Abstract
OBJECTIVES:
To examine automobile crash risk associated with cognition in older drivers without dementia.
DESIGN:
Retrospective secondary analysis of longitudinal cohort study.
SETTING:
Our study used data from the Adult Changes in Thought (ACT) Study merged with Washington State crash reports and licensure records. Data were available from 2002 to 2015.
PARTICIPANTS:
Group Health enrollees from Washington State aged 65 and older with active driver’s licenses (N=2,615).
MEASUREMENTS:
Cognitive function was assessed using the Cognitive Abilities Screening Instrument scored using item response theory (CASI-IRT). The study outcome was police-reported motor vehicle crash. We used a negative binomial mixed-effects model with robust standard errors clustered on the individual and considered associations between crash risk, level of cognition, and amount of decline since the previous study visit. Covariates included age, sex, education, alcohol, depression, medical comorbidities, eyesight, hearing, and physical function. Individuals were censored at dementia diagnosis, death, or failure to renew their license.
RESULTS:
Over an average of 7 years of follow-up, 350 (13%) people had at least one crash. A 1-unit lower CASI-IRT score was associated with a higher adjusted incidence rate ratio of crash of 1.26 (95% confidence interval = 1.08–1.51). Beyond level of cognition, amount of cognitive decline between study visits was not associated with crash risk.
CONCLUSION:
This study suggests that, in older drivers, poorer performance on the CASI-IRT may be a risk factor for motor vehicle crashes, even in individuals without diagnosed dementia. Further research is needed to understand driving behavior and inform driving decisions for older adults with poor cognitive function.
Keywords: older drivers, motor vehicle crash, dementia, cognition, cognitive measurement
Approximately 86% of adults aged 65 and older have an active license to operate a motor vehicle.1 Having the option to drive has benefits for older adults, including greater life satisfaction.2 Cessation is associated with negative health outcomes, including isolation, depression, early entry into a long-term care facility, and caregiver bur-den,1–3 although a crash can be devastating or deadly for fragile older adults and other road users.4 The fatal crash rate for older drivers per vehicle-mile traveled begins to rise at age 65. Drivers aged 85 and older have the highest fatal crash rate per mile of any age group.5 Older adults may self-regulate or cease driving because of health prob-lems.1,6 Social, logistical, and health factors must be considered as older individuals, families, and clinicians balance safety with the independence of driving.
Cognitive function influences ability to perceive hazards in the road, process visual cues (e.g., stop lights), focus on driving tasks, anticipate other road users’ actions, and make quick decisions.2,3,7–10 Individuals with dementia become lost more; have greater difficulties at intersections; are more likely to confuse pedals, maintain speed poorly, and stray from designated lanes; and have poorer safety judgment than older drivers without demen-tia.9,11–14 Cognitive impairment may also affect capacity to self-monitor and self-regulate driving.1,8,10,15
Healthcare providers and state licensing departments of older adults with prodromal or early-stage dementia may considered them able to drive,1 and they may pass driving tests.14 Prior studies found that drivers with dementia were at higher risk of a crash, although strength of association varied.2,3,9,10,16
There is limited understanding of how cognitive impairment before a dementia diagnosis translates to crash risk and how clinicians should advise older adults and their families about driving behavior as cognition changes.1–3,17 Research has focused primarily on the period after which a diagnosis of dementia has been made. Published studies are few and have used outcomes such as driving tests, driver tests, perceived driving ability, and simulated driving that may not translate to real-world crash risk or have had brief follow-up or small sample size.2,3,7,9–14,18–20
Guidelines as to when individuals with cognitive decline should stop driving are vague. The National Highway Traffic Safety Administration advises eventual cessation.1 Department of Motor Vehicle guidelines vary according to state.1 Research aimed at clinicians and nontechnical articles for families and drivers emphasize the importance of tapering and eventual cessation as cognition declines but provide limited guidance as to when specifically to stop driving.3,12,13,20–22 This vagueness reflects lack of evidence of the relationship between changing cognition and crash risk.
We evaluated the association between cognitive function and crash risk in drivers aged 65 and older without dementia in a cohort of older drivers over a 13-year period. We also assessed the association between change in cognitive function and crash risk.
METHODS
Study Design and Participants
The Adult Changes in Thought (ACT) Study is an ongoing, longitudinal study of adults aged 65 and older. Enrollees are examined biennially to identify incident dementia. We provide further details in Supplementary Appendix S1.
We linked ACT data from 2002 to 2015 to the Washington State crash database and to licensure information from the Washington State Department of Licensing. Participant last names and birth dates were used to generate driver’s license identification numbers.23 Participants were followed until they dropped out of ACT, died, failed to renew their license, or 2 years (the average length of time between study visits) after their last ACT visit, with the date of each serving as their end date (Figure 1).
