bronchoconstriction. ACh simultaneously activates presynaptic inhibitory M2 muscarinic receptors, which reduce further ACh release and serve as an auto-inhibitory feedback mechanism. Sensory nerves can also trigger bronchoconstriction by activating parasympathetic nerves via a central neuronal reflex pathway. In asthma, eosinophils are recruited to nerves by eotaxin-1 and release major basic protein (MBP) that is a parasympathetic nerve M2 receptor antagonist. Loss of M2 receptors’ inhibitory feedback results in excessive ACh release and increased bronchoconstriction. Eosinophils affect sensory nerve function as well by inducing nerve growth and increasing sensory neuropeptides such as substance P, which results in increased bronchoconstriction
Sensory and parasympathetic nerves communicate via a central pathway known as a neuronal reflex. Sensory nerves initiate “reflex bronchoconstriction” through this pathway by stimulating parasympathetic nerve ACh release (Fig. 2). Reflex bronchoconstriction occurs in response to a variety of stimuli, including methacholine,6 histamine,16 cold air,17 exercise,18 and allergens,19 and is increased in patients with asthma.20–22 Changes in either the sensory afferent limb or parasympathetic efferent limb of the reflex pathway can inhibit or exacerbate bronchoconstriction induced by triggering this reflex.
1.2 |. Eosinophils increase bronchoconstriction by potentiating ACh release from parasympathetic nerves
Eosinophil granules are filled with highly charged, cationic proteins including major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin.23 Major basic protein is of particular relevance since it is an allosteric antagonist of parasympathetic nerve M2 receptors.24 In the presence of major basic protein, M2 receptors no longer limit ACh release from parasympathetic nerves. Consequently, loss of M2 function potentiates parasympathetic nerve-mediated bronchoconstriction. The importance of this mechanism in clinical asthma is underscored by the observation that M2 receptors are dysfunctional in asthmatics25,26 and that muscarinic antagonists such as tiotropium, which block parasympathetic nerve signaling, reverse bronchoconstriction.27,28 M2 receptor dysfunction also occurs in all animal models of airway hyperreactivity, including in the setting of respiratory virus infections,29–36 antigen sensitization and challenge,4,8–11,37–43 ozone inhalation,44–47 pesticide exposure,48,49 and obesity.50 Treatment with the polyanionic substances heparin or poly-L-glutamic acid that neutralize major basic protein,37 or an antibody against major basic protein,9,46 preserve neuronal M2 receptor function and prevent airway hyperreactivity. Furthermore, M2 receptor and airway function are protected in experimental models by reducing the number of airway eosinophils using an anti-IL5 antibody,8 or by blocking eosinophil migration into airways with anti-CCR341 or anti-very late antigen-4 (VLA-4) antibodies.38
1.3 |. Nerve-associated eosinophils cause M2 receptor dysfunction
Eosinophil migration to airway nerves is critical for the development of M2 receptor dysfunction and airway hyperreactivity. In histologic airway sections from patients who died of acute asthma11 and in experimental models of antigen- and ozone-induced airway inflammation,9,39,42,43,45,51 eosinophils are found clustered around nerve axons and parasympathetic ganglia. This association is not simply due to more eosinophils in airways overall. Eosinophils are dis-proportionally associated with nerves (~2-fold greater number), compared to blood vessels and airway parenchyma, and the number of eosinophils along nerve axons corresponds with the degree of M2 receptor dysfunction.11
Airway nerves actively recruit eosinophils by releasing chemokines such as eotaxin-1. Eotaxin-1 binds eosinophil CCR3 receptors and is a potent mediator of eosinophil migration.52 Parasympathetic and sensory nerves constitutively express eotaxin-1 at baseline and increase expression after antigen challenge.41,53 These changes may be mediated by IL4, which increases eotaxin-1 expression in a cholinergic neuroblastoma model of parasympathetic nerves. Furthermore, in antigen-sensitized and challenged guinea pigs, CCR3 antagonists reduce eosinophils around nerves without affecting the number of eosinophils in lung tissue overall, indicating that CCR3 specifically regulates eosinophil migration to nerves in vivo. Importantly, CCR3 antagonists also block airway hyperactivity.41 Thus, the proximity of eosinophils to airway nerves is critical for mediating their effects.