Figure 1.
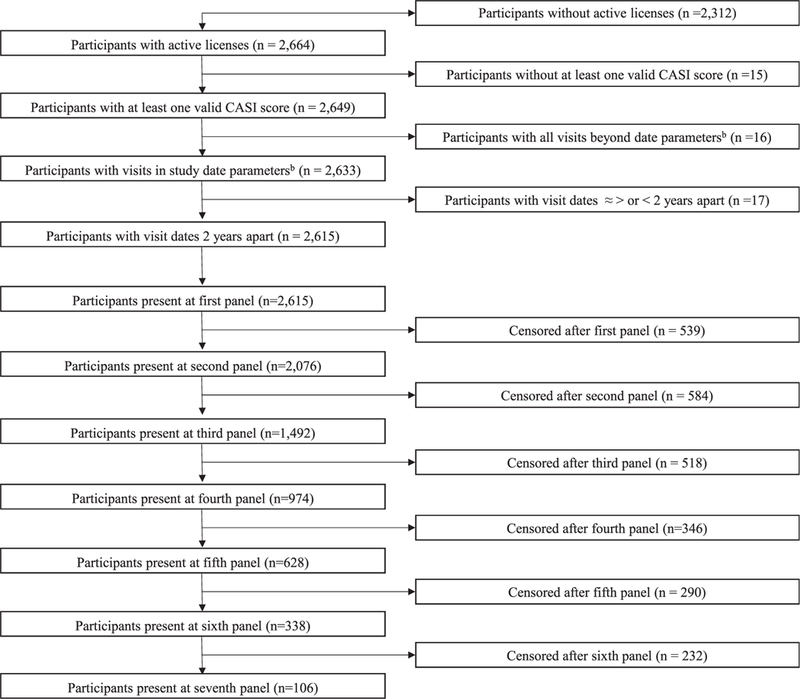
STrengthening the Reporting of OBservational studies in Epidemiology diagram detailing reasons for exclusion from study sample and number of participants included in each study panel. aTotal number of Adult Changes in Thought (ACT) study participants who matched between the Washington State Department of Licensure database and the ACT participant registry. bParticipants with licensure dates and ACT participation dates for the same time periods.
Measures
The outcome of interest was a motor vehicle crash in which the participant was the driver on any nonprivate road within Washington State reported by or to police or Washington State Patrol. Crashes are reported to Washington State Patrol if there is any injury, death, or property damage totaling at least $1,000.24
The primary risk factor of interest, cognitive function, was measured using the Cognitive Abilities Screening Instrument25 scored using item response theory (CASI-IRT).26 We provide further details in Supplementary Appendix S2.
Covariates were selected based on a conceptual model drawing from a framework for safe driving and health sta-tus10 and the Advanced Cognition Training for Independent and Vital Elderly study.27 Demographic covariates included age, years of education, sex, and race. We adjusted for ACT visit year to account for chronological trends linked to crash risk. Visit year was modeled linearly. An exploration found no trend or relationship between visit year and CASI-IRT score.
Self-reported health covariates included visual or hearing impairment, depressive symptoms, a current problem with alcohol, physical function, and comorbidities using the RxRisk score, a revised and expanded version of the Chronic Disease Score. (See Table 1 for further definitions.) We separately adjusted for use of 4 medication classes previously shown to be associated with higher crash risk: sedatives, benzodiazepines, opioids, and anti-psychotics.1,28 Individuals were considered to be users of a medication class if they had filled 2 or more prescriptions within a 4-month period. Medication use was calculated separately for each period between study visits individually for each person.
Table 1.