TNF-α is another cytokine that regulates eosinophil recruitment to nerves, albeit through multiple mechanisms. TNF-α promotes eosinophil chemotaxis and survival by stimulating eotaxin and IL5 production, respectively.54,55 Accordingly, eosinophil migration to nerves and subsequent development of airway hyperreactivity after antigen challenge and after ozone exposure are blocked by the TNF-α receptor antagonist etanercept.42,56 TNF-α also directly decreases M2 receptor expression in airway parasympathetic nerves independent of eosinophils. Therefore, TNF-α induces airway hyperreactivity through direct and indirect effects on airway nerves.
After eosinophils are recruited to airway nerves, they bind to neuronally expressed adhesion proteins VCAM-1 and ICAM-1 via complementary receptors VLA-4 and CD11b, respectively.57 VCAM-1 is constitutively expressed by parasympathetic nerves, whereas ICAM-1 is upregulated in response to TNF-α or IFN-γ.58 Similar de novo expression of ICAM-1 or VCAM-1 occurs on IMR-32 cholinergic neuroblastoma cells in response to TNF-α or IFN-γ,58 and on sensory nerves in response to nerve growth factor.53 The physiologic importance of these adhesion proteins is supported by studies showing that adhesion proteins are upregulated in the lungs after antigen sensitization,59 and that blocking eosinophil binding to VCAM-1 or ICAM-1 prevents airway hyperreactivity in antigen challenged guinea pigs38 and monkeys in vivo.60
The corticosteroid dexamethasone also reduces airway hyperreactivity after antigen sensitization in guinea pigs by down-regulating parasympathetic nerve ICAM-1 expression, which reduces eosinophil-nerve adhesion.58 This model provides additional evidence for the importance of physical interactions between eosinophils and airway nerves since the dose of dexamethasone that prevents airway hyperreactivity decreases the number of eosinophils associated with nerves, but not eosinophils in broncho-alveolar lavage or in airway tissue.
Eosinophil degranulation is triggered by adhesion to either neuronal ICAM-1 or VCAM-1, followed by an additional stimulus from neurons such as the release of reactive oxygen species.61,62 This secondary neuronal signal is essential since formaldehyde-fixed neurons bind eosinophils, but do not induce degranulation. However, binding ICAM-1 or VCAM-1 does augment other eosinophil functions such as leukotriene release, even in the absence of neuronal mediators.62 Thus, adhesion alone activates eosinophils while degranulation requires an additional neuronally released stimulus.
1.4 |. Eosinophils increase sensory innervation, neuropeptide expression, and reflex bronchoconstriction
The effects of eosinophils are not limited to the autonomic parasympathetic pathway. Eosinophils alter sensory nerve structure and regulation of reflex bronchoconstriction as well. Recently, we found that transgenic mice with airway eosinophilia driven by over-expression of IL5 from airway epithelium have markedly increased airway sensory innervation compared to wild-type mice. Innervation in eosinophil-deficient mice and eosinophil-deficient mice with elevated airway IL5 was similar to that in wild-type mice, indicating that eosinophils, not IL5, mediate changes in nerve structure (unpublished observation). Mice with more airway sensory nerves had increased reflex bronchoconstriction. Thus, increased innervation is linked to airway hyperreactivity. Airway biopsies from humans with eosinophilic asthma also had more sensory innervation compared to non-asthmatics, suggesting that eosinophils have similar effects on nerve structure in humans (unpublished observation).