Demographic Characteristics at First Visit Qualifying for Study, According to Cognitive Abilities Screening Instrument Scored Using Item Response Theory Score (N = 2,615)
| Characteristic | ≥−1, n = 62 | −1–0, n = 601 | 0–1, n = 1,373 | >1, n = 579 | Total, N = 2,615 |
|---|---|---|---|---|---|
| Age, median (IQR) | 81 (6) | 77 (10) | 74 (10) | 72 (9) | 74 (9) |
| Education, years, median (IQR) | 12(4) | 14(4) | 16(3) | 17(3) | 16(5) |
| RxRisk score, median (IQR)a | 3,906 (3,460) | 3,448 (3,497) | 3,037 (2,366) | 2,491 (1,653) | 3,054 (2,444) |
| PPF score, median (IQR)b | 8(5) | 9(4) | 10(4) | 11 (4) | 10(3) |
| Male, n (%) | 24 (44) | 272 (47) | 698 (52) | 358 (63) | 1,352 (53) |
| Race, n (%) | |||||
| White | 43 (69) | 514 (86) | 1,222 (89) | 546 (94) | 2,325 (89) |
| Black | 14 (23) | 29 (5) | 34 (3) | 6(1) | 83 (3) |
| Asian | 3 (5) | 20 (3) | 54 (4) | 13(2) | 90 (3) |
| Other, including mixed | 2 (3) | 38 (6) | 63 (4) | 14(2) | 117(4) |
| Self-reported vision problems, n (%) | 33 (61) | 364 (63) | 795 (59) | 355 (62) | 1547 (61) |
| Self-reported problem with alcohol, n (%)c | 1 (2) | 29 (5) | 116 (9) | 44 (8) | 190 (7) |
| Self-reported depression, n (%)d | 9(17) | 50 (9) | 92 (7) | 38 (7) | 189 (7) |
| Self-reported hearing problems, n (%) | 13 (24) | 248 (43) | 492 (37) | 188 (33) | 941 (37) |
| Older driver crash rate per 100,000 driver yearse | 13.55 | 7.55 | 6.23 | 5.36 | 6.53 |
This expansion of the Chronic Disease Score is a chronic comorbidity score that algorithmically combines age, sex, insurance-based prescription drug coverage, and chronic conditions measured using pharmacy data.49 For example, cardiac disease is associated with a weight of 2,914.48, and being a man aged 75 and older is associated with a weight of 2,842.19.49 The overall score was quartiled and modeled as categorical indicators.
Physical function was assessed using a performance-based physical function (PPF) score50 (range 0–16; higher scores indicating better physical function) based on grip strength in the dominant hand, 5 timed chair stands, standing balance, and average of two 10-foot timed walks.
An alcohol-related problem was characterized in the Adult Changes in Thought (ACT) Study as the respondent reporting a doctor suggesting a decrease in drinking; aggressive behaviors while intoxicated, 2 or more alcohol-related traffic violations; or social, marital, or work-related problems due to drinking.
Depression was measured using the Centers for Epidemiologic Studies Depression Scale and dichotomized at 10 by ACT.
This rate draws from each subject’s first panel only.
IQR = interquartile range.
Statistical Analyses
To assess the association between cognitive function and crash risk, we used a multilevel mixed-effects model with random intercepts and a negative binomial distribution clustered on the individual. We chose a count-based model because some individuals crashed multiple times during the 2-year period between study visits. Because of overdispersion, we used a negative binomial distribution. The use of a mixed-effects model with random intercepts adjusted for between-individual variation. We explored the potential for influential individuals or 2-year periods using a delta-beta test and a jackknife analysis but found no major variation in coefficient estimates. We explored zero-inflated models, but coefficients and confidence intervals (CIs) differed little from standard negative binomial models, and Vuong tests found no evidence of better fit. These multivariable analytical models were adjusted for the covariates CASI-IRT, age, education, sex, race, ACT visit year, visual impairment, hearing impairment, depressive symptoms, a current problem with alcohol, physical function, comorbidities, and medication use.
Each analysis period, referred to in this study as a panel, was approximately 2 years, reflecting the time between ACT visits. No individual had less than 1 year between study visits. Because some panels had unusually long lapses (up to 10 years and 93 days), we excluded panels with lapses longer than 3 years, ensuring that panel length remained relatively consistent. Excluding these removed 216 panels (2%). An analysis including these individuals using follow-up time as a log-transformed offset showed almost no difference.
Cognitive function was modeled linearly using CASI-IRT scores. We also modeled CASI-IRT scores using cubic splines and ordered categorical analyses to explore possible nonlinearity but identified no obvious cut-points or substantive differences in findings.
To assess the effect of change in CASI-IRT rather than cognitive level, we modeled the difference between the previous and current panel’s CASI-IRT score within subjects in addition to current CASI-IRT score, both continuous variables, using a negative binomial mixed-effects model. This analysis excluded baseline visits because previous CASI-IRT scores were not available.
In an additional analysis, we included a covariate denoting a previous crash during the study. This model included the number of previous panels during which a crash could have occurred to adjust for exposure. The first panel period was excluded.
Sensitivity Analyses
Study participants were limited to those with an active driving license, although we were not able to determine whether or how much driving occurred. To explore driving exposure, we conducted a series of sensitivity analyses using subsamples of participants likely to be active drivers. Each analysis involved different sets of data as inclusion criteria changed. First, in Washington State, at and after age 70, individuals must renew their licenses in person rather than online and pass a vision test at renewal.1 A higher proportion of individuals aged 70 and older who renewed their licenses may have been likely to drive actively, because they sought licensure in person. Thus, the first analysis included only individuals aged 70 and older. Second, we excluded individuals who reported using a handivan to arrive at an appointment. A third analysis included only older drivers reporting having no difficulty with activities of daily living (ADLs) and instrumental ADLs.