Eosinophil-mediated changes in sensory nerve structure are accompanied by alterations in neuropeptide expression. Neuropeptides, such as substance P, are small signaling molecules that are synthesized and secreted by sensory nerves. Nerves increase substance P expression in response to allergens,63 ozone,64 and respiratory viruses,65 and blocking the substance P receptor neurokinin-1 (NK1) suppresses airway hyperreactivity.35,39,64,66 Eosinophils mediate an increase in neuronal substance P after allergens and consequently, changes in neuronal substance P after allergens are absent in eosinophil-deficient mice (unpublished observation). Substance P causes airway hyperreactivity in several ways. Substance P activates NK1 receptors on parasympathetic nerves, which potentiates neuronal ACh release,67 and NK1 receptors on eosinophils, which triggers the release of major basic protein.68 Substance P also stimulates bronchoconstriction directly by activating NK1 receptors on airway smooth muscle.69,70
Neuropeptides also promote eosinophil recruitment. For example, eosinophil chemotaxis occurs along neuropeptide concentration gradients71 and neuropeptides enhance eosinophil responsiveness to other chemotactic factors such as platelet activating factor and leukotriene B4.72 In turn, neuropeptides stimulate eosinophils’ entry into tissues by upregulating ICAM-1,73 VCAM-1,74 and E-selectin75 binding molecules on vascular endothelium. This effect can be modeled in vivo by stimulating nerves in rat airways. Nerve stimulation increases eosinophil adherence to endothelium and is blocked by an antagonist of NK1.76 Thus, neuropeptides promote eosinophil recruitment by increasing eosinophil responsiveness and by modulating local tissue environments.
Eosinophils influence sensory nerve function by altering the threshold for nerve activation. Specifically, sensory nerves are more sensitive to depolarization by capsaicin, ATP, and electrical stimulation after exposure to major basic protein and eosinophil peroxidase.77,78 The effects of granule proteins are completely eliminated by the neutralizing polyanions heparin or poly-L-glutamic acid, further establishing that sensory nerves and eosinophils exert bidirectional effects on each other’s function.
1.5 |. Distinct eosinophil populations exert different effects in airways
Eosinophils perform a broad range of functions in the airways that have reshaped perceptions that they solely have cytotoxic effects in asthma.36,79–84 Their interactions with airway nerves and their role in mediating airway hyperreactivity are no exception. For example, Wicher et al. recently demonstrated that eosinophils paradoxically reduce airway hyperreactivity 3 days after ozone exposure in guinea pigs despite initially causing airway hyperreactivity after 1 day.56 Distinct eosinophil populations mediate these divergent effects. Initially, ozone triggers the release of eosinophil major basic protein from airway resident eosinophils, which results in M2 dysfunction and airway hyperresponsiveness. In contrast, an influx of newly divided bone marrow-derived eosinophils arrives 3 days later and attenuates airway hyperreactivity at this time point. TNF-α and IL5 mediate eosinophilopoiesis and the recruitment of protective eosinophils in nonsensitized hosts after ozone. However, this mechanism is fundamentally altered by antigen sensitization. Eosinophils fail to expand in bone marrow after ozone in antigen-sensitized animals. Consequently, newly divided bone marrow-derived eosinophils do not arrive in the airways and ozone-induced airway hyperreactivity persists in sensitized animals 3 days after exposure.85 These findings have important implications for asthmatics exposed to environmental ozone. Ozone is a well-established precipitant of asthma exacerbations and approximately half of all asthmatics are sensitized to aero-allergens.86 Atopic status may be an important determinant of treatment response in these patients.