Next, we focused on those who might be more likely to drive because of a dearth of other options. First, we limited the analysis to individuals without a spouse to serve as a possible alternative driver. We also conducted an analysis excluding individuals using a home health or nurse services or living in a nursing home. An analysis of participants living in rural areas was not conducted because of small sample size.
The Group Health Research Institute institutional review board reviewed and approved this study and waived consent. ACT Study participants were notified of this research through the study newsletter and could opt out of participation, although none did.
Analyses were performed using Stata version 13. 1 (StataCorp, College Station, TX).
RESULTS
Sample Characteristics
The overall sample included 2,615 individuals who were followed for an average of 6.7 years, for a total of 18,063 participant-years. Individuals had between 1 and 7 ACT-related study visits (Figure 1).
At baseline, median CASI-IRT score was 0.50 (range −2.71–0.50). Baseline characteristics of the study population are shown in Table 1 according to CASI-IRT score from the lowest- (≤−1) to the highest-scoring group (>1). Median age at study entry was lower in groups with higher levels of cognitive functioning.
Cognition Function and Crash Risk
Three hundred fifty participants (13%) had at least 1 crash. There were 422 crashes during the study, with as many as 3 crashes within a panel. The overall crash rate was 23.4 crashes per 1,000 participant-years.
For older drivers, a 1-unit difference in CASI-IRT score was associated with an adjusted incidence rate ratio (IRR) for a crash of 1.26 (95% CI= 1.08–1.51) (Table 2). Covariates such as RxRisk, age, and sex were not associated with crash risk. Adjusted for the number of previous panels, having crashed earlier in the study did not have a statistically significant association with current crash risk (IRR= 0.84, 95% CI=0.47–1.50).
Table 2.
Automobile Crashes of Older Drivers, Clustered on the Individual, with Robust Standard Errors, Adjusted for Fear of Adult Changes in Thought (ACT) Visit
| Factor | IRR (95% CI) | P-Value |
|---|---|---|
| CASI-IRT scorea | 1.26 (1.07–1.48) | <.01 |
| Medication classes | .92 | |
| Benzodiazepine | 0.82 (0.51–1.30) | |
| Opioids | 1.03 (0.78–1.34) | |
| Sedatives | 1.07 (0.57–1.99) | |
| Anti-psychotics | 0.83 (0.26–2.75) | |
| Depression | 1.26 (1.03–1.53) | .02 |
| Performance-based physical function | 1.01 (0.98–1.05) | .36 |
| RxRiskb quartile (reference 1 (1,119–2,132)) | .10 | |
| 2 (2,132–3,146) | 1.47 (0.98–2.20) | |
| 3 (3,146–4,543) | 1.60 (1.06–2.40) | |
| 4 (4,543–52,970) | 1.69 (1.10–2.58) | |
| Age | 0.99 (0.96–1.02) | .50 |
| Education, years (reference <13) | .14 | |
| 13–15 | 1.27 (0.95–1.71) | |
| ≥16 | 1.37 (1.00–1.88) | |
| Female | 0.88 (0.68–1.13) | .32 |
| Race (reference white) | .50 | |
| Black | 1.11 (0.62–1.94) | |
| Asian | 1.47 (0.89–2.43) | |
| Other | 0.98 (0.51–1.70) | |
| Vision problems | 1.08 (0.85–1.37) | .45 |
| Hearing problems | 0.89 (0.73–1.12) | .37 |
All presented variables were included in the model.
This analysis assumed that the length of time between study visits (the exposure period) was similar across panels. ACT study protocol involves assessment every 2 years. More details can be found in the Statistical Analysis section and in Supplementary Methods I. We performed sensitivity analyses in which we used log-transformed offsets for follow-up time. This approach accounted for variation in length of follow-up intervals between participants. Using this approach, there was no significant change in study findings. Every unit reduction in Cognitive Abilities Screening Instrument scored using item response theory (CASI-IRT) score was associated with greater risk of a crash (incidence rate ratio (IRR) = 1.27, 95% confidence interval (CI) 5 1.08–1.51).
CASI-IRT results have been flipped; each 1-unit lower CASI-IRT score is associated with a 26% higher crash rate.
This expansion of the Chronic Disease Score is a chronic comorbidity score that algorithmically combines age, sex, insurance-based prescription drug coverage, and chronic conditions measured using pharmacy data.49 For example, cardiac disease is associated with a weight of 2,914.48, and being a man aged 75 and older is associated with a weight of 2,842.19.49 The overall score was quartiled and modeled as categorical indicators.