The role of eosinophils during a respiratory virus infection is similarly dependent on the sensitization status of the host. While respiratory viruses cause airway hyperreactivity in both nonsensitized and antigen-sensitized guinea pigs, virus-induced airway hyperreactivity is only mediated by eosinophils in antigen-sensitized animals.36 Eosinophils’ effects in sensitized animals require the presence of CD8 T cells.34 CD8 T cells bind to eosinophils directly to induce degranulation87 and secrete cytokines that modulate eosinophils’ responses.88,89 Other noncanonical eosinophil functions are observed during viral infections as well, including antigen presentation to CD4 T cells90,91 and nitric oxide generation that contributes to antiviral immune defense.79
These studies highlight the evolving understanding that eosinophils have diverse phenotypes and functions. This concept builds in large part on the foundational work of Leeet al.,92 and is particularly evident in the lung. As Mesnil et al. elegantly illustrated, the lungs of mice at steady state contain a population of resident eosinophils that are IL5-independent and express Siglec-Fint CD62L+CD101low.93 In contrast, a second population of IL5-dependent, Siglec-Fhi CD62L−CD101hi eosinophils is recruited to lungs after house dust mite exposure. It is likely that these unique populations affect nerve function in different ways, particularly given that nerve function is preserved by blocking IL5-dependent eosinophils in some models (e.g. antigen-sensitized guinea pigs),8 but not in others (e.g. nonsensitized guinea pigs exposed to ozone).56 The relationships between eosinophil phenotypes and airway nerve function in asthma are an area of active, ongoing investigation.
1.6 |. Implications of eosinophil-nerve interactions for asthma phenotypes
Asthma is categorized into subgroups, or phenotypes, based on clinical features and biomarkers.94 Phenotypes identify groups with common immunologic mechanisms that drive disease and are used clinically to identify patients who may benefit from advanced therapies. Examples of such therapies include antibodies against IL5 (e.g. mepolizumab and reslizumab) and the IL5 receptor (e.g. benralizumab), which have been found to reduce the number of asthma exacerbations and systemic corticosteroid use in eosinophilic asthmatics.95–98 Several other treatments may enter clinical use soon, including antibodies directed against IL13, IL4 receptor-alpha, and thymic stromal lymphopoietin (TSLP).99–101 However, none of these agents were developed with eosinophil-nerve interactions specifically in mind. Indeed, targeted therapies that prevent eosinophil migration and binding to nerves have the potential to reduce airway hyperreactivity and improve airway function. These outcomes are distinct from exacerbations, contribute to patients’ daily symptom burden, and are insufficiently treated by current anti-IL5 therapies. Prime candidates include antagonists against CCR3 and ICAM-1 that have shown promise in preclinical animal models.41,60 Furthermore, existing therapies that block parasympathetic nerve-mediated bronchoconstriction, such as tiotropium, may have even greater efficacy in eosinophilic asthmatics with increased airway innervation. Therefore, it may be valuable to consider increased innervation as a distinct asthma phenotype in future clinical trials.
2 |. SUMMARY
Interactions between eosinophils and airway nerves lead to the development of airway hyperreactivity and excessive bronchoconstriction that are characteristic of asthma. Nerves release chemokines that actively recruit eosinophils, and stimulate eosinophil degranulation and release of major basic protein, which potentiates parasympathetic nerve-mediated bronchoconstriction. Eosinophil mediators also increase sensory nerve-induced reflex bronchoconstriction by stimulating nerve growth and neuropeptide expression. Therefore, eosinophils worsen bronchoconstriction through effects on both sensory afferent and parasympathetic efferent pathways. Interactions between airway nerves and eosinophils provide a rich opportunity for development of therapies that treat excessive bronchoconstriction in asthma.