We performed additional analyses to incorporate change in cognitive function in addition to level of cognitive functioning. Median change in CASI-IRT between 2 sequential panels was a decrease of 0.08 points (interquartile range −1.62–0.28). Adjusted for health and demographic factors, the IRR for the level of cognition was 1.30 (95% CI= 1.04–1.62), similar to the analyses reported above, and the IRR for change in CASI-IRT score from the previous visit was not significantly associated with crash (IRR=1.10, 95% CI=0.86–1.41).
Results were relatively robust across the sensitivity analyses compared with the primary analysis (displayed on the left of figure 2), with little change in risk estimates and significance.
Figure 2.
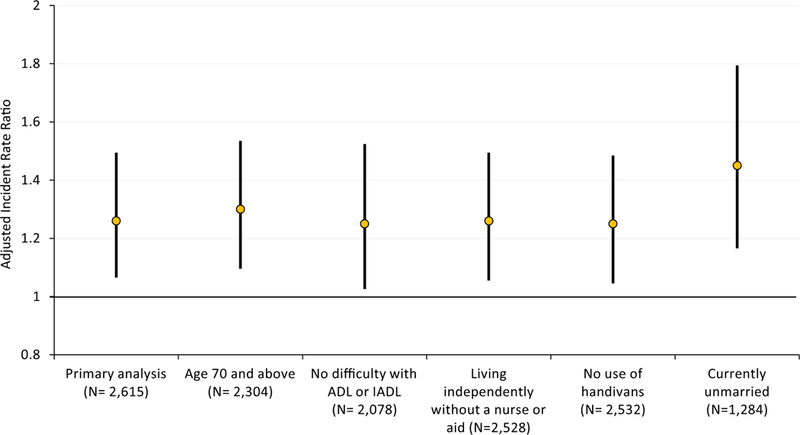
Point estimates and 95% confidence intervals for the adjusted incident rate ratio of crash associated with a 1-unit difference in Cognitive Abilities Screening Instrument scored using item response theory score for the primary analysis and sensitivity analyses. N refers to the number of clusters included in each analysis
DISCUSSION
In this large cohort study of older licensed drivers without dementia, poorer cognitive function was associated with greater risk of motor vehicle crash risk, controlling for age, sex, alcohol use, comorbidities, and medication use. Although we were not able to measure driving exposure, point estimates of crash risk were consistent across sensitivity analyses despite varying samples. This finding is in accord with other analyses evaluating predementia cognitive impairment (preclinical Alzheimer’s disease and mild cognitive impairment) and driving (road test-based driving performance and self-reported crash incidents up to 3 years before study visit).14,29,30
There is no widely accepted clinical examination, procedure, or laboratory test to evaluate driving and crash risk related to cognitive function.2 It is difficult to compare approaches because of the variety of end-points used in the literature, from state-or self-reported crash to simulator results to driving tests. Using driving tests and simulators, studies have found value in a range of methods specific to domains within cognition, including the Trail-Making Test Parts A and B; the Benton Visual Retention Test; and notably for mild cognitive impairment, the maze task. 2,14 Evidence of associations between driving performance and habits and the Montreal Cognitive Assessment, the global cognition test included in the National Highway Traffic Safety Administration Plan for Older Driver Safety,1 varies, notably in associations with future crashes.31,32 Cut-offs were established for crash risk within 2 years using the Motor-Free Visual Perception Test, the Trail-Making Test Part B, the Useful Field of View, and others using data from Maryland Pilot Older Driver Study, but neither cognition at baseline nor incremental change in cognitive status measures over time was reported.33
The Mini-Mental State Examination (MMSE) is the most widely used measure of cognitive function,2,16,34,35 although a review found mixed results using the MMSE to predict driving performance, with notably poor correlation when cognition was high.36 The longest, largest study to examine the association between crash risk and incremental change in MMSE score found no association.16 This study problematically used self-reported driving exposure and crash outcomes data, but other aspects were similar to the current study.16
The CASI and MMSE are similar in composition and coverage; they both measure global cognition, and the CASI was derived from the MMSE.25,35 The CASI includes items that assess judgment, visual perception, and verbal fluency, domains that the MMSE does not cover.25,35 The CASI may have slightly better sensitivity and specificity for dementia.34 We used IRT scoring to address curvilinear measurement properties that characterize standard scoring for the MMSE and the CASI.26
The CASI and the MMSE are composites designed to capture many elements of cognition and do not necessarily focus on domains related to crash risk. The CASI does not have sufficient measurement precision in specific cognitive domains to determine whether any particular domain was most responsible for study results. The Clinical Assessment of Driver-Related Skills (CADReS) test battery1 has been advocated as one approach to identify potentially unsafe drivers. CADReS assesses domains beyond cognition, and evaluation of its predecessor, Assessment of Driver-Related Skills, found no association with crash risk37 and poor specificity and sensitivity in predicting road test per-formance.38 More research is needed on evaluating and advising older drivers in relation to cognition.