Abbreviations:
- ACh
acetylcholine
- NK1
neurokinin-1 receptor
- VLA-4
very late antigen-4
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Undem BJ, Carr MJ, Kollarik M. Physiology and plasticity of putative cough fibres in the guinea pig. Pulm Pharmacol Ther. 2002;15: 193–198. [DOI] [PubMed] [Google Scholar]
- 2.Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev. 2016;96:975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984;83:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fryer AD, Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol (1985). 1991;71:2255–2261. [DOI] [PubMed] [Google Scholar]
- 5.Nadel JA, Barnes PJ. Autonomic regulation of the airways. Annu Rev Med. 1984;35:451–467. [DOI] [PubMed] [Google Scholar]
- 6.Wagner EM, Jacoby DB. Methacholine causes reflex bronchoconstriction. J Appl Physiol (1985). 1999;86:294–297. [DOI] [PubMed] [Google Scholar]
- 7.Scott GD, Blum ED, Fryer AD, Jacoby DB. Tissue optical clearing, three-dimensional imaging, and computer morphometry in whole mouse lungs and human airways. Am J Respir Cell Mol Biol. 2014;51:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbon CL, Jacoby DB, Fryer AD. Pretreatment with an antibody to interleukin-5 prevents loss of pulmonary M2 muscarinic receptor function in antigen-challenged guinea pigs. Am J Respir Cell Mol Biol. 1995;12:320–328. [DOI] [PubMed] [Google Scholar]
- 9.Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest. 1997;100:2254–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello RW, Evans CM, Yost BL, et al. Antigen-induced hyperreactivity to histamine: role of the vagus nerves and eosinophils. Am J Physiol. 1999;276:L709–L714. [DOI] [PubMed] [Google Scholar]
- 11.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol. 1997;273: L93–L103. [DOI] [PubMed] [Google Scholar]
- 12.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138:4S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol. 2002;2:211–215. [DOI] [PubMed] [Google Scholar]
- 14.Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–1948. [DOI] [PubMed] [Google Scholar]
- 15.Minette PA, Barnes PJ. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J Appl Physiol (1985). 1988;64:2532–2537. [DOI] [PubMed] [Google Scholar]
- 16.Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. Am Rev Respir Dis. 1980;121:389–413. [DOI] [PubMed] [Google Scholar]
- 17.Sheppard D, Epstein J, Holtzman MJ, Nadel JA, Boushey HA. Dose-dependent inhibition of cold air-induced bronchoconstriction by atropine. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:169–174. [DOI] [PubMed] [Google Scholar]
- 18.Simonsson BG, Skoogh BE, Ekstrom-Jodal B. Exercise-induced airways constriction. Thorax. 1972;27:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold WM. Vagally-mediated reflex bronchoconstriction in allergic asthma. Chest. 1973;63:11S. [DOI] [PubMed] [Google Scholar]
- 20.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol (1985). 1989;67: 2461–2465. [DOI] [PubMed] [Google Scholar]
- 21.Makker HK, Holgate ST. The contribution of neurogenic reflexes to hypertonic saline-induced bronchoconstriction in asthma. J Allergy Clin Immunol. 1993;92:82–88. [DOI] [PubMed] [Google Scholar]
- 22.Crimi N, Palermo F, Oliveri R, Polosa R, Settinieri I, Mistretta A. Protective effects of inhaled ipratropium bromide on bronchoconstriction induced by adenosine and methacholine in asthma. Eur Respir J. 1992;5:560–565. [PubMed] [Google Scholar]
- 23.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayala LE, Ahmed T. Is there loss of protective muscarinic receptor mechanism in asthma?. Chest. 1989;96:1285–1291. [DOI] [PubMed] [Google Scholar]
- 26.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but notin asthmatic subjects. J Appl Physiol (1985). 1989;67:2461–2465. [DOI] [PubMed] [Google Scholar]
- 27.Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerstjens HA, Disse B, Schroder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–314. [DOI] [PubMed] [Google Scholar]
- 29.Fryer AD, Jacoby DB. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1991;102:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rynko AE, Fryer AD, Jacoby DB. Interleukin-1β mediates virus-induced m2 muscarinic receptor dysfunction and airway hyperreactivity. Am J Respir Cell Mol Biol. 2014;51:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie Z, Scott GD, Weis PD, Itakura A, Fryer AD, Jacoby DB. Role of TNF-alpha in virus-induced airway hyperresponsiveness and neuronal M(2) muscarinic receptor dysfunction. Br J Pharmacol. 2011;164:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno-Vinasco L, Verbout NG, Fryer AD, Jacoby DB. Retinoic acid prevents virus-induced airway hyperreactivity and M2 receptor dysfunction via anti-inflammatory and antiviral effects. Am J Physiol Lung Cell Mol Physiol. 2009;297:L340–L346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno L, Jacoby DB, Fryer AD. Dexamethasone prevents virus-induced hyperresponsiveness via multiple mechanisms. Am J Physiol Lung Cell Mol Physiol. 2003;285:L451–L455. [DOI] [PubMed] [Google Scholar]
- 34.Adamko DJ, Fryer AD, Bochner BS, Jacoby DB. CD8+ T lymphocytes in viral hyperreactivity and M2 muscarinic receptor dysfunction. Am J Respir Crit Care Med. 2003;167:550–556. [DOI] [PubMed] [Google Scholar]
- 35.Jacoby DB, Yost BL, Elwood T, Fryer AD. Effects of neurokinin receptor antagonists in virus-infected airways. Am J Physiol Lung Cell Mol Physiol. 2000;279:L59–L65. [DOI] [PubMed] [Google Scholar]
- 36.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fryer AD, Jacoby DB. Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J Clin Invest. 1992;90:2292–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fryer AD, Costello RW, Yost BL, et al. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J Clin Invest. 1997;99:2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costello RW, Fryer AD, Belmonte KE, Jacoby DB. Effects of tachykinin NK1 receptor antagonists on vagal hyperreactivity and neuronal M2 muscarinic receptor function in antigen challenged guinea-pigs. Br J Pharmacol. 1998;124(2):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans CM, Jacoby DB, Fryer AD. Effects of dexamethasone on antigen-induced airway eosinophilia and M(2) receptor dysfunction. Am J Respir Crit Care Med. 2001;163:1484–1492. [DOI] [PubMed] [Google Scholar]
- 41.Fryer AD, Stein LH, Nie Z, et al. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nie Z, Jacoby DB, Fryer AD. Etanercept prevents airway hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. Br J Pharmacol. 2009;156:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbout NG, Jacoby DB, Gleich GJ, Fryer AD. Atropine-enhanced, antigen challenge-induced airway hyperreactivity in guinea pigs is mediated by eosinophils and nerve growth factor. Am J Physiol Lung Cell Mol Physiol. 2009;297:L228–L237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultheis AH, Bassett DJ, Fryer AD. Ozone-induced airway hyperresponsiveness and loss of neuronal M2 muscarinic receptor function. J Appl Physiol (1985). 1994;76:1088–1097. [DOI] [PubMed] [Google Scholar]
- 45.Yost BL, Gleich GJ, Jacoby DB, Fryer AD. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L627–L635. [DOI] [PubMed] [Google Scholar]
- 46.Yost BL, Gleich GJ, Fryer AD. Ozone-induced hyperresponsiveness and blockade of M2 muscarinic receptors by eosinophil major basic protein. J Appl Physiol (1985). 1999;87:1272–1278. [DOI] [PubMed] [Google Scholar]
- 47.Gambone LM, Elbon CL, Fryer AD. Ozone-induced loss of neuronal M2 muscarinic receptor function is prevented by cyclophosphamide. J Appl Physiol (1985). 1994;77:1492–1499. [DOI] [PubMed] [Google Scholar]
- 48.Proskocil BJ, Bruun DA, Thompson CM, Fryer AD, Lein PJ. Organophosphorus pesticides decrease M2 muscarinic receptor function in guinea pig airway nerves via indirect mechanisms. PLoS One. 2010;5:e10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci. 2005;83:166–176. [DOI] [PubMed] [Google Scholar]
- 50.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verhein KC, Jacoby DB, Fryer AD. IL-1 receptors mediate persistent, but not acute, airway hyperreactivity to ozone in guinea pigs. Am J Respir Cell Mol Biol. 2008;39:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–1172. [DOI] [PubMed] [Google Scholar]