The range of change in CASI-IRT scores showed that cognitive decline may progress at different rates in different people and also within a particular person over time. Serial assessment of cognition is generally recommended.1 When we controlled for level of cognition, change since the previous visit was not associated with crash risk. These findings suggest that cross-sectional cognitive evaluations that ignore prior levels of cognitive functioning could be used for risk stratification.
Beyond cognition, only depression was associated with crash risk in this cohort of older licensed drivers without dementia. The lack of association between crash risk and comorbidity as measured using the comorbidity scale RxRisk is consistent with other studies using comorbidity indices.39 This lack of association, when considered in tandem with the association between crash risk and specific conditions,6,40 suggests the importance of specific diagnoses and physical function rather than overall health.
Specific to eyesight, alcohol, instrumental ADLs, and hearing, it was possible that objectively measured, rather than self-reported, data were needed. Alternatively, individuals may be more aware of changes and the crash risk related to eyesight, balance, and other factors and hence more likely to stop or restrict their driving exposure.
Our study draws its primary strength from its data source, the longitudinal ACT Study. This mature and representative study has a large sample size with little attrition and detailed health, cognition, and lifestyle data.41 Because invited ACT participants represent a random sample of individuals aged 65 and older at Group Health,42 and because the Group Health population resembles the Washington State population in age, sex, and race,43 it is likely that results were generalizable beyond study participants.
The chief limitation of the present study was limited information on driving exposure. We limited inclusion in these analyses to licensed drivers, a common approach that other investigators have taken.7,12,13,19,32,33,44 Consequently, our study results are generalizable only to licensed older drivers. It is possible that older adults continue to drive without a license and are invisible to this study and to the Washington State Patrol (unless they crash). Of greater concern, older adults may be licensed but choose not to drive or to limit driving exposure in terms of mileage or environment,6,45,46 especially as cognition declines and other health conditions advance.27 This would result in an underestimation of the true crash risk. Prior research into driving exposure generated inconsistent results, drew from cross-sectional rather than longitudinal data, and focused on mileage to the exclusion of other factors that can affect crash risk (e.g., road type).47 Even with these limitations, cognitive impairment has been linked to lower driving exposure and ceasing to drive,13,19,27 although drivers may not avoid demanding driving situations.48 Point estimates of crash risk were consistent across sensitivity analyses despite varying sample sizes, increasing confidence in findings, although future research should explore changes in driving habits—objective and perceived—as cognition declines.
The available data do not include information on crashes beyond their occurrence. Also, although falls are associated with motor vehicle crashes,16 we did not have data to address their relationship.
In summary, older drivers with poorer performance on the CASI-IRT were somewhat more likely to sustain a crash. Older drivers with cognitive impairment, their family members, and clinicians must balance the benefits of autonomy, mobility, and social engagement with the risk of crashing as they make decisions about driving exposure. Study findings underscore the potential consequences of poor cognition in relation to driving and crash risk and suggest that discussions about this balance may be relevant to older drivers, their families, and clinicians, even before development of dementia.
Supplementary Material
Overview of the Adult Changes in Thought Study, Focusing on Cognitive Assessment
Description of the Cognitive Abilities Screening Instrument
ACKNOWLEDGMENTS
Sponsor’s Role: This paper was undertaken as part of Dr. Fraade-Blanar’s doctoral work. The sponsor provided funds for her tuition and stipend. Additional funds were used to pay for Group Health programming time to merge study datasets.
Footnotes
Financial Disclosure: Funding for this study was from the Institute of Translational Health Sciences through the TL1 Multidisciplinary Predoctoral Clinical Research Training Program (Grant TL1TR000422).
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Please note: Wiley-Blackwell is not responsible for the content, accuracy, errors, or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Conflict of Interest: None.
REFERENCES
- 1.Pomidor A, ed. Clinician’s Guide to Assessing and Counseling Older Driver, 3rd Ed. Washington, DC: American Geriatrics Society, 2016. [Google Scholar]
- 2.Martin A, Marottoli R, O’Neill D. Driving assessment for maintaining mobility and safety in drivers with dementia (review). Cochrane Database Syst Rev 2011;10:1–22. [DOI] [PubMed] [Google Scholar]
- 3.Breen DA, Breen DP, Moore JW, Breen PA, O’Neill D. Driving and dementia. Br Med J 2007;334:1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G, Braver ER, Chen L-H. Fragility versus excessive crash involvement as determinants of high death rates per vehicle-mile of travel among older drivers. Accid Anal Prev 2003;35:227–235. [DOI] [PubMed] [Google Scholar]
- 5.IIHS. Fatality Facts: Older People (online). Available at http://www.iihs.org/iihs/topics/t/older-drivers/fatalityfacts/older-people/2012 Accessed July 31, 2016.