- 53.Foster EL, Simpson EL, Fredrikson LJ, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011;6:e22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghaffar O, Hamid Q, Renzi PM, et al. Constitutive and cytokine-stimulated expression of eotaxin by human airway smooth muscle cells. Am J Respir Crit Care Med. 1999;159:1933–1942. [DOI] [PubMed] [Google Scholar]
- 55.Zhang K, Gharaee-Kermani M, McGarry B, Remick D, Phan SH. TNF-alpha-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol. 1997;158:954–959. [PubMed] [Google Scholar]
- 56.Wicher SA, Jacoby DB, Fryer AD. Newly divided eosinophils limit ozone-induced airway hyperreactivity in nonsensitized guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2017;312:L969–L982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawatzky DA, Kingham PJ, Court E, et al. Eosinophil adhesion to cholinergic nerves via ICAM-1 and VCAM-1 and associated eosinophil degranulation. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1279–L1288. [DOI] [PubMed] [Google Scholar]
- 58.Nie Z, Nelson CS, Jacoby DB, Fryer AD. Expression and regulation of intercellular adhesion molecule-1 on airway parasympathetic nerves. J Allergy Clin Immunol. 2007;119:1415–1422. [DOI] [PubMed] [Google Scholar]
- 59.Miao H, Xue QF, Hu QH, et al. In situ expression of ICAM-1 and its mRNA in the lung tissue of asthmatic rats. Clin Hemorheol Microcirc. 1997;17:325–331. [PubMed] [Google Scholar]
- 60.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247:456–459. [DOI] [PubMed] [Google Scholar]
- 61.Walsh MT, Curran DR, Kingham PJ,et al. Effect of eosinophil adhesion on intracellular signaling in cholinergic nerve cells. Am J Respir Cell Mol Biol. 2004;30:333–341. [DOI] [PubMed] [Google Scholar]
- 62.Kingham PJ, McLean WG, Sawatzky DA, Walsh MT, Costello RW. Adhesion-dependent interactions between eosinophils and cholinergic nerves. Am J Physiol Lung Cell Mol Physiol. 2002;282: L1229–L1238. [DOI] [PubMed] [Google Scholar]
- 63.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest. 1996;98:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu ZX, Maize DF Jr, Satterfield BE, Frazer DG, Fedan JS, Dey RD. Role of intrinsic airway neurons in ozone-induced airway hyperresponsiveness in ferret trachea. J Appl Physiol (1985). 2001;91: 371–378. [DOI] [PubMed] [Google Scholar]
- 65.Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med. 2002;165:1071–1075. [DOI] [PubMed] [Google Scholar]
- 66.Verhein KC, Hazari MS, Moulton BC, Jacoby IW, Jacoby DB, Fryer AD. Three days after a single exposure to ozone, the mechanism of airway hyperreactivity is dependent on substance P and nerve growth factor. Am J Physiol Lung Cell Mol Physiol. 2011;300: L176–L184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watson N, Maclagan J, Barnes PJ. Endogenous tachykinins facilitate transmission through parasympathetic ganglia in guinea-pig trachea. Br J Pharmacol. 1993;109:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans CM, Belmonte KE, Costello RW, Jacoby DB, Gleich GJ, Fryer AD. Substance P-induced airway hyperreactivity is mediated by neuronal M(2) receptor dysfunction. Am J Physiol Lung Cell Mol Physiol. 2000;279:L477–L486. [DOI] [PubMed] [Google Scholar]
- 69.Devillier P,Advenier C, Drapeau G, Marsac J, Regoli D. Comparison of the effects of epithelium removal and of an enkephalinase inhibitor on the neurokinin-induced contractions of guinea-pig isolated trachea. Br J Pharmacol. 1988;94:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amadesi S, Moreau J, Tognetto M, et al. NK1 receptor stimulation causes contraction and inositol phosphate increase in mediumsize human isolated bronchi. Am J Respir Crit Care Med. 2001;163: 1206–1211. [DOI] [PubMed] [Google Scholar]
- 71.Dunzendorfer S, Meierhofer C, Wiedermann CJ. Signaling in neuropeptide-induced migration of human eosinophils. J Leukoc Biol. 1998;64:828–834. [DOI] [PubMed] [Google Scholar]