- 6.Lyman JM, McGwin G, Sims R V. Factors related to driving difficulty and habits in older drivers. Accid Anal Prev 2001;33:413–421. [DOI] [PubMed] [Google Scholar]
- 7.Ott BR, Heindel WC, Papandonatos GD, et al. A longitudinal study of drivers with Alzheimer disease. Neurology 2008;70:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horswill M, Marrington S, McCullough C, et al. The hazard perception ability of older drivers. J Gerontol B Psychol Sci Soc Sci 2008;63B:P212–P218. [DOI] [PubMed] [Google Scholar]
- 9.Withaar F, Brouwer W, van Zomeren A. Fitness to drive in older drivers with cognitive impairment. J Int Neuropsychol Soc 2000;6:480^90. [DOI] [PubMed] [Google Scholar]
- 10.Anstey KJ, Wood J, Lord S, Walker JG. Cognitive, sensory and physical factors enabling driving safety in older adults. Clin Psychol Rev 2005;25: 45–65. [DOI] [PubMed] [Google Scholar]
- 11.Dawson JD, Anderson SW, Uc EY, Dastrup E, Rizzo M. Predictors of driving safety in early Alzheimer disease. Neurology 2009;72:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo M, McGehee D V, Dawson JD, Anderson SN. Simulated car crashes at intersections in drivers with Alzheimer disease. Alzheimer Dis Assoc Disord 2001;15:10–20. [DOI] [PubMed] [Google Scholar]
- 13.Carr D, Duchek J, Morris J. Characteristics of motor vehicle crashes of drivers with dementia of the Alzheimer type. J Am Geriatr Soc 2000;48: 18–22. [DOI] [PubMed] [Google Scholar]
- 14.Snellgrove CA. Cognitive Screening for the Safe Driving Competence of Older People with Mild Cognitive Impairment or Early Dementia. Canberra; 2005. [Google Scholar]
- 15.O’Connor ML, Edwards JD, Bannon Y. Self-rated driving habits among older adults with clinically-defined mild cognitive impairment, clinically-defined dementia, and normal cognition. Accid Anal Prev 2013;61:197–202. [DOI] [PubMed] [Google Scholar]
- 16.Joseph PG, O’Donnell MJ, Teo KK, et al. The Mini-Mental State Examination, clinical factors, and motor vehicle crash risk. J Am Geriatr Soc 2014; 62:1419–1426. [DOI] [PubMed] [Google Scholar]
- 17.Molnar FJ, Patel A, Marshall SC, Man-Son-Hing M, Wilson KG. Systematic review of the optimal frequency of follow-up in persons with mild dementia who continue to drive. Alzheimer Dis Assoc Disord 2006;20: 295–297. [DOI] [PubMed] [Google Scholar]
- 18.Levy DT, Vernick JS, Howard KA. Relationship between driver’s license renewal policies and fatal crashes involving drivers 70 years or older. JAMA 1995;274:1026–1030. [PubMed] [Google Scholar]
- 19.Duchek JM, Carr DB, Hunt L, et al. Longitudinal driving performance in early-stage dementia of the Alzheimer type. J Am Geriatr Soc 2003;51: 1342–1347. [DOI] [PubMed] [Google Scholar]
- 20.Lincoln N, Radford K, Lee E. The assessment of fitness to drive in people with dementia. Int J Geriatr Psychiatry 2006;21:1044–1051. [DOI] [PubMed] [Google Scholar]
- 21.Dickerson AE. Driving with dementia: Evaluation, referral, and resources. Occup Med (Lond) 2014;28:62–76. [DOI] [PubMed] [Google Scholar]
- 22.The Hartford. At the Crossroads: Family Conversations About Alzheimer’s Disease, Dementia, and Driving (online). Available at http://hartfordauto.thehartford.com/UI/Downloads/Crossroads.pdf Accessed March 26, 2018.
- 23.Gallian JA. Assigning driver’s license numbers. Math Mag 1991;64:13–22. [Google Scholar]
- 24.Washington State Patrol. Motor Vehicle Collision Reporting (OMVCR) (online). Available at https://fortress.wa.gov/wsp/wrecr/OMVCR/ Accessed September 6, 2017.