- 72.Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149:3309–3315. [PubMed] [Google Scholar]
- 73.Nakagawa N, Sano H, Iwamoto I. Substance P induces the expression of intercellular adhesion molecule-1 on vascular endothelial cells and enhances neutrophil transendothelial migration. Peptides. 1995;16:721–725. [DOI] [PubMed] [Google Scholar]
- 74.Quinlan KL, Song IS, Naik SM, et al. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol. 1999;162: 1656–1661. [PubMed] [Google Scholar]
- 75.Smith CH, Barker JN, Morris RW, MacDonald DM, Lee TH. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151:3274–3282. [PubMed] [Google Scholar]
- 76.Baluk P, Bertrand C,Geppetti P, McDonald DM, Nadel JA. NK1 receptors mediate leukocyte adhesion in neurogenic inflammation in the rat trachea. Am J Physiol. 1995;268:L263–L269. [DOI] [PubMed] [Google Scholar]
- 77.Gu Q, Lim ME, Gleich GJ, Lee LY. Mechanisms of eosinophil major basic protein-induced hyperexcitability of vagal pulmonary chemosensitive neurons. Am J Physiol Lung Cell Mol Physiol. 2009;296:L453–L461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu Q, Wiggers ME, Gleich GJ, Lee LY. Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am J Physiol Lung Cell Mol Physiol. 2008;294:L544–L552. [DOI] [PubMed] [Google Scholar]
- 79.Drake MG, Bivins-Smith ER, Proskocil BJ, et al. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am J Respir Cell Mol Biol. 2016;55:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Percopo CM, Dyer KD, Ochkur SI, et al. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yousefi S, Gold JA, Andina N, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. [DOI] [PubMed] [Google Scholar]
- 83.Farhan RK, Vickers MA, Ghaemmaghami AM, Hall AM, Barker RN, Walsh GM. Effective antigen presentation to helper T cells by human eosinophils. Immunology. 2016;149:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wicher SA, Lawson KL, Jacoby DB, Fryer AD, Drake MG. Ozone-induced eosinophil recruitment to airways is altered by antigen sensitization and tumor necrosis factor-alpha blockade. Physiol Rep. 2017;5:e13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol. 2014;134:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mengelers HJ, Maikoe T, Raaijmakers JA, Lammers JW, Koenderman L. Cognate interaction between human lymphocytes and eosinophils is mediated by beta 2-integrins and very late antigen-4. J Lab Clin Med. 1995;126:261–268. [PubMed] [Google Scholar]
- 88.Till S, Li B, Durham S, et al. Secretion of the eosinophil-active cytokines interleukin-5, granulocyte/macrophage colony-stimulating factor and interleukin-3 by bronchoalveolar lavage CD4+ and CD8+ T cell lines in atopic asthmatics, and atopic and non-atopic controls. Eur J Immunol. 1995;25:2727–2731. [DOI] [PubMed] [Google Scholar]
- 89.Mbawuike IN, Wells J, Byrd R, Cron SG, Glezen WP, Piedra PA. HLA-restricted CD8+ cytotoxic T lymphocyte, interferon-gamma, and interleukin-4 responses to respiratory syncytial virus infection in infants and children. J Infect Dis. 2001;183:687–696. [DOI] [PubMed] [Google Scholar]
- 90.Handzel ZT, Busse WW, Sedgwick JB, et al. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998; 160:1279–1284. [PubMed] [Google Scholar]
- 91.Shi HZ. Eosinophils function as antigen-presenting cells. J Leukoc Biol. 2004;76:520–527. [DOI] [PubMed] [Google Scholar]
- 92.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 2013;26:710–715. [DOI] [PubMed] [Google Scholar]
- 95.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. [DOI] [PubMed] [Google Scholar]
- 96.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. [DOI] [PubMed] [Google Scholar]
- 97.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. [DOI] [PubMed] [Google Scholar]
- 98.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. [DOI] [PubMed] [Google Scholar]
- 99.Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–796. [DOI] [PubMed] [Google Scholar]
- 100.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. [DOI] [PubMed] [Google Scholar]
- 101.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377:936–946. [DOI] [PubMed] [Google Scholar]