- 25.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994;6:45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- 26.Crane PK, Narasimhalu K, Gibbons LE, et al. Item response theory facilitated co-calibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol 2008;61:1081–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards JD, Ross Lesley A, Ackerman ML, et al. Longitudinal predictors of driving cessation among older adults from the ACTIVE clinical trial. J Gerontol B Psychol Sci Soc Sci 2008;63B:P6–P12. [DOI] [PubMed] [Google Scholar]
- 28.Gibson JE, Hubbard RB, Smith CJP, Tata LJ, Britton JR, Fogarty AW. Use of self-controlled analytical techniques to assess the association between use of prescription medications and the risk of motor vehicle crashes. Am J Epidemiol 2009;169:761–768. [DOI] [PubMed] [Google Scholar]
- 29.Ott BR, Jones RN, Noto RB, et al. Brain amyloid in preclinical Alzheimer’s disease is associated with increased driving risk. Alzheimer’s Dement (Amst) 2017;6:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roe CM, Babulal GM, Head DM, et al. Preclinical Alzheimer’s disease and longitudinal driving decline. Alzheimers Dement (N Y) 2017;3:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollis AM, Duncanson H, Kapust LR, Xi PM, O’Connor MG. Validity of the Mini-Mental State Examination and the Montreal Cognitive Assessment in the prediction of driving test outcome. J Am Geriatr Soc 2015;63: 988–992. [DOI] [PubMed] [Google Scholar]
- 32.Rapoport MJ, Naglie G, Weegar K, et al. The relationship between cognitive performance, perceptions of driving comfort and abilities, and selfreported driving restrictions among healthy older drivers. Accid Anal Prev 2013;61:288–295. [DOI] [PubMed] [Google Scholar]
- 33.Staplin L, Gish KW, Wagner EK. MaryPODS revisited: Updated crash analysis and implications for screening program implementation. J Safety Res 2003;34:389–397. [DOI] [PubMed] [Google Scholar]
- 34.Woodford HJ, George J. Cognitive assessment in the elderly: A review of clinical methods. QJM 2007;100:469–484. [DOI] [PubMed] [Google Scholar]
- 35.Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor B. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry 2007;78: 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobbs BM, Carr DB, Morris JC. Evaluation and management of the driver with dementia. Neurologist 2002;8:61–70. [DOI] [PubMed] [Google Scholar]
- 37.Woolnough A, Salim D, Marshall SC, et al. Determining the validity of the AMA guide: A historical cohort analysis of the Assessment of Driving Related Skills and crash rate among older drivers. Accid Anal Prev 2013; 61:311–316. [DOI] [PubMed] [Google Scholar]
- 38.Ott BR, Davis JD, Papandonatos GD, et al. Assessment of driving-related skills prediction of unsafe driving in older adults in the office setting. J Am Geriatr Soc 2013;61:1164–1169. [DOI] [PubMed] [Google Scholar]
- 39.Papa M, Boccardi V, Prestano R, et al. Comorbidities and crash involvement among younger and older drivers. PLoS One 2014;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGwin G, Sims R V, Pulley L, Roseman JM. Relations among chronic medical conditions, medications, and automobile crashes in the elderly: A population-based case-control study. Am J Epidemiol 2000;152:424–431. [DOI] [PubMed] [Google Scholar]
- 41.Crane P, Walker R, Hubbard R, et al. Glucose levels and risk of dementia. N Engl J Med 2013;369:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonnen JA, Larson EB, Haneuse S, et al. Neuropathology in the Adult Changes in Thought study: A review. J Alzheimers Dis 2009;18:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen RN, Boudreau DM, Ebel BE, Grossman DC, Sullivan SD. Sedative hypnotic medication use and the risk of motor vehicle crash. Am J Public Health 2015;105:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung I, McCartt AT. Declines in fatal crashes of older drivers: Changes in crash risk and survivability. Accid Anal Prev 2011;43:666–674. [DOI] [PubMed] [Google Scholar]
- 45.Braitman K a McCartt AT. Characteristics of older drivers who self-limit their driving. Ann Adv Automot Med 2008;52:245–254. [PMC free article] [PubMed] [Google Scholar]
- 46.Hakamies-Blomqvist L, Johansson K, Lundberg C. Driver licenses as a measure of older drivers’ exposure: A methodological note. Accid Anal Prev 1995;27:853–857. [DOI] [PubMed] [Google Scholar]
- 47.Shinar D Traffic Safety and Human Behavior, 2nd Ed. Emerald Group Publishing Limited, Bingley, UK; 2007. [Google Scholar]
- 48.O’Connor ML, Edwards JD, Wadley VG, Crowe M. Changes in mobility among older adults with psychometrically defined mild cognitive impairment. J Gerontol BPsychol Sci Soc Sci 2010;65:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fishman P, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O’Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: The RxRisk model. Med Care 2003;41:84–99. [DOI] [PubMed] [Google Scholar]
- 50.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of the Adult Changes in Thought Study, Focusing on Cognitive Assessment
Description of the Cognitive Abilities Screening Instrument


